Role of Human Macrophage Polarization in Inflammation during Infectious Diseases
Abstract
:1. Introduction
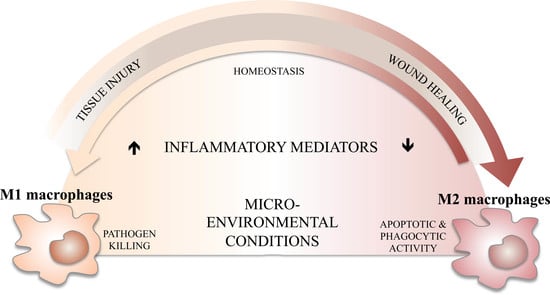
2. Macrophage Polarization and Its Role in Inflammation
2.1. M1 Like Macrophages and Inflammation
2.2. M2 Like Macrophages and Resolution of Inflammation
3. M1/M2 Balance, Inflammation, and Its Resolution
4. Swinging Macrophage Polarization During Infections
4.1. Bacterial and Fungal Infections
4.2. Viral Infections
4.3. Parasitic Infections
4.4. Leishmaniasis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Nathan, C. Metchnikoff’s legacy in 2008. Nat. Immunol. 2008, 9, 695–698. [Google Scholar] [CrossRef] [PubMed]
- David, J.R. Lymphocyte mediators and cellular hypersensitivity. N. Engl. J. Med. 1973, 288, 143–149. [Google Scholar] [PubMed]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative activation of macrophages: An immunologic functional perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef] [PubMed]
- Duffield, J.S.; Lupher, M.; Thannickal, V.J.; Wynn, T.A. Host responses in tissue repair and fibrosis. Annu. Rev. Pathol. 2013, 8, 241–276. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J. Macrophage polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Mosser, D.M. Macrophage activation by endogenous danger signals. J. Pathol. 2008, 214, 161–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, W.; Zhao, X.; Daha, M.R.; van Kooten, C. Reversible differentiation of pro- and anti-inflammatory macrophages. Mol. Immunol. 2013, 53, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Beyer, M.; Mallmann, M.R.; Xue, J.; Staratschek-Jox, A.; Vorholt, D.; Krebs, W.; Sommer, D.; Sander, J.; Mertens, C.; Nino-Castro, A.; et al. High-resolution transcriptome of human macrophages. PLoS ONE 2012, 7, e45466. [Google Scholar] [CrossRef] [PubMed]
- Tarique, A.A.; Logan, J.; Thomas, E.; Holt, P.G.; Sly, P.D.; Fantino, E. Phenotypic, functional, and plasticity features of classical and alternatively activated human macrophages. Am. J. Respir. Cell Mol. Biol. 2015, 53, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Vogel, D.Y.; Glim, J.E.; Stavenuiter, A.W.; Breur, M.; Heijnen, P.; Amor, S.; Dijkstra, C.D.; Beelen, R.H. Human macrophage polarization in vitro: Maturation and activation methods compared. Immunobiology 2014, 219, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Roussel, M.; Ferrell, P.B., Jr.; Greenplate, A.R.; Lhomme, F.; Le Gallou, S.; Diggins, K.E.; Johnson, D.B.; Irish, J.M. Mass cytometry deep phenotyping of human mononuclear phagocytes and myeloid-derived suppressor cells from human blood and bone marrow. J. Leukoc. Biol. 2017, 102, 437–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiratori, H.; Feinweber, C.; Luckhardt, S.; Linke, B.; Resch, E.; Geisslinger, G.; Weigert, A.; Parnham, M.J. THP-1 and human peripheral blood mononuclear cell-derived macrophages differ in their capacity to polarize in vitro. Mol. Immunol. 2017, 88, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Italiani, P.; Mazza, E.M.; Lucchesi, D.; Cifola, I.; Gemelli, C.; Grande, A.; Battaglia, C.; Bicciato, S.; Boraschi, D. Transcriptomic profiling of the development of the inflammatory response in human monocytes in vitro. PLoS ONE 2014, 9, e87680. [Google Scholar] [CrossRef] [PubMed]
