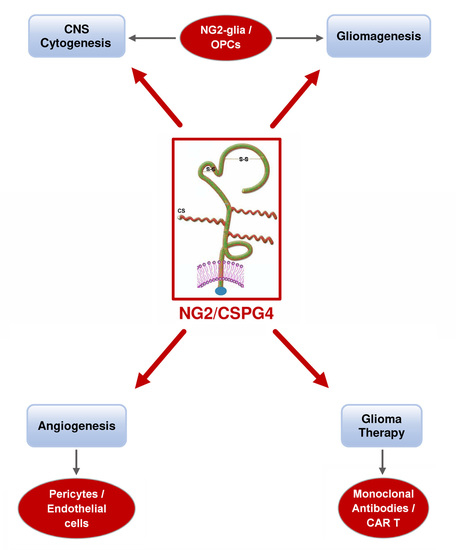
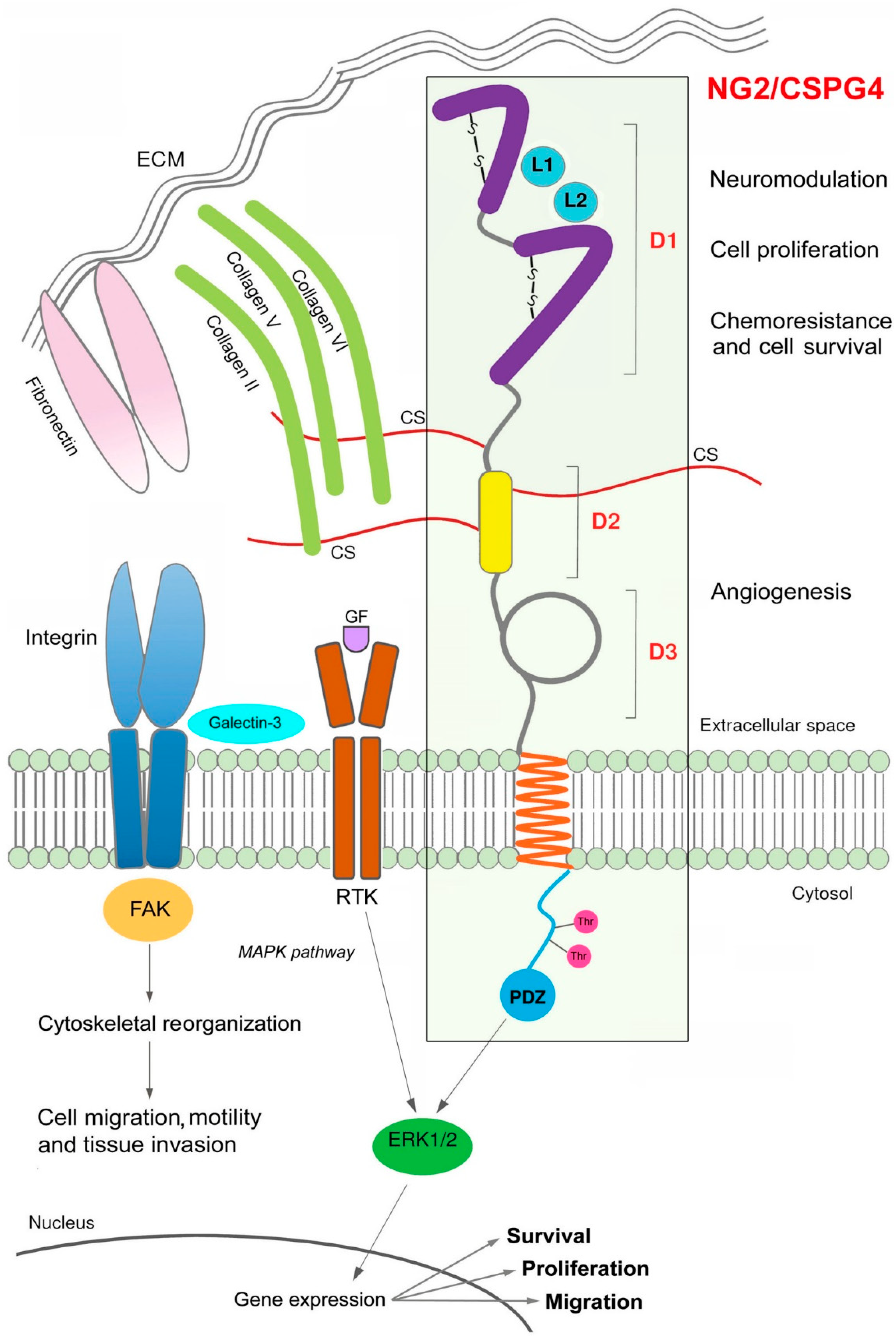
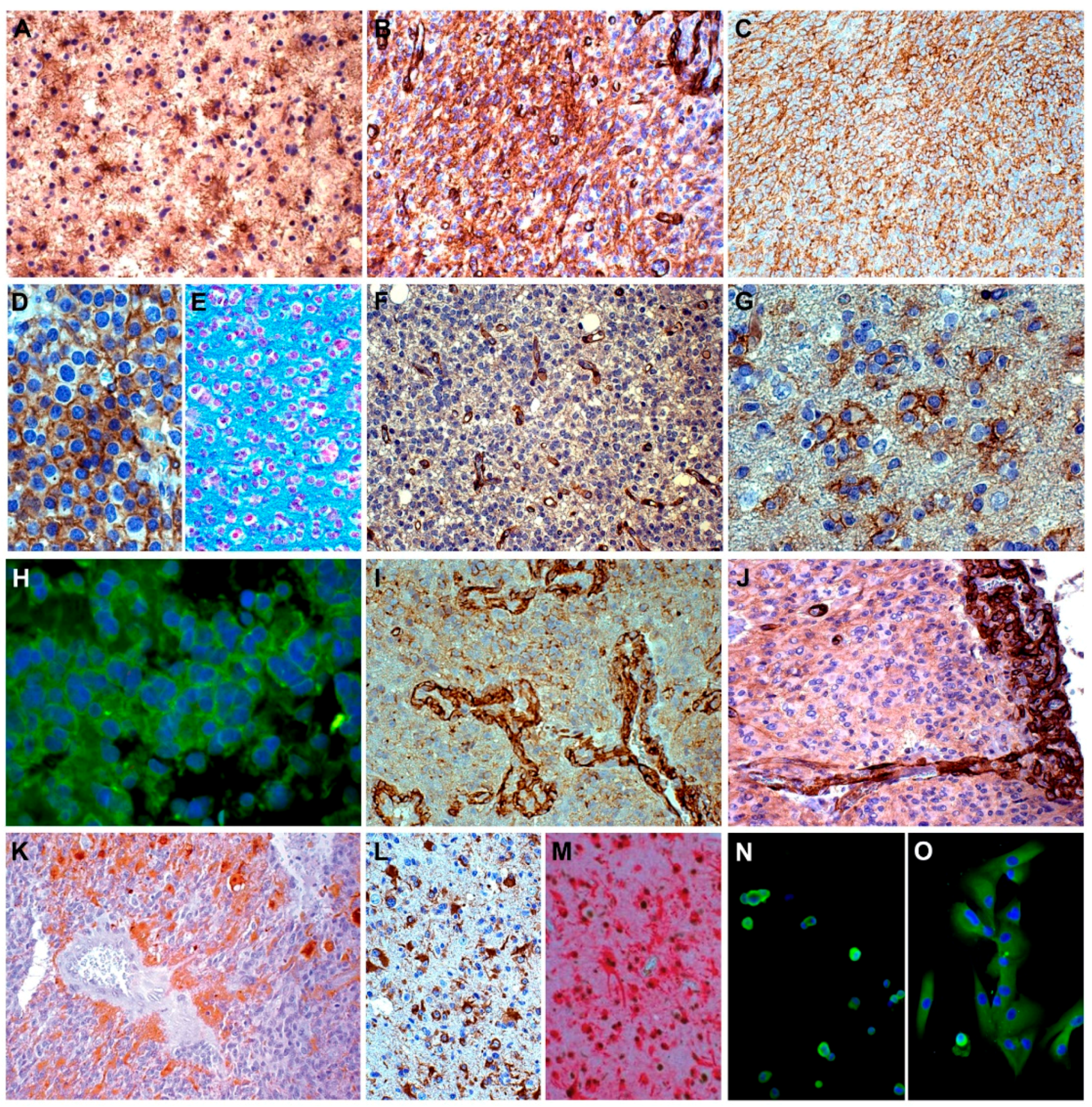
The Significance of Chondroitin Sulfate Proteoglycan 4 (CSPG4) in Human Gliomas
Abstract
:1. Introduction
2. NG2/CSPG4 Gene
3. NG2/CSPG4 Structural and Functional Features
4. NG2/CSPG4 Expression Pattern
5. NG2/CSPG4 in the CNS Biology
6. NG2 /CSPG4 in Gliomas
7. NG2/CSPG4 in Blood Vessel Development
8. NG2/CSPG4 in the Treatment of Gliomas
9. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| CAR-T | chimeric antigen receptor-based T cell |
| CNPase | 2′,3′-cyclic nucleotide phosphodiesterase |
| CNS | central nervous system |
| CS | chondroitin sulphate |
| CSPG4 | chondroitin sulphate proteoglycan 4 |
| EGFR | epidermal growth factor receptor |
| ENU | N-ethyl-N-nitrosourea |
| ERK | extracellular signal-regulated kinase |
| FAK | focal adhesion kinase |
| FGF | fibroblast growth factor |
| GAG | glycosaminoglycan |
| GFAP | glial fibrillary acidic protein |
| GB | glioblastoma |
| GSC | glioblastoma stem cell |
| NG2 | neuron glial antigen 2 |
| Nkx2.2 | homeobox protein Nkx2.2 |
| O2A | oligodendrocyte-type 2 astrocyte |
| OLIG2 | oligodendrocyte lineage transcription factor 2 |
| OPC | oligodendroglial precursor cell |
| PDGFR | platelet-derived growth factor receptor |
| PKCα | protein kinase C-alpha |
| SOX10 | SRY-related HMG-box 10 |
| SVZ | subventricular zone |
References
- Raff, M.C.; Miller, R.H.; Noble, M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium. Nature 1983, 303, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Richardson, W.D.; Young, K.M.; Tripathi, R.B.; McKenzie, I. NG2-glia as Multipotent Neural Stem Cells: Fact or Fantasy? Neuron 2011, 70, 661–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawson, M.R.; Polito, A.; Levine, J.M.; Reynolds, R. NG2-expressing glial progenitor cells: An abundant and widespread population of cycling cells in the adult rat CNS. Mol. Cell. Neurosci. 2003, 24, 476–488. [Google Scholar] [CrossRef]
- Nishiyama, A.; Watanabe, M.; Yang, Z.; Bu, J. Identity, distribution, and development of polydendrocytes: NG2-expressing glial cells. J. Neurocytol. 2002, 31, 437–455. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.; Nishiyama, A.; Peterson, J.; Prineas, J.; Trapp, B.D. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J. Neurosci. 2000, 20, 6404–6412. [Google Scholar] [CrossRef] [PubMed]
- Etxeberria, A.; Mangin, J.M.; Aguirre, A.; Gallo, V. Adult-born SVZ progenitors receive transient synapses during remyelination in corpus callosum. Nat. Neurosci. 2010, 13, 287–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Biase, L.M.; Nishiyama, A.; Bergles, D.E. Excitability and synaptic communication within the oligodendrocyte lineage. J. Neurosci. 2010, 30, 3600–3611. [Google Scholar] [CrossRef] [PubMed]
