Protective Effects of Peroxiredoxin 4 (PRDX4) on Cholestatic Liver Injury
Abstract
:1. Introduction
2. Results
2.1. Reduced Liver Injury in Tg BDL Mice
2.2. Attenuation of Cholestasis-Induced Oxidative Stress by the Overexpression of PRDX4
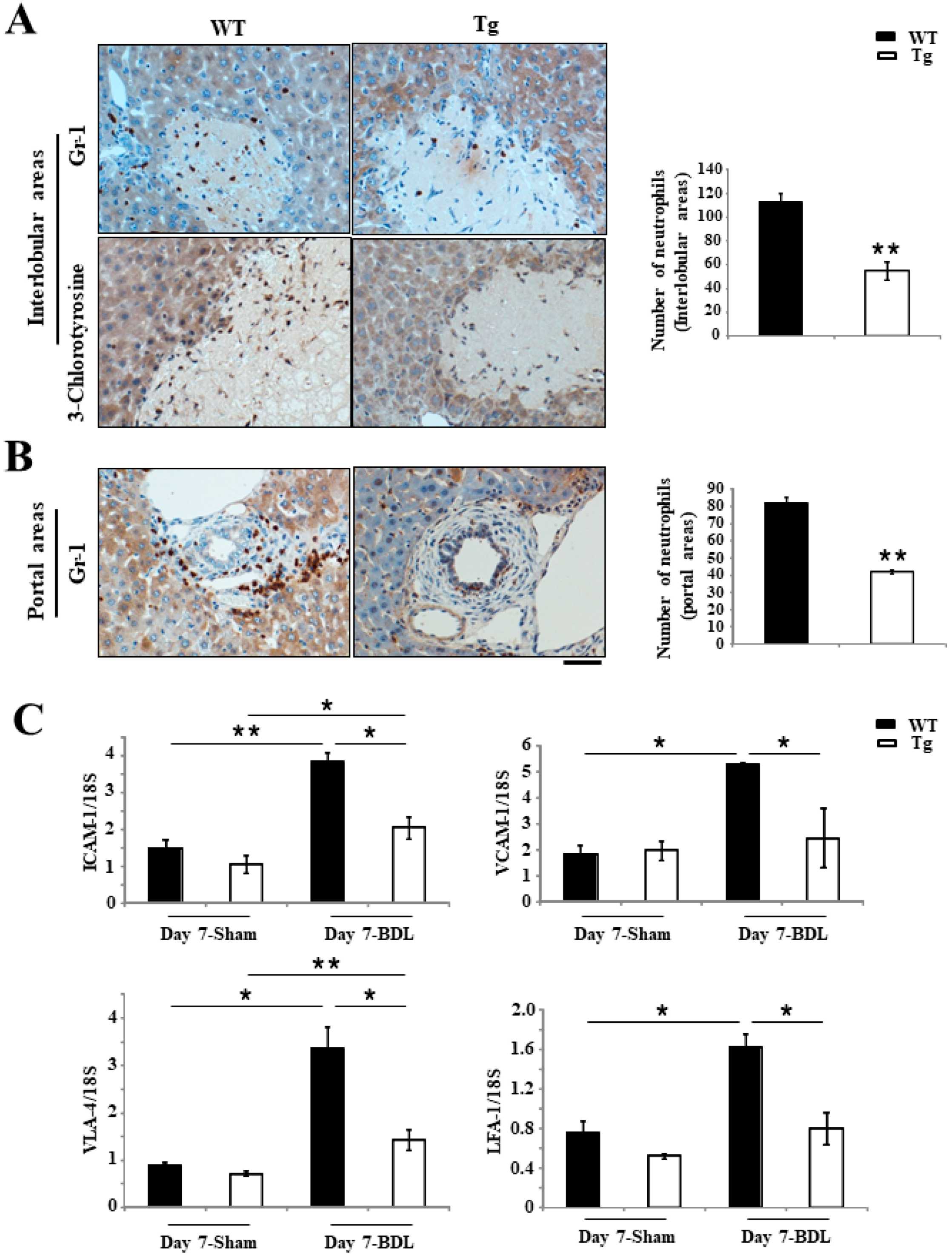
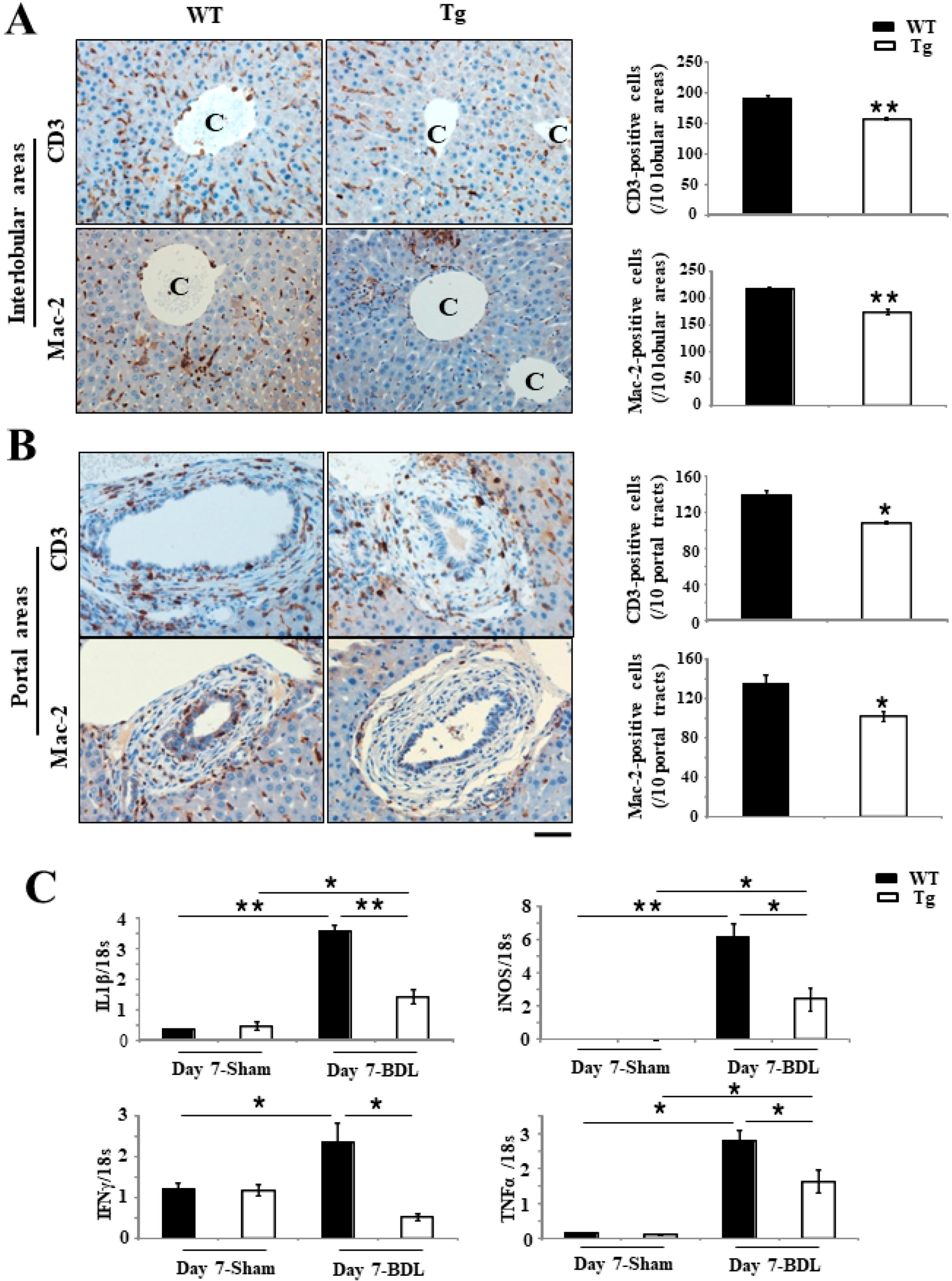
2.3. Inhibition of Inflammation Responses in Tg BDL Mice
2.4. Repressed Proliferation of Bile Ducts and Liver Cells in Tg BDL Mice
2.5. An Improved Prognosis of BDL-Induced Cholestasis in Tg Mice Compared with WT Mice
2.6. Changes in the BA Metabolism in Tg Mice
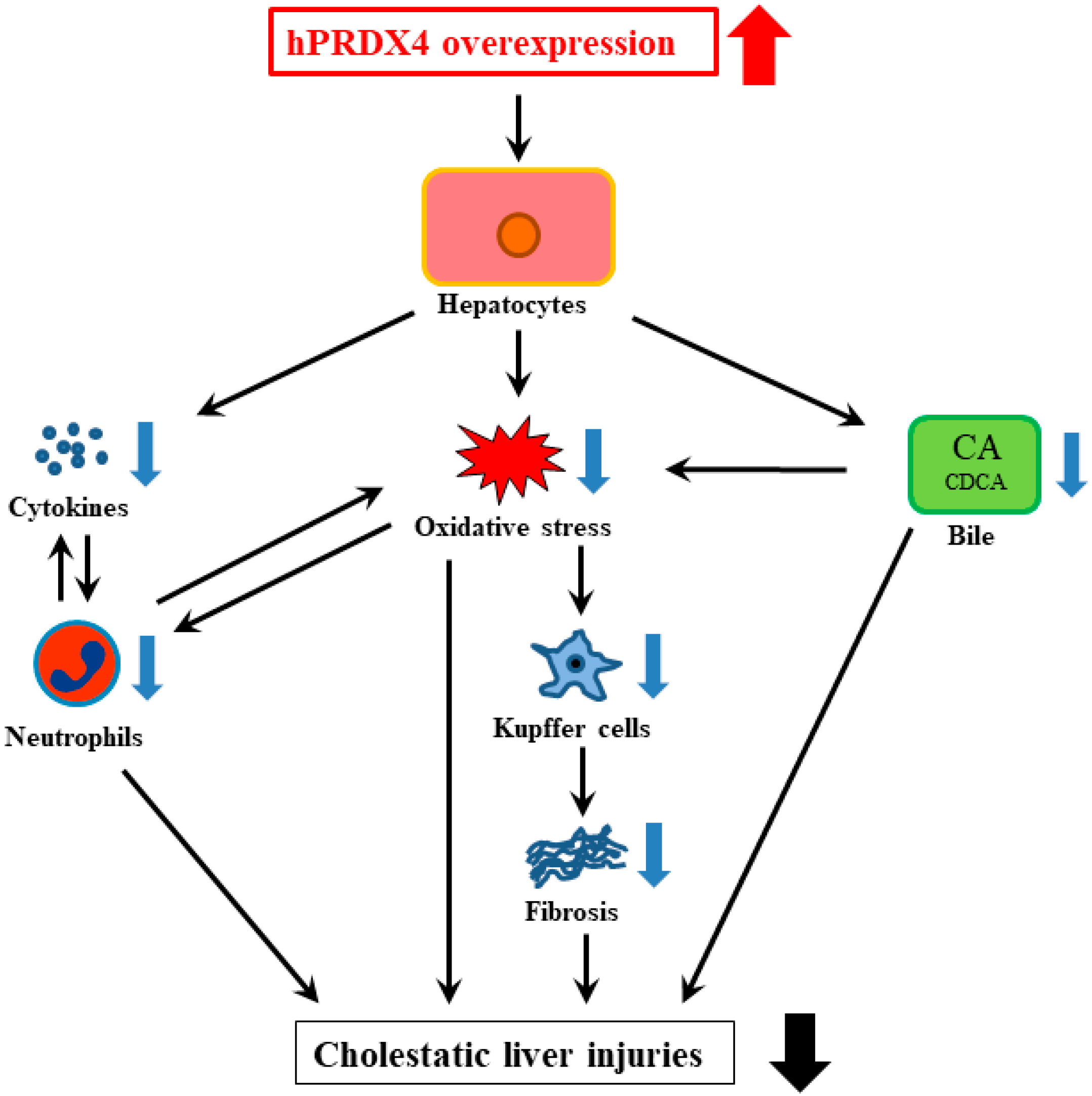
3. Discussion
4. Materials and Methods
4.1. Animals and BDL Model
4.2. Histopathology
4.3. Analyses of Liver Injury Induced by Cholestasis
4.4. Histology and IHC
4.5. ROS Detection
4.6. RT-PCR and Real-Time RT-PCR
4.7. Western Blotting
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PRDX4 | Peroxiredoxin 4 |
| ER | Endoplasmic reticulum |
| WT | Wild-type |
| UDCA | Ursodeoxycholic acid |
| BDL | Bile duct ligation |
| ROS | Reactive oxygen species |
| BAs | Bile acids |
| NAC | N-acetylcysteine |
| GGT | γ-Glutamyl transpeptidase |
| ALP | Alkaline phosphatase |
| Mn-SOD | Manganese-dependent superoxide dismutase |
| DHE | Dihydroethidium |
| TBARS | Thiobarbituric acid reactive substances |
| MDA | Malondialdehyde |
| ALT | Alanine aminotransferase |
| AST | Aspartate aminotransferase |
| IL-1β | Interleukin 1 β |
| iNOS | Inducible nitric oxide synthase |
| IFNγ | Tumor necrosis factor-α |
| TNF-α | Tumor necrosis factor-α |
| CA | Cholic acid |
| CDCA | Chenodeoxycholic acid |
| 8-OHdG | 8-hydroxy-20-deoxyguanosine |
| CK19 | Cytokeratin 19 |
| IBDM | Intrahepatic bile duct mass |
| α-SMA | α-Smooth muscle actin |
| LDL | Low-density lipoprotein |
| dROM | Diacron reactive oxygen metabolites |
| PCNA | Proliferating cell nuclear antigen |
| DDC | 3,5-Diethoxycarbonyl-1,4-dihydrocollidine |
| ANIT | α-Naphthyl-isothiocyanate |
References