- Rackov, G.; Hernandez-Jimenez, E.; Shokri, R.; Carmona-Rodriguez, L.; Manes, S.; Alvarez-Mon, M.; Lopez-Collazo, E.; Martinez, A.C.; Balomenos, D. P21 mediates macrophage reprogramming through regulation of p50-p50 NF-κB and IFN-β. J. Clin. Investig. 2016, 126, 3089–3103. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Hisamatsu, T.; Chiba, S.; Mori, K.; Kitazume, M.T.; Shimamura, K.; Nakamoto, N.; Matsuoka, K.; Ebinuma, H.; Naganuma, M.; et al. Glycolytic pathway affects differentiation of human monocytes to regulatory macrophages. Immunol. Lett. 2016, 176, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J.A.; Riquelme, P.; Sawitzki, B.; Tomiuk, S.; Miqueu, P.; Zuhayra, M.; Oberg, H.H.; Pascher, A.; Lutzen, U.; Janssen, U.; et al. Cutting edge: Immunological consequences and trafficking of human regulatory macrophages administered to renal transplant recipients. J. Immunol. 2011, 187, 2072–2078. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Hu, M.; Huang, D.; Zhao, Y.; Heng, B.; Guillemin, G.; Lim, C.K.; Hawthorne, W.J.; Yi, S. Human regulatory macrophages are potent in suppression of the xenoimmune response via indoleamine-2,3-dioxygenase-involved mechanism(s). Xenotransplantation 2017, 24, e12326. [Google Scholar] [CrossRef] [PubMed]
- Riquelme, P.; Amodio, G.; Macedo, C.; Moreau, A.; Obermajer, N.; Brochhausen, C.; Ahrens, N.; Kekarainen, T.; Fandrich, F.; Cuturi, C.; et al. DHRS9 is a stable marker of human regulatory macrophages. Transplantation 2017, 101, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Erbel, C.; Tyka, M.; Helmes, C.M.; Akhavanpoor, M.; Rupp, G.; Domschke, G.; Linden, F.; Wolf, A.; Doesch, A.; Lasitschka, F.; et al. CXCL4-induced plaque macrophages can be specifically identified by co-expression of MMP7+S100A8+ in vitro and in vivo. Innate Immun. 2015, 21, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Boyle, J.J.; Johns, M.; Kampfer, T.; Nguyen, A.T.; Game, L.; Schaer, D.J.; Mason, J.C.; Haskard, D.O. Activating transcription factor 1 directs mhem atheroprotective macrophages through coordinated iron handling and foam cell protection. Circ. Res. 2012, 110, 20–33. [Google Scholar] [CrossRef] [PubMed]
- Boyle, J.J.; Harrington, H.A.; Piper, E.; Elderfield, K.; Stark, J.; Landis, R.C.; Haskard, D.O. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am. J. Pathol. 2009, 174, 1097–1108. [Google Scholar] [CrossRef] [PubMed]
- Verreck, F.A.; de Boer, T.; Langenberg, D.M.; Hoeve, M.A.; Kramer, M.; Vaisberg, E.; Kastelein, R.; Kolk, A.; de Waal-Malefyt, R.; Ottenhoff, T.H. Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc. Natl. Acad. Sci. USA 2004, 101, 4560–4565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verreck, F.A.; de Boer, T.; Langenberg, D.M.; van der Zanden, L.; Ottenhoff, T.H. Phenotypic and functional profiling of human proinflammatory type-1 and anti-inflammatory type-2 macrophages in response to microbial antigens and IFN-γ- and CD40L-mediated costimulation. J. Leukoc. Biol. 2006, 79, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Martinez, F.O.; Gordon, S.; Locati, M.; Mantovani, A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: New molecules and patterns of gene expression. J. Immunol. 2006, 177, 7303–7311. [Google Scholar] [CrossRef] [PubMed]
- Jaguin, M.; Houlbert, N.; Fardel, O.; Lecureur, V. Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell Immunol. 2013, 281, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Vogel, D.Y.; Vereyken, E.J.; Glim, J.E.; Heijnen, P.D.; Moeton, M.; van der Valk, P.; Amor, S.; Teunissen, C.E.; van Horssen, J.; Dijkstra, C.D. Macrophages in inflammatory multiple sclerosis lesions have an intermediate activation status. J. Neuroinflamm. 2013, 10, 809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambarus, C.A.; Noordenbos, T.; de Hair, M.J.; Tak, P.P.; Baeten, D.L. Intimal lining layer macrophages but not synovial sublining macrophages display an IL-10 polarized-like phenotype in chronic synovitis. Arthritis Res. Ther. 2012, 14, R74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durafourt, B.A.; Moore, C.S.; Zammit, D.A.; Johnson, T.A.; Zaguia, F.; Guiot, M.C.; Bar-Or, A.; Antel, J.P. Comparison of polarization properties of human adult microglia and blood-derived macrophages. Glia 2012, 60, 717–727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros, M.H.; Hauck, F.; Dreyer, J.H.; Kempkes, B.; Niedobitek, G. Macrophage polarisation: An immunohistochemical approach for identifying M1 and M2 macrophages. PLoS ONE 2013, 8, e80908. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, C.A.; Kumar, A. Characterization of in vitro generated human polarized macrophages. J. Clin. Cell Immunol. 2015, 6, 380. [Google Scholar] [CrossRef]