- Dimou, L.; Gallo, V. NG2-glia and their functions in the central nervous system. Glia 2015, 63, 1429–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimou, L.; Simon, C.; Kirchhoff, F.; Takebayashi, H.; Gotz, M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J. Neurosci. 2008, 28, 10434–10442. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Fukaya, M.; Yang, J.K.; Rothstein, J.D.; Bergles, D.E. NG2+ CNS glial progenitors remain committed to the oligodendrocyte lineage in postnatal life and following neurodegeneration. Neuron 2010, 68, 668–681. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Gotz, M.; Dimou, L. Progenitors in the adult cerebral cortex: Cell cycle properties and regulation by physiological stimuli and injury. Glia 2011, 59, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.E.; Young, K.M.; Hamilton, N.B.; Li, H.; Richardson, W.D.; Attwell, D. Properties and fate of oligodendrocyte progenitor cells in the corpus callosum, motor cortex, and piriform cortex of the mouse. J. Neurosci. 2012, 32, 8173–8185. [Google Scholar] [CrossRef] [PubMed]
- Simon, C.; Lickert, H.; Gotz, M.; Dimou, L. Sox10-iCreERT2: A mouse line to inducibly trace the neural crest and oligodendrocyte lineage. Genesis 2012, 50, 506–515. [Google Scholar] [CrossRef] [PubMed]
- Young, K.M.; Psachoulia, K.; Tripathi, R.B.; Dunn, S.J.; Cossell, L.; Attwell, D.; Tohyama, K.; Richardson, W.D. Oligodendrocyte dynamics in the healthy adult CNS: Evidence for myelin remodeling. Neuron 2013, 77, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.M.; Zhang, W.; Levine, J.M. NG2: A component of the glial scar that inhibits axon growth. J. Anat. 2005, 207, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Bergles, D.E.; Nishiyama, A. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development 2008, 135, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Behar, T.; McMorris, F.A.; Novotny, E.A.; Barker, J.L.; Dubois-Dalcq, M. Growth and differentiation properties of O-2A progenitors purified from rat cerebral hemispheres. J. Neurosci. Res. 1988, 21, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Power, J.; Mayer-Proschel, M.; Smith, J.; Noble, M. Oligodendrocyte precursor cells from different brain regions express divergent properties consistent with the differing time courses of myelination in these regions. Dev. Biol. 2002, 245, 362–375. [Google Scholar] [CrossRef] [PubMed]