- Gossard, A.A.; Talwalkar, J.A. Cholestatic liver disease. Med. Clin. N. Am. 2014, 98, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Poupon, R.; Chazouillères, O.; Poupon, R.E. Chronic cholestatic diseases. J. Hepatol. 2000, 32, 129–140. [Google Scholar] [CrossRef]
- Hirschfield, G.M.; Heathcote, E.J.; Gershwin, M.E. Pathogenesis of cholestatic liver disease and therapeutic approaches. Gastroenterology 2010, 139, 1481–1496. [Google Scholar] [CrossRef] [PubMed]
- Aboutwerat, A.; Pemberton, P.W.; Smith, A.; Burrows, P.C.; McMahon, R.F.; Jain, S.K.; Warnes, T.W. Oxidant stress is a significant feature of primary biliary cirrhosis. Biochim. Biophys. Acta. 2003, 1637, 142–150. [Google Scholar] [CrossRef]
- Jaeschke, H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J. Gastroenterol. Hepatol. 2011, 26, 173–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sokol, R.J.; Devereaux, M.; Khandwala, R.A. Effect of dietary lipid and vitamin E on mitochondrial lipid peroxidation and hepatic injury in the bile duct-ligated rat. J. Lipid Res. 1991, 32, 1349–1357. [Google Scholar] [PubMed]
- Nieminen, A.L.; Saylor, A.K.; Tesfai, S.A.; Herman, B.; Lemasters, J.J. Contribution of the mitochondrial permeability transition to lethal injury after exposure of hepatocytes to t-butylhydroperoxide. Biochem. J. 1995, 307, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Parola, M.; Pinzani, M.; Casini, A.; Leonarduzzi, G.; Marra, F.; Caligiuri, A.; Ceni, E.; Biondi, P.; Poli, G.; Dianzani, M.U. Induction of procollagen type I gene expression and synthesis in human hepatic stellate cells by 4-hydroxy-2,3-nonenal and other 4-hydroxy-2,3-alkenals is related to their molecular structure. Biochem. Biophys. Res. Commun. 1996, 222, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Attili, A.F.; Angelico, M.; Cantafora, A.; Alvaro, D.; Capocaccia, L. Bile acidinduced liver toxicity: Relation to the hydrophobic-hydrophilic balance of bile acids. Med. Hypotheses 1986, 19, 57–69. [Google Scholar] [CrossRef]