- Duluc, D.; Delneste, Y.; Tan, F.; Moles, M.P.; Grimaud, L.; Lenoir, J.; Preisser, L.; Anegon, I.; Catala, L.; Ifrah, N.; et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood 2007, 110, 4319–4330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pannellini, T.; Iezzi, M.; Di Carlo, E.; Eleuterio, E.; Coletti, A.; Modesti, A.; Rosini, S.; Neri, M.; Musiani, P. The expression of LEC/CCL16, a powerful inflammatory chemokine, is upregulated in ulcerative colitis. Int. J. Immunopathol. Pharmacol. 2004, 17, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Sunakawa, Y.; Stintzing, S.; Cao, S.; Heinemann, V.; Cremolini, C.; Falcone, A.; Yang, D.; Zhang, W.; Ning, Y.; Stremitzer, S.; et al. Variations in genes regulating tumor-associated macrophages (TAMs) to predict outcomes of bevacizumab-based treatment in patients with metastatic colorectal cancer: Results from tribe and fire3 trials. Ann. Oncol. 2015, 26, 2450–2456. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xu, J.B.; He, Y.L.; Peng, J.J.; Zhang, X.H.; Chen, C.Q.; Li, W.; Cai, S.R. Tumor-associated macrophages promote angiogenesis and lymphangiogenesis of gastric cancer. J. Surg. Oncol. 2012, 106, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Schraufstatter, I.U.; Zhao, M.; Khaldoyanidi, S.K.; Discipio, R.G. The chemokine CCL18 causes maturation of cultured monocytes to macrophages in the M2 spectrum. Immunology 2012, 135, 287–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, B.; Yao, G.; Zhang, Y.; Gao, J.; Yang, B.; Rao, Z. M2-polarized tumor-associated macrophages are associated with poor prognoses resulting from accelerated lymphangiogenesis in lung adenocarcinoma. Clinics 2011, 66, 1879–1886. [Google Scholar] [CrossRef] [PubMed]
- Bogels, M.; Braster, R.; Nijland, P.G.; Gul, N.; van de Luijtgaarden, W.; Fijneman, R.J.; Meijer, G.A.; Jimenez, C.R.; Beelen, R.H.; van Egmond, M. Carcinoma origin dictates differential skewing of monocyte function. Oncoimmunology 2012, 1, 798–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, A.; Hsiao, Y.J.; Chen, H.Y.; Chen, H.W.; Ho, C.C.; Chen, Y.Y.; Liu, Y.C.; Hong, T.H.; Yu, S.L.; Chen, J.J.; et al. Opposite effects of M1 and M2 macrophage subtypes on lung cancer progression. Sci. Rep. 2015, 5, 14273. [Google Scholar] [CrossRef] [PubMed]
- Patel, U.; Rajasingh, S.; Samanta, S.; Cao, T.; Dawn, B.; Rajasingh, J. Macrophage polarization in response to epigenetic modifiers during infection and inflammation. Drug Discov. Today 2017, 22, 186–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madore, A.M.; Perron, S.; Turmel, V.; Laviolette, M.; Bissonnette, E.Y.; Laprise, C. Alveolar macrophages in allergic asthma: An expression signature characterized by heat shock protein pathways. Hum. Immunol. 2010, 71, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Shaykhiev, R.; Krause, A.; Salit, J.; Strulovici-Barel, Y.; Harvey, B.G.; O’Connor, T.P.; Crystal, R.G. Smoking-dependent reprogramming of alveolar macrophage polarization: Implication for pathogenesis of chronic obstructive pulmonary disease. J. Immunol. 2009, 183, 2867–2883. [Google Scholar] [CrossRef] [PubMed]