- Levison, S.W.; Goldman, J.E. Both oligodendrocytes and astrocytes develop from progenitors in the subventricular zone of postnatal rat forebrain. Neuron 1993, 10, 201–212. [Google Scholar] [CrossRef]
- Menn, B.; Garcia-Verdugo, J.M.; Yaschine, C.; Gonzalez-Perez, O.; Rowitch, D.; Alvarez-Buylla, A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 2006, 26, 7907–7918. [Google Scholar] [CrossRef] [PubMed]
- Hughes, E.G.; Kang, S.H.; Fukaya, M.; Bergles, D.E. Oligodendrocyte progenitors balance growth with self-repulsion to achieve homeostasis in the adult brain. Nat. Neurosci. 2013, 16, 668–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grako, K.A.; Ochiya, T.; Barritt, D.; Nishiyama, A.; Stallcup, W.B. PDGF (alpha)-receptor is unresponsive to PDGF–AA in aortic smooth muscle cells from the NG2 knockout mouse. J. Cell Sci. 1999, 112, 905–915. [Google Scholar] [PubMed]
- Leoni, G.; Rattray, M.; Fulton, D.; Rivera, A.; Butt, A.M. Immunoablation of cells expressing the NG2 chondroitin sulphate proteoglycan. J. Anat. 2014, 224, 216–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozerdem, U.; Stallcup, W.B. Pathological angiogenesis is reduced by targeting pericytes via the NG2 proteoglycan. Angiogenesis 2004, 7, 269–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivera, Z.; Ferrone, S.; Wang, X.; Jube, S.; Yang, H.; Pass, H.I.; Kanodia, S.; Gaudino, G.; Carbone, M. CSPG4 as a target of antibody-based immunotherapy for malignant mesothelioma. Clin. Cancer Res. 2012, 18, 5352–5463. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Svendsen, A.; Kmiecik, J.; Immervoll, H.; Skaftnesmo, K.O.; Planagumà, J.; Reed, R.K.; Bjerkvig, R.; Miletic, H.; Enger, P.Ø.; et al. Targeting the NG2/CSPG4 proteoglycan retards tumour growth and angiogenesis in preclinical models of GBM and melanoma. PLoS ONE 2011, 6, e23062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Yang, J. Development of novel antigen receptors for CAR T-cell therapy directed toward solid malignancies. Transl. Res. 2017, 187, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Lichtman, E.I.; Dotti, G. Chimeric antigen receptor T-cells for B-cell malignancies. Transl. Res. 2017, 187, 59–82. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, K.M.; Cheung, A.; Mele, S.; Chiaruttini, G.; Crescioli, S.; Griffin, M.; Nakamura, M.; Spicer, J.F.; Tsoka, S.; Lacy, K.E.; et al. Chondroitin Sulfate Proteoglycan 4 and Its Potential As an Antibody Immunotherapy Target across Different Tumor Types. Front Immunol. 2018, 8, 1911. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, A.; Brown, C.; Badie, B. Chimeric antigen receptor T-cell therapy for glioblastoma. Transl. Res. 2017, 187, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Pellegatta, S.; Savoldo, B.; Di Ianni, N.; Corbetta, C.; Chen, Y.; Patané, M.; Sun, C.; Pollo, B.; Ferrone, S.; DiMeco, F.; et al. Constitutive and TNFα-inducible expression of chondroitin sulfate proteoglycan 4 in glioblastoma and neurospheres: Implications for CAR-T cell therapy. Sci. Transl. Med. 2018, 10, 430. [Google Scholar] [CrossRef] [PubMed]
