Coexistence of TERT Promoter Mutations and the BRAF V600E Alteration and Its Impact on Histopathological Features of Papillary Thyroid Carcinoma in a Selected Series of Polish Patients
Abstract
:1. Introduction
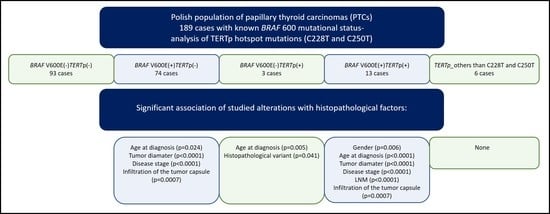
2. Results
2.1. TERT Promoter Status in PTCs and Its Relation to the Presence of the BRAF V600E Mutation
2.2. Association of the BRAF V600E Mutation and TERTp Alterations with Histopathological Factors in the Cohort of Polish Patients with PTC
2.3. Further Analysis of the Presence of TERTp Alterations in a Subgroup of Patients with PTMC
3. Discussion
4. Material and Methods
4.1. DNA Isolation
4.2. DNA Isolation
4.3. Detection of TERTp Mutations
4.4. Detection of BRAF V600E Mutation
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ATC | Anaplastic thyroid carcinoma |
| ETS | E26 transformation-specific |
| FTC | Follicular thyroid carcinoma |
| HCC | Hürthle cell carcinoma |
| LNM | Lymph node metastases |
| PDTC | Poorly differentiated thyroid carcinoma |
| PTC | Papillary thyroid carcinoma |
| PTMC | Papillary thyroid microcarcinoma |
| TC | Thyroid carcinoma |
| TERTp | TERT promoter |
References
- Loh, K.C.; Greenspan, F.S.; Gee, L.; Miller, T.R.; Yeo, P.P. Pathological tumor-node-metastasis (pTNM) staging for papillary and follicular thyroid carcinomas: A retrospective analysis of 700 patients. J. Clin. Endocrinol. Metab. 1997, 82, 3553–3562. [Google Scholar] [CrossRef] [PubMed]
- Durante, C.; Haddy, N.; Baudin, E.; Leboulleux, S.; Hartl, D.; Travagli, J.P.; Caillou, B.; Ricard, M.; Lumbroso, J.D.; De Vathaire, F.; et al. Long-term outcome of 444 patients with distant metastases from papillary and follicular thyroid carcinoma: Benefits and limits of radioiodine therapy. J. Clin. Endocrinol. Metab. 2006, 91, 2892–2899. [Google Scholar] [CrossRef] [PubMed]
- Su, D.-H.; Chang, S.-H.; Chang, T.-C. The impact of locoregional recurrences and distant metastases on the survival of patients with papillary thyroid carcinoma. Clin. Endocrinol. 2015, 82, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Machens, A.; Hinze, R.; Thomusch, O.; Dralle, H. Pattern of nodal metastasis for primary and reoperative thyroid cancer. World J. Surg. 2002, 26, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Xing, M.; Westra, W.H.; Tufano, R.P.; Cohen, Y.; Rosenbaum, E.; Rhoden, K.J.; Carson, K.A.; Vasko, V.; Larin, A.; Tallini, G.; et al. BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J. Clin. Endocrinol. Metab. 2005, 90, 6373–6379. [Google Scholar] [CrossRef] [PubMed]
- Durante, C.; Puxeddu, E.; Ferretti, E.; Morisi, R.; Moretti, S.; Bruno, R.; Barbi, F.; Avenia, N.; Scipioni, A.; Verrienti, A.; et al. BRAF mutations in papillary thyroid carcinomas inhibit genes involved in iodine metabolism. J. Clin. Endocrinol. Metab. 2007, 92, 2840–2843. [Google Scholar] [CrossRef] [PubMed]