- Stoger, J.L.; Gijbels, M.J.; van der Velden, S.; Manca, M.; van der Loos, C.M.; Biessen, E.A.; Daemen, M.J.; Lutgens, E.; de Winther, M.P. Distribution of macrophage polarization markers in human atherosclerosis. Atherosclerosis 2012, 225, 461–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukui, S.; Iwamoto, N.; Takatani, A.; Igawa, T.; Shimizu, T.; Umeda, M.; Nishino, A.; Horai, Y.; Hirai, Y.; Koga, T.; et al. M1 and M2 monocytes in rheumatoid arthritis: A contribution of imbalance of M1/M2 monocytes to osteoclastogenesis. Front. Immunol. 2017, 8, 1958. [Google Scholar] [CrossRef] [PubMed]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ren, Y.; Khan, F.A.; Pandupuspitasari, N.S.; Zhang, S. Immune evasion strategies of pathogens in macrophages: The potential for limiting pathogen transmission. Curr. Issues Mol. Biol. 2017, 21, 21–40. [Google Scholar] [PubMed]
- Chandra, V.; Mahajan, S.; Saini, A.; Dkhar, H.K.; Nanduri, R.; Raj, E.B.; Kumar, A.; Gupta, P. Human il10 gene repression by rev-erbalpha ameliorates mycobacterium tuberculosis clearance. J. Biol. Chem. 2013, 288, 10692–10702. [Google Scholar] [CrossRef] [PubMed]
- Ting, L.M.; Kim, A.C.; Cattamanchi, A.; Ernst, J.D. Mycobacterium tuberculosis inhibits IFN-γ transcriptional responses without inhibiting activation of STAT1. J. Immunol. 1999, 163, 3898–3906. [Google Scholar] [PubMed]
- Tang, Y.; Hua, S.C.; Qin, G.X.; Xu, L.J.; Jiang, Y.F. Different subsets of macrophages in patients with new onset tuberculous pleural effusion. PLoS ONE 2014, 9, e88343. [Google Scholar] [CrossRef] [PubMed]
- Mattila, J.T.; Ojo, O.O.; Kepka-Lenhart, D.; Marino, S.; Kim, J.H.; Eum, S.Y.; Via, L.E.; Barry, C.E., 3rd; Klein, E.; Kirschner, D.E.; et al. Microenvironments in tuberculous granulomas are delineated by distinct populations of macrophage subsets and expression of nitric oxide synthase and arginase isoforms. J. Immunol. 2013, 191, 773–784. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Luo, Q.; Guo, Y.; Chen, J.; Xiong, G.; Peng, Y.; Ye, J.; Li, J. Mycobacterium tuberculosis-induced polarization of human macrophage orchestrates the formation and development of tuberculous granulomas in vitro. PLoS ONE 2015, 10, e0129744. [Google Scholar] [CrossRef] [PubMed]
- Quiding-Jarbrink, M.; Raghavan, S.; Sundquist, M. Enhanced M1 macrophage polarization in human helicobacter pylori-associated atrophic gastritis and in vaccinated mice. PLoS ONE 2010, 5, e15018. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.; Drobbe, L.; Moos, V.; Renner Viveros, P.; Hagen, J.; Beigier-Bompadre, M.; Pang, E.; Belogolova, E.; Churin, Y.; Schneider, T.; et al. Comparative analysis of the interaction of helicobacter pylori with human dendritic cells, macrophages, and monocytes. Infect. Immun. 2012, 80, 2724–2734. [Google Scholar] [CrossRef] [PubMed]
- Saliba, A.E.; Li, L.; Westermann, A.J.; Appenzeller, S.; Stapels, D.A.; Schulte, L.N.; Helaine, S.; Vogel, J. Single-cell RNA-seq ties macrophage polarization to growth rate of intracellular salmonella. Nat. Microbiol. 2016, 2, 16206. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.J.; Dunstan, S.J.; Dolecek, C.; Perkins, T.; House, D.; Dougan, G.; Nguyen, T.H.; Tran, T.P.; Doan, C.D.; Le, T.P.; et al. Transcriptional response in the peripheral blood of patients infected with salmonella enterica serovar typhi. Proc. Natl. Acad. Sci. USA 2009, 106, 22433–22438. [Google Scholar] [CrossRef] [PubMed]
- Reales-Calderon, J.A.; Aguilera-Montilla, N.; Corbi, A.L.; Molero, G.; Gil, C. Proteomic characterization of human proinflammatory M1 and anti-inflammatory M2 macrophages and their response to candida albicans. Proteomics 2014, 14, 1503–1518. [Google Scholar] [CrossRef] [PubMed]
- Wagener, J.; MacCallum, D.M.; Brown, G.D.; Gow, N.A. Candida albicans chitin increases arginase-1 activity in human macrophages, with an impact on macrophage antimicrobial functions. MBio 2017, 8, e01820-16. [Google Scholar] [CrossRef] [PubMed]