- Trotter, J.; Karram, K.; Nishiyama, A. NG2 cells: Properties, progeny and origin. Brain Res. Rev. 2010, 63, 72–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campoli, M.; Ferrone, S.; Wang, X. Functional and clinical relevance of chondroitin sulphate proteoglycan 4. Adv. Cancer Res. 2010, 109, 73–121. [Google Scholar] [PubMed]
- Yadavilli, S.; Scafidi, J.; Becher, O.J.; Saratsis, A.M.; Hiner, R.L.; Kambhampati, M.; Mariarita, S.; MacDonald, T.J.; Codispoti, K.E.; Magge, S.N.; et al. The emerging role of NG2 in pediatric diffuse intrinsic pontine glioma. Oncotarget 2015, 6, 12141–12155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stallcup, W.B. The NG2 antigen, a putative lineage marker: Immunofluorescent localization in primary cultures of rat brain. Dev. Biol. 1981, 83, 154–165. [Google Scholar] [CrossRef]
- Wilson, B.S.; Ruberto, G.; Ferrone, S. Immunochemical characterization of a human high molecular weight--melanoma associated antigen identified with monoclonal antibodies. Cancer Immunol. Immunother. 1983, 14, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, A.; Dahlin, K.J.; Prince, J.T.; Johnstone, S.R.; Stallcup, W.B. The primary structure of NG2, a novel membrane-spanning proteoglycan. J. Cell Biol. 1991, 114, 359–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stallcup, W.B.; Dahlin-Huppe, K. Chondroitin sulfate and cytoplasmic domain-dependent membrane targeting of the NG2 proteoglycan promotes retraction fiber formation and cell polarization. J. Cell Sci. 2001, 114, 2315–2325. [Google Scholar] [PubMed]
- Sakry, D.; Neitz, A.; Singh, J.; Frischknecht, R.; Marongiu, D.; Binamé, F.; Perera, S.S.; Endres, K.; Lutz, B.; Radyushkin, K.; et al. Oligodendrocyte precursor cells modulate the neuronal network by activity-dependent ectodomain cleavage of glial NG2. PLoS Biol. 2014, 12, e1001993. [Google Scholar] [CrossRef] [PubMed]
- Buffo, A.; Vosko, M.R.; Ertürk, D.; Hamann, G.F.; Jucker, M.; Rowitch, D.; Götz, M. Expression pattern of the transcription factor Olig2 in response to brain injuries: Implications for neuronal repair. Proc. Natl. Acad. Sci. USA 2005, 102, 18183–18188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakry, D.; Trotter, J. The role of the NG2 proteoglycan in OPC and CNS network function. Brain Res. 1638, 161–166. [Google Scholar] [CrossRef] [PubMed]