- Faubion, W.A.; Guicciardi, M.E.; Miyoshi, H.; Bronk, S.F.; Roberts, P.J.; Svingen, P.A.; Kaufmann, S.H.; Gores, G.J. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J. Clin. Investing. 1999, 103, 137–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higuchi, H.; Bronk, S.F.; Takikawa, Y.; Werneburg, N.; Takimoto, R.; El-Deiry, W.; Gores, G.J. The bile acid glycochenodeoxycholate induces trail-receptor 2/DR5 expression and apoptosis. J. Biol. Chem. 2001, 276, 38610–38618. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, H.; Yamada, S.; Nabeshima, A.; Guo, X.; Tanimoto, A.; Wang, K.Y.; Kitada, S.; Tasaki, T.; Takama, T.; Shimajiri, S.; et al. Depletion of apoptosis signal-regulating kinase 1 prevents bile duct ligation-induced necroinflammation and subsequent peribiliary fibrosis. Am. J. Pathol. 2014, 184, 644–661. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.D.; Moon, J.O.; Slitt, A.L.; Copple, B.L. Early growth response factor-1 is critical for cholestatic liver injury. Toxicol. Sci. 2006, 90, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.; Jaeschke, H.; Copple, B.L. Bile acids induce inflammatory genes in hepatocytes: A novel mechanism of inflammation during obstructive cholestasis. Am. J. Pathol. 2011, 178, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.Y.; Ouyang, X.; Chen, Y.; Soroka, C.J.; Wang, J.; Mennone, A.; Yucheng, W.; Wajahat, Z.M.; Dhanpat, J.; James, L.B. Bile acids initiate cholestatic liver injury by triggering a hepatocyte-specific inflammatory response. JCI Insight 2017, 2, e90780. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Kawaguchi, H.; Yamada, T.; Guo, X.; Matsuo, K.; Hamada, T.; Miura, N.; Tasaki, T.; Tanimoto, A. Cholic Acid Enhances Visceral Adiposity, Atherosclerosis and Nonalcoholic Fatty Liver Disease in Microminipigs. J. Atheroscler. Thromb. 2017, 24, 1150–1166. [Google Scholar] [CrossRef] [PubMed]