- Cassol, E.; Cassetta, L.; Alfano, M.; Poli, G. Macrophage polarization and HIV-1 infection. J. Leukoc. Biol. 2010, 87, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Brockman, M.A.; Kwon, D.S.; Tighe, D.P.; Pavlik, D.F.; Rosato, P.C.; Sela, J.; Porichis, F.; Le Gall, S.; Waring, M.T.; Moss, K.; et al. IL-10 is up-regulated in multiple cell types during viremic HIV infection and reversibly inhibits virus-specific T cells. Blood 2009, 114, 346–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flynn, J.K.; Dore, G.J.; Hellard, M.; Yeung, B.; Rawlinson, W.D.; White, P.A.; Kaldor, J.M.; Lloyd, A.R.; Ffrench, R.A. Early IL-10 predominant responses are associated with progression to chronic hepatitis C virus infection in injecting drug users. J. Viral. Hepat. 2011, 18, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wang, Y.; Zhai, N.; Song, H.; Li, H.; Yang, Y.; Li, T.; Guo, X.; Chi, B.; Niu, J.; et al. HCV core protein inhibits polarization and activity of both M1 and M2 macrophages through the TLR2 signaling pathway. Sci. Rep. 2016, 6, 36160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, B.; Kodys, K.; Szabo, G. Hepatitis C virus-induced monocyte differentiation into polarized M2 macrophages promotes stellate cell activation via TGF-β. Cell. Mol. Gastroenterol. Hepatol. 2016, 2, 302–316. [Google Scholar] [CrossRef] [PubMed]
- Hoeve, M.A.; Nash, A.A.; Jackson, D.; Randall, R.E.; Dransfield, I. Influenza virus a infection of human monocyte and macrophage subpopulations reveals increased susceptibility associated with cell differentiation. PLoS ONE 2012, 7, e29443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Bao, Y.J.; Tong, A.H.; Zuyderduyn, S.; Bader, G.D.; Malik Peiris, J.S.; Lok, S.; Lee, S.M. Whole transcriptome analysis reveals differential gene expression profile reflecting macrophage polarization in response to influenza a H5N1 virus infection. BMC Med. Genom. 2018, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.; Bivins-Smith, E.R.; Smith, M.S.; Smith, P.M.; Yurochko, A.D. Transcriptome analysis reveals human cytomegalovirus reprograms monocyte differentiation toward an M1 macrophage. J. Immunol. 2008, 181, 698–711. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.; Nogalski, M.T.; Yurochko, A.D. Human cytomegalovirus stimulates monocyte-to-macrophage differentiation via the temporal regulation of caspase 3. J. Virol. 2012, 86, 10714–10723. [Google Scholar] [CrossRef] [PubMed]
- Avdic, S.; Cao, J.Z.; McSharry, B.P.; Clancy, L.E.; Brown, R.; Steain, M.; Gottlieb, D.J.; Abendroth, A.; Slobedman, B. Human cytomegalovirus interleukin-10 polarizes monocytes toward a deactivated M2c phenotype to repress host immune responses. J. Virol. 2013, 87, 10273–10282. [Google Scholar] [CrossRef] [PubMed]
- Poglitsch, M.; Weichhart, T.; Hecking, M.; Werzowa, J.; Katholnig, K.; Antlanger, M.; Krmpotic, A.; Jonjic, S.; Horl, W.H.; Zlabinger, G.J.; et al. CMV late phase-induced mTOR activation is essential for efficient virus replication in polarized human macrophages. Am. J. Transplant. 2012, 12, 1458–1468. [Google Scholar] [CrossRef] [PubMed]
- Cassol, E.; Cassetta, L.; Rizzi, C.; Alfano, M.; Poli, G. M1 and M2A polarization of human monocyte-derived macrophages inhibits HIV-1 replication by distinct mechanisms. J. Immunol. 2009, 182, 6237–6246. [Google Scholar] [CrossRef] [PubMed]
- Herbein, G.; Varin, A. The macrophage in HIV-1 infection: From activation to deactivation? Retrovirology 2010, 7, 33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.C.; Zou, X.B.; Chai, Y.F.; Yao, Y.M. Macrophage polarization in inflammatory diseases. Int. J. Biol. Sci. 2014, 10, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Almeida, S.; Nejsum, P.; Williams, A.R. Modulation of human macrophage activity by ascaris antigens is dependent on macrophage polarization state. Immunobiology 2018, 223, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Kimutai, A.; Ngure, P.K.; Tonui, W.K.; Gicheru, M.M.; Nyamwamu, L.B. Leishmaniasis in northern and western africa: A review. Afr. J. Infect. Dis. 2009, 3, 14–25. [Google Scholar] [CrossRef]