- Fugazzola, L.; Mannavola, D.; Cirello, V.; Vannucchi, G.; Muzza, M.; Vicentini, L.; Beck-Peccoz, P. BRAF mutations in an Italian cohort of thyroid cancers. Clin. Endocrinol. 2004, 61, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.Y.; Kim, W.B.; Rhee, Y.S.; Song, J.Y.; Kim, J.M.; Gong, G.; Lee, S.; Kim, S.Y.; Kim, S.C.; Hong, S.J.; et al. The BRAF mutation is useful for prediction of clinical recurrence in low-risk patients with conventional papillary thyroid carcinoma. Clin. Endocrinol. 2006, 65, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Bishop, J.; Shan, Y.; Pai, S.; Liu, D.; Murugan, A.K.; Sun, H.; El-Naggar, A.K.; Xing, M. Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr. Relat. Cancer 2013, 20, 603–610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, F.W.; Hodis, E.; Xu, M.J.; Kryukov, G.V.; Chin, L.; Garraway, L.A. Highly Recurrent TERT Promoter Mutations in Human Melanoma. Science 2013, 339, 957–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horn, S.; Figl, A.; Rachakonda, P.S.; Fischer, C.; Sucker, A.; Gast, A.; Kadel, S.; Moll, I.; Nagore, E.; Hemminki, K.; et al. TERT Promoter Mutations in Familial and Sporadic Melanoma. Science 2013, 339, 959–961. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Xing, M. TERT promoter mutations in thyroid cancer. Endocr. Relat. Cancer 2016, 23, R143–R145. [Google Scholar] [CrossRef] [PubMed]
- Blasco, M.A. Telomeres and human disease: Ageing, cancer and beyond. Nat. Rev. Genet. 2005, 6, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Low, K.C.; Tergaonkar, V. Telomerase: Central regulator of all of the hallmarks of cancer. Trends Biochem. Sci. 2013, 38, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Vinagre, J.; Almeida, A.; Populo, H.; Batista, R.; Lyra, J.; Pinto, V.; Coelho, R.; Celestino, R.; Prazeres, H.; Lima, L.; et al. Frequency of TERT promoter mutations in human cancers. Nat. Commun. 2013, 4, 2185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Biase, D.; Gandolfi, G.; Ragazzi, M.; Eszlinger, M.; Sancisi, V.; Gugnoni, M.; Visani, M.; Pession, A.; Casadei, G.; Durante, C.; et al. TERT Promoter Mutations in Papillary Thyroid Microcarcinomas. Thyroid 2015, 25, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Macerola, E.; Loggini, B.; Giannini, R.; Garavello, G.; Giordano, M.; Proietti, A.; Niccoli, C.; Basolo, F.; Fontanini, G. Coexistence of TERT promoter and BRAF mutations in cutaneous melanoma is associated with more clinicopathological features of aggressiveness. Virchows Arch. 2015, 467, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Griewank, K.G.; Murali, R.; Puig-Butille, J.A.; Schilling, B.; Livingstone, E.; Potrony, M.; Carrera, C.; Schimming, T.; Moller, I.; Schwamborn, M.; et al. TERT promoter mutation status as an independent prognostic factor in cutaneous melanoma. J. Natl. Cancer Inst. 2014, 106. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Bishop, J.; Zhu, G.; Zhang, T.; Ladenson, P.W.; Xing, M. Mortality Risk Stratification by Combining BRAF V600E and TERT Promoter Mutations in Papillary Thyroid Cancer: Genetic Duet of BRAF and TERT Promoter Mutations in Thyroid Cancer Mortality. JAMA Oncol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Liu, R.; Xing, M. A six-genotype genetic prognostic model for papillary thyroid cancer. Endocr. Relat. Cancer 2017, 24, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Marques, I.J.; Moura, M.M.; Cabrera, R.; Pinto, A.E.; Simões-Pereira, J.; Santos, C.; Menezes, F.D.; Montezuma, D.; Henrique, R.; Rodrigues Teixeira, M.; et al. Identification of somatic TERT promoter mutations in familial nonmedullary thyroid carcinomas. Clin. Endocrinol. 2017, 87, 394–399. