Chitosan Inhibits the Rehabilitation of Damaged Microbes Induced by Photodynamic Inactivation
Abstract
:1. Introduction
2. Results
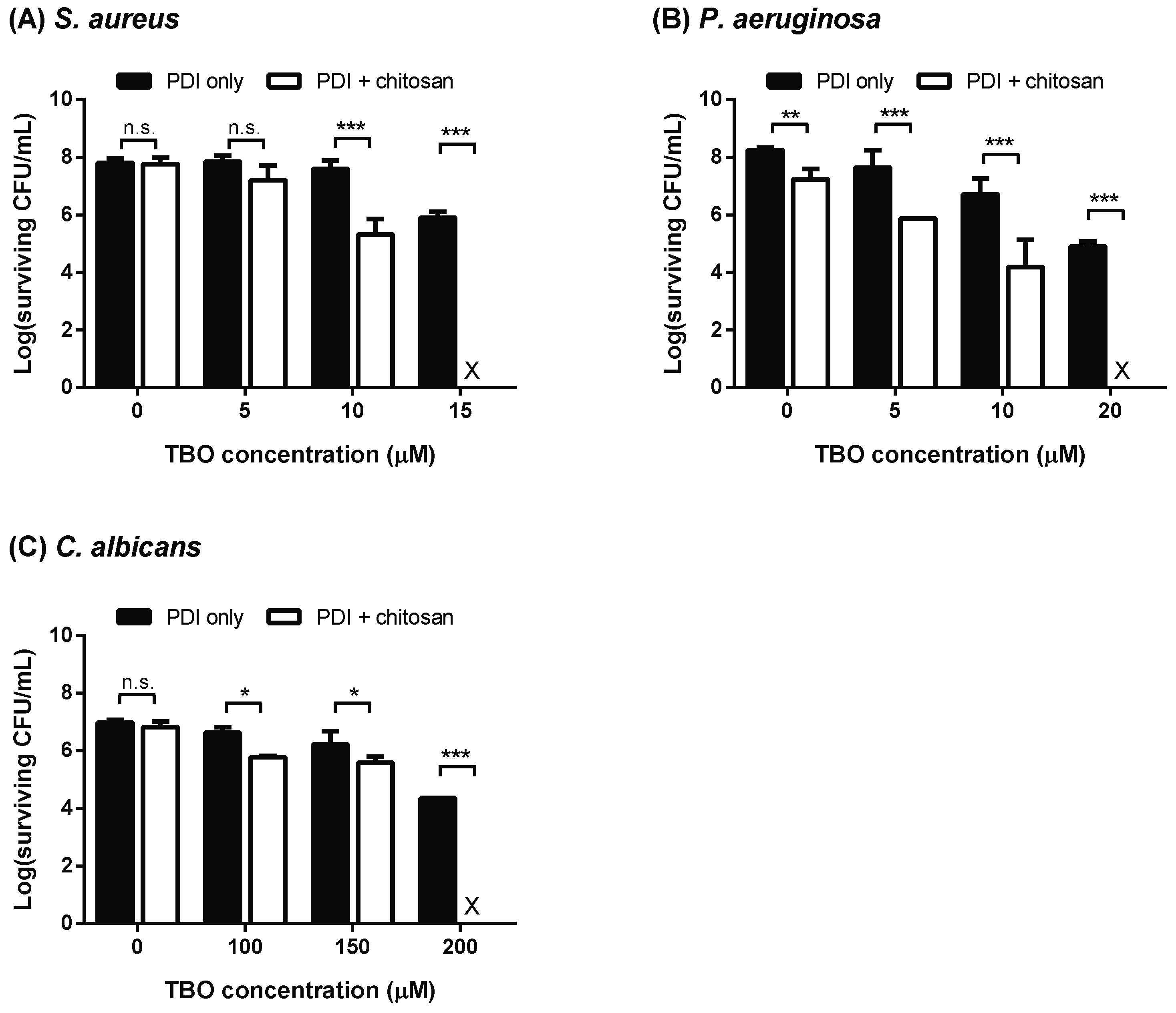
2.1. Chitosan Treatment after PDI
2.2. Morphologic Aspects Observed by TEM
2.3. Increasing the Incubation Time or Concentration of Chitosan in PDI-Induced Cytotoxicity
2.4. Prolonged Lag Phase in PDI-Surviving Cells
2.5. Chitosan Inhibits Recovery of Damaged Cells
3. Discussion
4. Materials and Methods
4.1. Strains and Reagents
4.2. PDI in Planktonic Microbial Cells
4.3. Effect of Chitosan on TBO-Mediated PDI
4.4. Survival Assay
4.5. Transmission Electron Microscopy (TEM)
4.6. Effects of Chitosan on Recovery of TBO-Mediated PDI Microbial Cells
4.7. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Revie, N.M.; Iyer, K.R.; Robbins, N.; Cowen, L.E. Antifungal drug resistance: Evolution, mechanisms and impact. Curr. Opin. Microbiol. 2018, 45, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Ferber, D. Antibiotic resistance. Superbugs on the hoof? Science 2000, 288, 792–794. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, D.P. Current challenges in the management of the infected patient. Curr. Opin. Infect. Dis. 2011, 24 (Suppl. S1), S1–S10. [Google Scholar] [CrossRef]
- Yayan, J.; Ghebremedhin, B.; Rasche, K. Antibiotic resistance of Pseudomonas aeruginosa in pneumonia at a single university hospital center in Germany over a 10-year period. PLoS ONE 2015, 10, e0139836. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Cuenca-Estrella, M. Antifungal drug resistance mechanisms in pathogenic fungi: From bench to bedside. Clin. Microbiol. Infect. 2014, 20 (Suppl. S6), 54–59. [Google Scholar] [CrossRef]
- Dolmans, D.E.; Fukumura, D.; Jain, R.K. Photodynamic therapy for cancer. Nat. Rev. Cancer 2003, 3, 380. [Google Scholar] [CrossRef] [PubMed]
- Jori, G.; Fabris, C.; Soncin, M.; Ferro, S.; Coppellotti, O.; Dei, D.; Fantetti, L.; Chiti, G.; Roncucci, G. Photodynamic therapy in the treatment of microbial infections: Basic principles and perspective applications. Lasers Surg. Med. 2006, 38, 468–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Huang, Y.Y.; Wang, Y.; Wang, X.; Hamblin, M.R. Antimicrobial photodynamic therapy to control clinically relevant biofilm infections. Front. Microbiol. 2018, 9, 1299. [Google Scholar] [CrossRef] [PubMed]
- Dobson, J.; de Queiroz, G.F.; Golding, J.P. Photodynamic therapy and diagnosis: Principles and comparative aspects. Vet. J. 2018, 233, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Winckler, K.D. Special section: Focus on anti-microbial photodynamic therapy (PDT). J. Photochem. Photobiol. B Biol. 2007, 86, 43–44. [Google Scholar] [CrossRef] [PubMed]
- Denis, T.G.S.; Dai, T.H.; Izikson, L.; Astrakas, C.; Anderson, R.R.; Hamblin, M.R.; Tegos, G.P. All you need is light antimicrobial photoinactivation as an evolving and emerging discovery strategy against infectious disease. Virulence 2011, 2, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M.; Maisch, T.; Nonell, S.; Plaetzer, K.; Almeida, A.; Tegos, G.P.; Hamblin, M.R. Photoantimicrobials—Are we afraid of the light? Lancet Infect. Dis. 2017, 17, e49–e55. [Google Scholar] [CrossRef]