- Louzir, H.; Aoun, K.; Spath, G.F.; Laouini, D.; Prina, E.; Victoir, K.; Bouratbine, A. [leishmania epidemiology, diagnosis, chemotherapy and vaccination approaches in the international network of pasteur institutes]. Med. Sci. 2013, 29, 1151–1160. [Google Scholar]
- Olivier, M.; Gregory, D.J.; Forget, G. Subversion mechanisms by which leishmania parasites can escape the host immune response: A signaling point of view. Clin. Microbiol. Rev. 2005, 18, 293–305. [Google Scholar] [CrossRef] [PubMed]
- Kropf, P.; Fuentes, J.M.; Fahnrich, E.; Arpa, L.; Herath, S.; Weber, V.; Soler, G.; Celada, A.; Modolell, M.; Muller, I. Arginase and polyamine synthesis are key factors in the regulation of experimental leishmaniasis in vivo. FASEB J. 2005, 19, 1000–1002. [Google Scholar] [CrossRef] [PubMed]
- Guerfali, F.Z.; Laouini, D.; Guizani-Tabbane, L.; Ottones, F.; Ben-Aissa, K.; Benkahla, A.; Manchon, L.; Piquemal, D.; Smandi, S.; Mghirbi, O.; et al. Simultaneous gene expression profiling in human macrophages infected with leishmania major parasites using sage. BMC Genom. 2008, 9, 238. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.J.; Wynn, T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011, 11, 723–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Uzonna, J.E. The early interaction of leishmania with macrophages and dendritic cells and its influence on the host immune response. Front. Cell. Infect. Microbiol. 2012, 2, 83. [Google Scholar] [CrossRef] [PubMed]
- Muraille, E.; Leo, O.; Moser, M. TH1/TH2 paradigm extended: Macrophage polarization as an unappreciated pathogen-driven escape mechanism? Front. Immunol. 2014, 5, 603. [Google Scholar] [CrossRef] [PubMed]
- Maspi, N.; Abdoli, A.; Ghaffarifar, F. Pro- and anti-inflammatory cytokines in cutaneous leishmaniasis: A review. Pathog. Glob. Health 2016, 110, 247–260. [Google Scholar] [CrossRef] [PubMed]
- Farrow, A.L.; Rana, T.; Mittal, M.K.; Misra, S.; Chaudhuri, G. Leishmania-induced repression of selected non-coding RNA genes containing B-box element at their promoters in alternatively polarized M2 macrophages. Mol. Cell. Biochem. 2011, 350, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, D.; Mukherjee, S.; Roy, S.; Dalton, J.E.; Kundu, S.; Sarkar, A.; Das, N.K.; Kaye, P.M.; Chatterjee, M. M2 polarization of monocytes-macrophages is a hallmark of indian post kala-azar dermal leishmaniasis. PLoS Negl. Trop. Dis. 2015, 9, e0004145. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Saldarriaga, O.A.; Spratt, H.; Osorio, E.Y.; Travi, B.L.; Luxon, B.A.; Melby, P.C. Transcriptional profiling in experimental visceral leishmaniasis reveals a broad splenic inflammatory environment that conditions macrophages toward a disease-promoting phenotype. PLoS Pathog. 2017, 13, e1006165. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atri, C.; Guerfali, F.Z.; Laouini, D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. Int. J. Mol. Sci. 2018, 19, 1801. https://doi.org/10.3390/ijms19061801
Atri C, Guerfali FZ, Laouini D. Role of Human Macrophage Polarization in Inflammation during Infectious Diseases. International Journal of Molecular Sciences. 2018; 19(6):1801. https://doi.org/10.3390/ijms19061801
Chicago/Turabian StyleAtri, Chiraz, Fatma Z. Guerfali, and Dhafer Laouini. 2018. "Role of Human Macrophage Polarization in Inflammation during Infectious Diseases" International Journal of Molecular Sciences 19, no. 6: 1801. https://doi.org/10.3390/ijms19061801