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, N.; Nobusawa, S.; Nakata, S.; Nakada, M.; Yamazaki, T.; Matsumura, N.; Harada, K.; Matsuda, H.; Funata, N.; Nagai, S.; et al. BRAF V600E, TERT promoter mutations and CDKN2A/B homozygous deletions are frequent in epithelioid glioblastomas: A histological and molecular analysis focusing on intratumoral heterogeneity. Brain Pathol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Casuscelli, J.; Becerra, M.F.; Manley, B.J.; Zabor, E.C.; Reznik, E.; Redzematovic, A.; Arcila, M.E.; Tennenbaum, D.M.; Ghanaat, M.; Kashan, M.; et al. Characterization and Impact of TERT Promoter Region Mutations on Clinical Outcome in Renal Cell Carcinoma. Eur. Urol. Focus 2017. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Kim, D.; Park, W.; Lee, H.; Choi, I.; Park, J.; Lee, J. Mutation of the TERT promoter leads to poor prognosis of patients with non-small cell lung cancer. Oncol. Lett. 2017, 14, 1609–1614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, M.; da Rocha, A.G.; Vinagre, J.; Batista, R.; Peixoto, J.; Tavares, C.; Celestino, R.; Almeida, A.; Salgado, C.; Eloy, C.; et al. TERT promoter mutations are a major indicator of poor outcome in differentiated thyroid carcinomas. J. Clin. Endocrinol. Metab. 2014, 99, E754–E765. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Jiang, X.; Wang, W.; Wang, H.; Xu, X.; Lin, A.; Teng, X.; Wu, H.; Teng, L. Association of telomerase reverse transcriptase promoter mutations with clinicopathological features and prognosis of thyroid cancer: A meta-analysis. OncoTargets Ther. 2016, 9, 6965–6976. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yuan, X.; Xu, D. Cancer-Specific Telomerase Reverse Transcriptase (TERT) Promoter Mutations: Biological and Clinical Implications. Genes 2016, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Nasirden, A.; Saito, T.; Fukumura, Y.; Hara, K.; Akaike, K.; Kurisaki-Arakawa, A.; Asahina, M.; Yamashita, A.; Tomomasa, R.; Hayashi, T.; et al. In Japanese patients with papillary thyroid carcinoma, TERT promoter mutation is associated with poor prognosis, in contrast to BRAF V600E mutation. Virchows Arch. 2016, 469, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Landa, I.; Ganly, I.; Chan, T.A.; Mitsutake, N.; Matsuse, M.; Ibrahimpasic, T.; Ghossein, R.A.; Fagin, J.A. Frequent somatic TERT promoter mutations in thyroid cancer: Higher prevalence in advanced forms of the disease. J. Clin. Endocrinol. Metab. 2013, 98, E1562–E1566. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, G.; Ragazzi, M.; Frasoldati, A.; Piana, S.; Ciarrocchi, A.; Sancisi, V. TERT promoter mutations are associated with distant metastases in papillary thyroid carcinoma. Eur. J. Endocrinol. 2015, 172, 403–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, R.; Zhang, T.; Zhu, G.; Xing, M. Regulation of mutant TERT by BRAF V600E/MAP kinase pathway through FOS/GABP in human cancer. Nat. Commun. 2018, 9, 579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haugen, B.R.; Alexander, E.K.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Pacini, F.; Randolph, G.W.; Sawka, A.M.; Schlumberger, M.; et al. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Team, R.C. A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available online: https://www.r-project.org/ (accessed on 15 December 2017).
- Gordon, M. Gmisc: Descriptive Statistics, Transition Plots, and More. R Package, Version 1.4.1; 2016. Available online: https://rdrr.io/cran/Gmisc/ (accessed on 5 September 2018).