- Ragas, X.; Sanchez-Garcia, D.; Ruiz-Gonzalez, R.; Dai, T.H.; Agut, M.; Hamblin, M.R.; Nonell, S. Cationic porphycenes as potential photosensitizers for antimicrobial photodynamic therapy. J. Med. Chem. 2010, 53, 7796–7803. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.C.; Gomes, M.C.; Faustino, M.A.; Neves, M.G.; Cunha, A.; Cavaleiro, J.A.; Almeida, A.; Tome, J.P. Comparative photodynamic inactivation of antibiotic resistant bacteria by first and second generation cationic photosensitizers. Photochem. Photobiol. Sci. 2012, 11, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Harris, F.; Chatfield, L.K.; Phoenix, D.A. Phenothiazinium based photosensitisers--photodynamic agents with a multiplicity of cellular targets and clinical applications. Curr. Drug Targets 2005, 6, 615–627. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.; Schmitz, C.; Facius, R.; Horneck, G.; Milow, B.; Funken, K.H.; Ortner, J. Systematic study of parameters influencing the action of Rose Bengal with visible light on bacterial cells: Comparison between the biological effect and singlet-oxygen production. Photochem. Photobiol. 2000, 71, 514–523. [Google Scholar] [CrossRef]
- Gold, M.H. Photodynamic therapy for cosmetic uses on the skin: An update 2010. G. Ital. Dermatol. Venereol. 2010, 145, 525–541. [Google Scholar] [PubMed]
- Soukos Nikolaos, S.; Wilson, M.; Burns, T.; Speight Paul, M. Photodynamic effects of toluidine blue on human oral keratinocytes and fibroblasts and Streptococcus sanguis evaluated in vitro. Lasers Surg. Med. 1996, 18, 53–259. [Google Scholar]
- Zeina, B.; Greenman, J.; Corry, D.; Purcell, W.M. Cytotoxic effects of antimicrobial photodynamic therapy on keratinocytes in vitro. Br. J. Dermatol. 2002, 146, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Zeina, B.; Greenman, J.; Corry, D.; Purcell, W.M. Antimicrobial photodynamic therapy: Assessment of genotoxic effects on keratinocytes in vitro. Br. J. Dermatol. 2003, 148, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Morley, K.L.; Chauve, G.; Kazlauskas, R.; Dupont, C.; Shareck, F.; Marchessault, R.H. Acetyl xylan esterase-catalyzed deacetylation of chitin and chitosan. Carbohyd. Polym. 2006, 63, 310–315. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [PubMed]
- Tikhonov, V.E.; Stepnova, E.A.; Babak, V.G.; Yamskov, I.A.; Palma-Guerrero, J.; Jansson, H.B.; Lopez-Llorca, L.V.; Salinas, J.; Gerasimenko, D.V.; Avdienko, I.D.; et al. Bactericidal and antifungal activities of a low molecular weight chitosan and its N-/2(3)-(dodec-2-enyl)succinoyl/-derivatives. Carbohyd. Polym. 2006, 64, 66–72. [Google Scholar] [CrossRef]
- Muzzarelli, R.; Tarsi, R.; Filippini, O.; Giovanetti, E.; Biagini, G.; Varaldo, P.E. Antimicrobial properties of N-carboxybutyl chitosan. Antimicrob. Agents Chemother. 1990, 34, 2019–2023. [Google Scholar] [CrossRef] [PubMed]
- Rabea, E.I.; Badawy, M.E.T.; Stevens, C.V.; Smagghe, G.; Steurbaut, W. Chitosan as antimicrobial agent: Applications and mode of action. Biomacromolecules 2003, 4, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Anitha, A.; Sowmya, S.; Kumar, P.T.S.; Deepthi, S.; Chennazhi, K.P.; Ehrlich, H.; Tsurkan, M.; Jayakumar, R. Chitin and chitosan in selected biomedical applications. Prog. Polym. Sci. 2014, 39, 1644–1667. [Google Scholar] [CrossRef]