- You, W.K.; Yotsumoto, F.; Sakimura, K.; Adams, R.H.; Stallcup, W.B. NG2 proteoglycan promotes tumor vascularization via integrin–dependent effects on pericyte function. Angiogenesis 2014, 17, 61–76. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Tamura, Y.; Yamato, M.; Kume, S.; Eguchi, A.; Takata, K.; Watanabe, Y.; Kataoka, Y. NG2 glial cells regulate neuroimmunological responses to maintain neuronal function and survival. Sci. Rep. 2017, 7, 42041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makagiansar, I.T.; Williams, S.; Mustelin, T.; Stallcup, W.B. Differential phosphorylation of NG2 proteoglycan by ERK and PKCalpha helps balance cell proliferation and migration. J. Cell Biol. 2007, 178, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Stallcup, W.B. NG2 Proteoglycan Enhances Brain Tumor Progression by Promoting Beta–1 Integrin Activation in both Cis and Trans Orientations. Cancers 2017, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.H.; Dahlin-Huppe, K.; Stallcup, W.B. Interaction of the NG2 proteoglycan with the actin cytoskeleton. J. Cell Biochem. 1996, 63, 463–477. [Google Scholar] [CrossRef]
- Fukushi, J.; Makagiansar, I.T.; Stallcup, W.B. NG2 proteoglycan promotes endothelial cell motility and angiogenesis via engagement of galectin-3 and alpha3beta1 integrin. Mol. Biol. Cell 2004, 15, 3580–3590. [Google Scholar] [CrossRef] [PubMed]
- Nicolosi, P.A.; Dallatomasina, A.; Perris, R. Theranostic impact of NG2/CSPG4 proteoglycan in cancer. Theranostics 2015, 5, 530–544. [Google Scholar] [CrossRef] [PubMed]
- Stallcup, W.B.; You, W.K.; Kucharova, K.; Cejudo-Martin, P.; Yotsumoto, F. Proteoglycan-dependent contributions of pericytes and macrophages to brain tumor vascularization and progression. Microcirculation 2016, 23, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Ampofo, E.; Schmitt, B.M.; Menger, M.D.; Laschke, M.W. The regulatory mechanisms of NG2/CSPG4 expression. Cell Mol. Biol. Lett. 2017, 22, 4. [Google Scholar] [CrossRef] [PubMed]
- Stallcup, W.B.; Huang, F.J. A role for the NG2 proteoglycan in glioma progression. Cell Adh. Migr. 2008, 2, 192–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishiyama, A.; Lin, X.H.; Giese, N.; Heldin, C.H.; Stallcup, W.B. Interaction between NG2 proteoglycan and PDGF alpha-receptor on O2A progenitor cells is required for optimal response to PDGF. J. Neurosci. Res. 1996, 43, 315–330. [Google Scholar] [CrossRef]