| TERT/BRAF Status | BRAF V600E-Positive | BRAF V600E-Negative | p-Value |
| (n = 87) | (n = 96) | ||
| TERTp hotspot mutation | 0.007 | ||
| TERTp-positive | 13 (14.9%) | 3 (3.1%) | |
| TERTp-negative | 74 (85.1%) | 93 (96.9%) | |
| TERTp-Positive | TERTp-Negative | p-Value | |
| (n = 16) | (n = 167) | ||
| BRAF mutation | 0.007 | ||
| BRAF V600E-positive | 13 (81.2%) | 74 (44.3%) | |
| BRAF V600E-negative | 3 (18.8%) | 93 (55.7%) |
| Histopathological Features | No Mutation | BRAF + TERTp− | BRAF − TERTp+ | BRAF + TERTp+ | TERTp_Others | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. (%) | No. of Missing Cases | No. (%) | No. of Missing Cases | p-Value | No. (%) | No. of Missing Cases | p-Value | No. (%) | No. of Missing Cases | p-Value | No. (%) | No. of Missing Cases | p-Value | |
| Total No. Of Cases | 93 | 74 | 3 | 13 | 6 | |||||||||
| Sex | 0.3 | 0.23 | 0.006 | 1 | ||||||||||
| Female | 86 (92.5%) | 64 (86.5%) | 2 (66.7%) | 8 (61.5%) | 6 (100.0%) | |||||||||
| Male | 7 (7.5%) | 10 (13.5%) | 1 (33.3%) | 5 (38.5%) | 0 (0.0%) | |||||||||
| Age at diagnosis (years) | 50.0 (36.0–58.0) | 54.0 (40.2–64.0) | 0.024 | 70.0 (68.0–76.5) | 0.005 | 71.0 (63.0–75.0) | <0.0001 | 48.5 (34.0–60.8) | 0.94 | |||||
| Tumor diameter (mm) | 7.0 (4.0–14.0) | 1 | 13.0 (8.0–17.8) | <0.0001 | 12.0 (11.0–13.0) | 0.21 | 25.0 (17.2–31.5) | 1 | <0.0001 | 10.0 (3.5–18.8) | 0.91 | |||
| Histopathological variant | 10 | 6 | 0.97 | 0.041 | 2 | 0.14 | 1 | |||||||
| Classical (CPTC) | 34 (41.0%) | 29 (42.6%) | 1 (33.3%) | 8 (72.7%) | 3 (50.0%) | |||||||||
| Follicular (FVPTC) | 23 (27.7%) | 21 (30.9%) | 0 (0.0%) | 1 (9.1%) | 2 (33.3%) | |||||||||
| Classical and follicular | 16 (19.3%) | 12 (17.6%) | 0 (0.0%) | 0 (0.0%) | 1 (16.7%) | |||||||||
| Oxyphilic | 5 (6.0%) | 3 (4.4%) | 2 (66.7%) | 1 (9.1%) | 0 (0.0%) | |||||||||
| Others | 5 (6.0%) | 3 (4.4%) | 0 (0.0%) | 1 (9.1%) | 0 (0.0%) | |||||||||
| pT | 1 | 1 | <0.0001 | 0.14 | <0.0001 | 0.14 | ||||||||
| 1a | 58 (63.0%) | 19 (26.0%) | 1 (33.3%) | 0 (0.0%) | 2 (33.3%) | |||||||||
| 1b | 12 (13.0%) | 23 (31.5%) | 2 (66.7%) | 3 (23.1%) | 2 (33.3%) | |||||||||
| 2 | 9 (9.8%) | 5 (6.8%) | 0 (0.0%) | 1 (7.7%) | 0 (0.0%) | |||||||||
| 3 | 13 (14.1%) | 24 (32.9%) | 0 (0.0%) | 7 (53.8%) | 2 (33.3%) | |||||||||
| 4a | 0 (0.0%) | 2 (2.7%) | 0 (0.0%) | 2 (15.4%) | 0 (0.0%) | |||||||||
| pT | 1 | 1 | 0.002 | 1 | <0.0001 | 0.23 | ||||||||
| T1/T2 | 79 (85.9%) | 47 (64.4%) | 3 (100.0%) | 4 (30.8%) | 4 (66.7%) | |||||||||
| T3/T4 | 13 (14.1%) | 26 (35.6%) | 0 (0.0%) | 9 (69.2%) | 2 (33.3%) | |||||||||