- Soylu, A.R.; Aydogdu, N.; Basaran, U.N.; Altaner, S.; Tarcin, O.; Gedik, N.; Umit, H.; Tezel, A.; Dokmeci, G.; Baloglu, H.; et al. Antioxidants vitamin E and C attenuate hepatic fibrosis in biliaryobstructed rats. World J. Gastroenterol. 2006, 12, 6835–6841. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Lee, K.C.; Huang, Y.T.; Wang, Y.W.; Hou, M.C.; Lee, F.Y.; Lin, H.C.; Lee, S.D. Effects of Nacetylcysteine administration in hepatic microcirculation of rats with biliary cirrhosis. J. Hepatol. 2008, 49, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Froh, M.; Lehnert, M.; Schoonhoven, R.; Yang, L.; Lind, H.; Lemasters, J.J.; Thurman, R.G. Polyphenols from Camellia sinenesis attenuate experimental cholestasisinduced liver fibrosis in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G1004–G1013. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Froh, M.; Wheeler, M.D.; Smutney, O.; Lehmann, T.G.; Thurman, R.G. Viral gene delivery of superoxide dismutase attenuates experimental cholestasis-induced liver fibrosis in the rat. Gene Ther. 2002, 9, 183–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, S.; Guo, X. Peroxiredoxin 4 (PRDX4): Its critical in vivo roles in animal models of metabolic syndrome ranging from atherosclerosis to nonalcoholic fatty liver disease. Pathol. Int. 2018, 68, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Fujii, J.; Ikeda, Y.; Kurahashi, T.; Homma, T. Physiological and pathological views of peroxiredoxin 4. Free. Radic. Biol. Med. 2015, 83, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yamada, S.; Wang, K.Y.; Shimajiri, S.; Guo, X.; Tanimoto, A.; Murata, Y.; Kitajima, S.; Watanabe, T.; Izumi, H.; et al. Overexpression of peroxiredoxin 4 protects against high-dose streptozotocin-induced diabetes by suppressing oxidative stress and cytokines in transgenic mice. Antioxid. Redox. Signal. 2010, 13, 1477–1490. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Yamada, S.; Tanimoto, A.; Ding, Y.; Wang, K.Y.; Shimajiri, S.; Murata, Y.; Kimura, S.; Tasaki, T.; Nabeshima, A.; et al. Overexpression of peroxiredoxin 4 attenuates atherosclerosis in apolipoprotein E knockout mice. Antioxid. Redox. Signal. 2012, 17, 1362–1375. [Google Scholar] [CrossRef] [PubMed]