- Raafat, D.; von Bargen, K.; Haas, A.; Sahl, H.-G. Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef] [PubMed]
- Raafat, D.; Sahl, H.-G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [PubMed]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.; Chien, H.F.; Wang, T.H.; Huang, C.T.; Ker, Y.B.; Chen, C.T. Chitosan augments photodynamic inactivation of gram-positive and gram-negative bacteria. Antimicrob. Agents Chemother. 2011, 55, 1883–1890. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.P.; Hsieh, C.M.; Tsai, T.; Yang, J.C.; Chen, C.T. Optimization and evaluation of a chitosan/hydroxypropyl methylcellulose hydrogel containing toluidine blue O for antimicrobial photodynamic inactivation. Int. J. Mol. Sci. 2015, 16, 20859–20872. [Google Scholar] [CrossRef] [PubMed]
- Chien, H.F.; Chen, C.P.; Chen, Y.C.; Chang, P.H.; Tsai, T.M.; Chen, C.T. The use of chitosan to enhance photodynamic inactivation against Candida albicans and its drug-resistant clinical isolates. Int. J. Mol. Sci. 2013, 14, 7445–7456. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Verlee, A.; Mincke, S.; Stevens, C.V. Recent developments in antibacterial and antifungal chitosan and its derivatives. Carbohyd. Polym. 2017, 164, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Nikolaidis, I.; Favini-Stabile, S.; Dessen, A. Resistance to antibiotics targeted to the bacterial cell wall. Protein Sci. 2014, 23, 243–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hopke, A.; Brown, A.J.P.; Hall, R.A.; Wheeler, R.T. Dynamic fungal cell wall architecture in stress adaptation and immune evasion. Trends Microbiol. 2018, 26, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xuan, Y.; Koide, Y.; Zhiyentayev, T.; Tanaka, M.; Hamblin, M.R. Type i and type ii mechanisms of antimicrobial photodynamic therapy: An in vitro study on gram-negative and gram-positive bacteria. Lasers Surg. Med. 2012, 44, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Kashef, N.; Hamblin, M.R. Can microbial cells develop resistance to oxidative stress in antimicrobial photodynamic inactivation? Drug Resist. Updat 2017, 31, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Malik, Z.; Ladan, H.; Nitzan, Y. Photodynamic inactivation of Gram-negative bacteria: Problems and possible solutions. J. Photochem. Photobiol. B 1992, 14, 262–266. [Google Scholar] [CrossRef]





© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-H.; Chien, H.-F.; Lin, M.-H.; Chen, C.-P.; Shen, M.; Chen, C.-T. Chitosan Inhibits the Rehabilitation of Damaged Microbes Induced by Photodynamic Inactivation. Int. J. Mol. Sci. 2018, 19, 2598. https://doi.org/10.3390/ijms19092598
Lin C-H, Chien H-F, Lin M-H, Chen C-P, Shen M, Chen C-T. Chitosan Inhibits the Rehabilitation of Damaged Microbes Induced by Photodynamic Inactivation. International Journal of Molecular Sciences. 2018; 19(9):2598. https://doi.org/10.3390/ijms19092598
Chicago/Turabian StyleLin, Ching-Hsuan, Hsiung-Fei Chien, Ming-Hsuan Lin, Chueh-Pin Chen, Mandy Shen, and Chin-Tin Chen. 2018. "Chitosan Inhibits the Rehabilitation of Damaged Microbes Induced by Photodynamic Inactivation" International Journal of Molecular Sciences 19, no. 9: 2598. https://doi.org/10.3390/ijms19092598