| Lymph node metastases | 14 | 21 | 0.42 | 2 | 1 | 6 | <0.0001 | 2 | 0.16 | |||||
| 0 | 59 (74.7%) | 34 (64.2%) | 1 (100.0%) | 0 (0.0%) | 2 (50.0%) | |||||||||
| 1a | 11 (13.9%) | 10 (18.9%) | 0 (0.0%) | 2 (28.6%) | 0 (0.0%) | |||||||||
| 1b | 9 (11.4%) | 9 (17.0%) | 0 (0.0%) | 5 (71.4%) | 2 (50.0%) | |||||||||
| Infiltration of the tumor capsule | 0.0007 | 0.51 | 0.0007 | 0.6 | ||||||||||
| Yes | 19 (20.4%) | 34 (45.9%) | 1 (33.3%) | 9 (69.2%) | 2 (33.3%) | |||||||||
| No | 74 (79.6%) | 40 (54.1%) | 2 (66.7%) | 4 (30.8%) | 4 (66.7%) | |||||||||
| Angioinvasion | 1 | 0.26 | 0.13 | 1 | ||||||||||
| Yes | 8 (8.6%) | 6 (8.1%) | 1 (33.3%) | 3 (23.1%) | 0 (0.0%) | |||||||||
| No | 85 (91.4%) | 68 (91.9%) | 2 (66.7%) | 10 (76.9%) | 6 (100.0%) | |||||||||
| Multifocality | 0.83 | 1 | 0.69 | 0.59 | ||||||||||
| Yes | 14 (15.1%) | 12 (16.2%) | 0 (0.0%) | 1 (7.7%) | 0 (0.0%) | |||||||||
| No | 79 (84.9%) | 62 (83.8%) | 3 (100.0%) | 12 (92.3%) | 6 (100.0%) | |||||||||
| TERT Status and Histopathological Features | Total | BRAF V600E-Negative | BRAF V600E-Positive | |||
|---|---|---|---|---|---|---|
| No. (%) | No. of Missing Cases | No. (%) | No. of Missing Cases | No. (%) | No. of Missing Cases | |
| Total No. of cases | 189 | 100 | 89 | |||
| Sex | ||||||
| Female | 166 (87.8%) | 92 (92.0%) | 74 (83.1%) | |||
| Male | 23 (12.2%) | 8 (8.0%) | 15 (16.9%) | |||
| Age at diagnosis [years] | 53.0 (40.0–62.0) | 50.5 (37.5–59.0) | 57.0 (44.0–66.0) | |||
| TERT promoter hotspot mutation (C228T and C250T) | ||||||
| Lack of mutation | 173 (91.5%) | 97 (97.0%) | 76 (85.4%) | |||
| Mutation | 16 (8.5%) | 3 (3.0%) | 13 (14.6%) | |||
| Type of TERT promoter alteration | ||||||
| Number of negative samples | 167 | 93 | 74 | |||
| C228T | 13 (59.1%) | 3 (42.9%) | 10 (66.7%) | |||
| C250T | 3 (13.6%) | 0 (0.0%) | 3 (20.0%) | |||
| alteration −100 C → T; rs2735943 | 1 (4.5%) | 0 (0.0%) | 1 (6.7%) | |||
| alteration −162 C->T | 1 (4.5%) | 0 (0.0%) | 1 (6.7%) | |||
| alteration −77 C->T | 2 (9.1%) | 2 (28.6%) | 0 (0.0%) | |||
| alteration −80 C->T | 2 (9.1%) | 2 (28.6%) | 0 (0.0%) | |||
| Tumor diameter (mm) | 10.0 (5.0–17.0) | 2 | 8.0 (4.5–14.0) | 1 | 13.0 (8.8–20.0) | 1 |
| Histopathological variant | 18 | 10 | 8 | |||
| classical (CPTC) | 75 (43.9%) | 37 (41.1%) | 38 (46.9%) | |||
| follicular (FVPTC) | 47 (27.5%) | 24 (26.7%) | 23 (28.4%) | |||
| classical and follicular | 29 (17.0%) | 17 (18.9%) | 12 (14.8%) | |||
| oxyphilic | 11 (6.4%) | 7 (7.8%) | 4 (4.9%) | |||
| others | 9 (5.3%) | 5 (5.6%) | 4 (4.9%) | |||