- Nabeshima, A.; Yamada, S.; Guo, X.; Tanimoto, A.; Wang, K.Y.; Shimajiri, S.; Kimura, S.; Tasaki, T.; Noguchi, H.; Kitada, S.; et al. Peroxiredoxin 4 protects against nonalcoholic steatohepatitis and type 2 diabetes in a nongenetic mouse model. Antioxid. Redox. Signal. 2013, 19, 1983–1998. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Noguchi, H.; Ishii, N.; Homma, T.; Hamada, T.; Hiraki, T.; Zhang, J.; Matsuo, K.; Yokoyama, S.; Ishibashi, H.; et al. The Association of Peroxiredoxin 4 with the Initiation and Progression of Hepatocellular Carcinoma. Antioxid. Redox. Signal. 2018. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S. Histopathology on atherosclerosis to metabolic syndrome using genetically modified animal models. J. Kanazawa Med. Univ. 2018, 43, 1–8. [Google Scholar]
- Nguyen, K.D.; Sundaram, V.; Ayoub, W.S. Atypical causes of cholestasis. World J. Gastroenterol. 2014, 20, 9418–9426. [Google Scholar] [PubMed]
- Wang, H.; Vohra, B.P.S.; Zhang, Y.; Heuckeroth, R.O. Transcriptional profiling after bile duct ligation identifies PAI-1 as a contributor to cholestatic injury in mice. Hepatology 2005, 42, 1099–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, H.; Zhang, N.; Xu, Y.; Chen, Q.; Khan, M.; Potter, J.J.; Nayar, S.K.; Cornish, T.; Alpini, G.; Bronk, S.; et al. Yes-associated protein regulates the hepatic response after bile duct ligation. Hepatology 2012, 56, 1097–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgiev, P.; Jochum, W.; Heinrich, S.; Jang, J.H.; Nocito, A.; Dahm, F.; Clavien, P.A. Characterization of time-related changes after experimental bile duct ligation. Br. J. Surg. 2008, 95, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Copple, B.L.; Jaeschke, H.; Klaassen, C.D. Oxidative stress and the pathogenesis of cholestasis. Semin. Liver Dis. 2010, 30, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Rudraiah, S.; Moscovitz, J.E.; Donepudi, A.C.; Campion, S.N.; Slitt, A.L.; Aleksunes, L.M.; Manautou, J.E. Differential Fmo3 gene expression in various liver injury models involving hepatic oxidative stress in mice. Toxicology 2014, 325, 85–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaeschke, H. Reactive oxygen and mechanisms of inflammatory liver injury. J. Gastroenterol. Hepatol. 