- Horner, P.J.; Thallmair, M.; Gage, F.H. Defining the NG2-expressing cell of the adult CNS. J. Neurocytol. 2002, 31, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Shoshan, Y.; Nishiyama, A.; Chang, A.; Mörk, S.; Barnett, G.H.; Cowell, J.K.; Trapp, B.D.; Staugaitis, S.M. Expression of oligodendrocyte progenitor cell antigens by gliomas: Implications for the histogenesis of brain tumors. Proc. Natl. Acad. Sci. USA 1999, 96, 10361–10366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, A. A fourth type of neuroglial cell in the adult central nervous system. J. Neurocytol. 2004, 33, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Gensert, J.M.; Goldman, J.E. Endogenous progenitors remyelinate demyelinated axons in the adult CNS. Neuron 1997, 19, 197–203. [Google Scholar] [CrossRef]
- Ong, W.Y.; Levine, J.M. A light and electron microscopic study of NG2 chondroitin sulphate proteoglycan–positive oligodendrocyte precursor cells in the normal and kainite-lesioned rat hippocampus. Neuroscience 1999, 92, 83–95. [Google Scholar] [CrossRef]
- Butt, A.M.; Kiff, J.; Hubbard, P.; Berry, M. Synantocytes: New functions for novel NG2 expressing glia. J. Neurocytol. 2002, 31, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Paukert, M.; Bergles, D.E. Synaptic communication between neurons and NG2+ cells. Curr. Opin. Neurobiol. 2006, 16, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Aguirre, A.A.; Chittajallu, R.; Belachew, S.; Gallo, V. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J. Cell Biol. 2004, 165, 575–589. [Google Scholar] [CrossRef] [PubMed]
- Balenci, L.; Saoudi, Y.; Grunwald, D.; Deloulme, J.C.; Bouron, A.; Bernards, A.; Baudier, J. IQGAP1 regulates adult neural progenitors in vivo and vascular endothelial growth factor-triggered neural progenitor migration in vitro. J. Neurosci. 2007, 27, 4716–4724. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.C.; Scolding, N.J.; Raine, C.S. Co-expression of PDGF alpha receptor and NG2 by oligodendrocyte precursors in human CNS and multiple sclerosis lesions. J. Neuroimmunol. 2006, 176, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Baracskay, K.L.; Kidd, G.J.; Miller, R.H.; Trapp, B.D. NG2-positive cells generate A2B5-positive oligodendrocyte precursor cells. Glia 2007, 55, 1001–1010. [Google Scholar] [CrossRef] [PubMed]
- Scherer, S.S.; Braun, P.E.; Grinspan, J.; Collarini, E.; Wang, D.Y.; Kamholz, J. Differential regulation of the 2′,3′-cyclic nucleotide 3’-phosphodiesterase gene during oligodendrocyte development. Neuron 1994, 12, 1363–1375. [Google Scholar] [CrossRef]
- Sugiarto, S.; Persson, A.I.; Munoz, E.G.; Waldhuber, M.; Lamagna, C.; Andor, N.; Hanecker, P.; Ayers-Ringler, J.; Phillips, J.; Siu, J.; et al. Asymmetry-defective oligodendrocyte progenitors are glioma precursors. Cancer Cell. 2011, 20, 328–340. [Google Scholar] [CrossRef] [PubMed]
- Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [Google Scholar]