| pT | 2 | 1 | 1 | |||
| 1a | 80 (42.8%) | 60 (60.6%) | 20 (22.7%) | |||
| 1b | 42 (22.5%) | 16 (16.2%) | 26 (29.5%) | |||
| 2 | 15 (8.0%) | 9 (9.1%) | 6 (6.8%) | |||
| 3 | 46 (24.6%) | 14 (14.1%) | 32 (36.4%) | |||
| 4a | 4 (2.1%) | 0 (0.0%) | 4 (4.5%) | |||
| pT | 2 | 1 | 1 | |||
| T1/T2 | 137 (73.3%) | 85 (85.9%) | 52 (59.1%) | |||
| T3/T4 | 50 (26.7%) | 14 (14.1%) | 36 (40.9%) | |||
| Lymph node metastases | ||||||
| 0 | 96 (66.7%) | 61 (74.4%) | 35 (56.5%) | |||
| 1a | 23 (16.0%) | 11 (13.4%) | 12 (19.4%) | |||
| 1b | 25 (17.4%) | 10 (12.2%) | 15 (24.2%) | |||
| Distant metastases | 2 | 2 | ||||
| 0 | 187 (100.0%) | 98 (100.0%) | 89 (100.0%) | |||
| Infiltration of the tumor capsule | ||||||
| yes | 65 (34.4%) | 21 (21.0%) | 44 (49.4%) | |||
| no | 124 (65.6%) | 79 (79.0%) | 45 (50.6%) | |||
| Angioinvasion | ||||||
| yes | 18 (9.5%) | 9 (9.0%) | 9 (10.1%) | |||
| no | 171 (90.5%) | 91 (91.0%) | 80 (89.9%) | |||
| Multifocality | ||||||
| yes | 27 (14.3%) | 14 (14.0%) | 13 (14.6%) | |||
| no | 162 (85.7%) | 86 (86.0%) | 76 (85.4%) | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rusinek, D.; Pfeifer, A.; Krajewska, J.; Oczko-Wojciechowska, M.; Handkiewicz-Junak, D.; Pawlaczek, A.; Zebracka-Gala, J.; Kowalska, M.; Cyplinska, R.; Zembala-Nozynska, E.; et al. Coexistence of TERT Promoter Mutations and the BRAF V600E Alteration and Its Impact on Histopathological Features of Papillary Thyroid Carcinoma in a Selected Series of Polish Patients. Int. J. Mol. Sci. 2018, 19, 2647. https://doi.org/10.3390/ijms19092647
Rusinek D, Pfeifer A, Krajewska J, Oczko-Wojciechowska M, Handkiewicz-Junak D, Pawlaczek A, Zebracka-Gala J, Kowalska M, Cyplinska R, Zembala-Nozynska E, et al. Coexistence of TERT Promoter Mutations and the BRAF V600E Alteration and Its Impact on Histopathological Features of Papillary Thyroid Carcinoma in a Selected Series of Polish Patients. International Journal of Molecular Sciences. 2018; 19(9):2647. https://doi.org/10.3390/ijms19092647
Chicago/Turabian StyleRusinek, Dagmara, Aleksandra Pfeifer, Jolanta Krajewska, Malgorzata Oczko-Wojciechowska, Daria Handkiewicz-Junak, Agnieszka Pawlaczek, Jadwiga Zebracka-Gala, Malgorzata Kowalska, Renata Cyplinska, Ewa Zembala-Nozynska, and et al. 2018. "Coexistence of TERT Promoter Mutations and the BRAF V600E Alteration and Its Impact on Histopathological Features of Papillary Thyroid Carcinoma in a Selected Series of Polish Patients" International Journal of Molecular Sciences 19, no. 9: 2647. https://doi.org/10.3390/ijms19092647