2000, 15, 718–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tag, C.G.; Sauer-Lehnen, S.; Weiskirchen, S.; Borkham-Kamphorst, E.; Tolba, R.H.; Tacke, F.; Weiskirchen, R. Bile duct ligation in mice: Induction of inflammatory liver injury and fibrosis by obstructive cholestasis. J. Vis. Exp. 2015. [Google Scholar] [CrossRef] [PubMed]
- Geerts, A.M.; Vanheule, E.; Praet, M.; Van, V.H.; De, V.M.; Colle, I. Comparison of three research models of portal hypertension in mice: Macroscopic, histological and portal pressure evaluation. Int. J. Exp. Pathol. 2008, 89, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Wuestefeld, T.; Klein, C.; Streetz, K.L.; Beraza, N.; Schölmerich, J.; Burgart, L.J.; Zender, L.; Kubicka, S.; Baskin-Bey, E.; Gores, G.J.; et al. Lack of gp130 expression results in more bacterial infection and higher mortality during chronic cholestasis in mice. Hepatology 2005, 42, 1082–1090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosler, P. Diagnosis and management of acute cholangitis. Curr. Gastroenterol. Rep. 2011, 13, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Yokoyama, Y.; Kokuryo, T.; Kawai, K.; Kitagawa, T.; Seki, T.; Nakagawa, A.; Nagino, M. 15-deoxy-delta 12,14-prostaglandin J2 prevents inflammatory response and endothelial cell damage in rats with acute obstructive cholangitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G410–G418. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.J.; Briz, O. Bile-acid-induced cell injury and protection. World J. Gastroenterol. 2009, 15, 1677–1689. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.Y.L.; Ferrell, J.M. Bile Acid Metabolism in Liver Pathobiology. Gene Expr. 2018, 18, 71–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawata, A.; Noguchi, H.; Mazaki, Y.; Kurahashi, T.; Izumi, H.; Wang, K.Y.; Guo, X.; Uramoto, H.; Kohno, K.; Taniguchi, H.; et al. Overexpression of Peroxiredoxin 4 Affects Intestinal Function in a Dietary Mouse Model of Nonalcoholic Fatty Liver Disease. PLoS ONE 2016, 11, e0152549. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, A.F.; Hagey, L.R. Bile acids: Chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell. Mol. Life Sci. 2008, 65, 2461–2483. [Google Scholar] [CrossRef] [PubMed]