- Lindberg, N.; Kastemar, M.; Olofsson, T.; Smits, A.; Uhrbom, L. Oligodendrocyte progenitor cells can act as cell of origin for experimental glioma. Oncogene 2009, 28, 2266–2275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, C.; Celestino, J.C.; Okada, Y.; Louis, D.N.; Fuller, G.N.; Holland, E.C. PDGF autocrine stimulation dedifferentiates cultured astrocytes and induces oligodendrogliomas and oligoastrocytomas from neural progenitors and astrocytes in vivo. Genes Dev. 2001, 15, 1913–1925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, F.K.; Göransson, H.; Westermark, B. Expression analysis of genes involved in brain tumor progression driven by retroviral insertional mutagenesis in mice. Oncogene 2005, 24, 3896–3905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, X.; Holland, E.C. Applications of mouse glioma models in preclinical trials. Mutat. Res. 2005, 576, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Shih, A.H.; Holland, E.C. Platelet-derived growth factor (PDGF) and glial tumorigenesis. Cancer Lett. 2006, 232, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Briançon-Marjollet, A.; Balenci, L.; Fernandez, M.; Estève, F.; Honnorat, J.; Farion, R.; Beaumont, M.; Barbier, E.; Rémy, C.; Baudier, J. NG2-expressing glial precursor cells are a new potential oligodendroglioma cell initiating population in N-ethyl-N-nitrosourea-induced gliomagenesis. Carcinogenesis 2010, 31, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Engelhardt, A. Detection of acid mucopolysaccharides in human brain tumors by histochemical methods. Acta Neuropathol. 1980, 49, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Böck, P.; Jellinger, K. Detection of glycosaminoglycans in human gliomas by histochemical methods. Acta Neuropathol. Suppl. 1981, 7, 81–84. [Google Scholar] [PubMed]
- Giordana, M.T.; Mauro, A.; Schiffer, D. Glycosaminoglycans of brain tumors transplacentally induced by ENU in the rat. Acta Neuropathol. Suppl. 1981, 7, 79–80. [Google Scholar] [PubMed]
- Giordana, M.T.; Bertolotto, A.; Mauro, A.; Migheli, A.; Pezzotta, S.; Racagni, G.; Schiffer, D. Glycosaminoglycans in human cerebral tumors. Part II. Histochemical findings and correlations. Acta Neuropathol. 1982, 57, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A.; Bertolotto, A.; Giordana, M.T.; Magrassi, M.L.; Migheli, A.; Schiffer, D. Biochemical and histochemical evaluation of glycosaminoglycans in brain tumors induced in rats by nitrosourea derivatives. J. Neurooncol. 1983, 1, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Nioka, H.; Matsumura, K.; Nakasu, S.; Handa, J. Immunohistochemical localization of glycosaminoglycans in experimental rat glioma models. J. Neurooncol. 