- Nuño-Lámbarri, N.; Barbero-Becerra, V.J.; Uribe, M.; Chávez-Tapia, N.C. Elevated cholesterol levels have a poor prognosis in a cholestasis scenario. J. Biochem. Mol. Toxicol. 2017, 31, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Fickert, P.; Thueringer, A.; Moustafa, T.; Silbert, D.; Gumhold, J.; Tsybrovskyy, O.; Lebofsky, M.; Jaeschke, H.; Denk, H.; Trauner, M. The role of osteopontin and tumor necrosis factor α receptor-1 in xenobiotic-induced cholangitis and biliary fibrosis in mice. Lab. Investig. 2010, 90, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Carpenter-Deyo, L.; Marchand, D.H.; Jean, P.A.; Roth, R.A.; Reed, D.J. Involvement of glutathione in 1-naphthylisothiocyanate (ANIT) metabolism and toxicity to isolated hepatocytes. Biochem. Pharmacol. 1991, 42, 2171–2180. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Guo, X.; Hamada, T.; Yokoyama, S.; Nakamura, Y.; Zheng, J.; Kurose, N.; Ishigaki, Y.; Uramoto, H.; Tanimoto, A.; et al. Protective Effects of Peroxiredoxin 4 (PRDX4) on Cholestatic Liver Injury. Int. J. Mol. Sci. 2018, 19, 2509. https://doi.org/10.3390/ijms19092509
Zhang J, Guo X, Hamada T, Yokoyama S, Nakamura Y, Zheng J, Kurose N, Ishigaki Y, Uramoto H, Tanimoto A, et al. Protective Effects of Peroxiredoxin 4 (PRDX4) on Cholestatic Liver Injury. International Journal of Molecular Sciences. 2018; 19(9):2509. https://doi.org/10.3390/ijms19092509
Chicago/Turabian StyleZhang, Jing, Xin Guo, Taiji Hamada, Seiya Yokoyama, Yuka Nakamura, Jianbo Zheng, Nozomu Kurose, Yasuhito Ishigaki, Hidetaka Uramoto, Akihide Tanimoto, and et al. 2018. "Protective Effects of Peroxiredoxin 4 (PRDX4) on Cholestatic Liver Injury" International Journal of Molecular Sciences 19, no. 9: 2509. https://doi.org/10.3390/ijms19092509