1994, 21, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Bertolotto, A.; Goia, L.; Schiffer, D. Immunohistochemical study of chondroitin sulphate in human gliomas. Acta Neuropathol. 1986, 72, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Persson, A.I.; Petritsch, C.; Swartling, F.J.; Itsara, M.; Sim, F.J.; Auvergne, R.; Goldenberg, D.D.; Vandenberg, S.R.; Nguyen, K.N.; Yakovenko, S.; et al. Non–stem cell origin for oligodendroglioma. Cancer Cell 2010, 18, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Guha, A.; Feldkamp, M.M.; Lau, N.; Boss, G.; Pawson, A. Proliferation of human malignant astrocytomas is dependent on Ras activation. Oncogene 1997, 15, 2755–2765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadavilli, S.; Hwang, E.I.; Packer, R.J.; Nazarian, J. The Role of NG2 Proteoglycan in Glioma. Transl. Oncol. 2016, 9, 57–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Chekenya, M.; Pilkington, G.J. NG2 precursor cells in neoplasia: Functional, histogenesis and therapeutic implications for malignant brain tumours. J. Neurocytol. 2002, 31, 507–521. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, C.; Bartoli, C.; Aguirre-Cruz, L.; Virard, I.; Colin, C.; Fernandez, C.; Gouvernet, J.; Figarella-Branger, D. Shared oligodendrocyte lineage gene expression in gliomas and oligodendrocyte progenitor cells. J. Neurosurg. 2003, 99, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Ligon, K.L.; Alberta, J.A.; Kho, A.T.; Weiss, J.; Kwaan, M.R.; Nutt, C.L.; Louis, D.N.; Stiles, C.D.; Rowitch, D.H. The oligodendroglial lineage marker OLIG2 is universally expressed in diffuse gliomas. J. Neuropathol. Exp. Neurol. 2004, 63, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Schrappe, M.; Klier, F.G.; Spiro, R.C.; Waltz, T.A.; Reisfeld, R.A.; Gladson, C.L. Correlation of chondroitin sulphate proteoglycan expression on proliferating brain capillary endothelial cells with the malignant phenotype of astroglial cells. Cancer Res. 1991, 51, 4986–4993. [Google Scholar] [PubMed]
- Tsidulko, A.Y.; Kazanskaya, G.M.; Kostromskaya, D.V.; Aidagulova, S.V.; Kiselev, R.S.; Volkov, A.M.; Kobozev, V.V.; Gaitan, A.S.; Krivoshapkin, A.L.; Grigorieva, E.V. Prognostic relevance of NG2/CSPG4, CD44 and Ki-67 in patients with glioblastoma. Tumour Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svendsen, A.; Verhoeff, J.J.; Immervoll, H.; Brøgger, J.C.; Kmiecik, J.; Poli, A.; Netland, I.A.; Prestegarden, L.; Planagumà, J.; Torsvik, A.; et al. Expression of the progenitor marker NG2/CSPG4 predicts poor survival and resistance to ionising radiation in glioblastoma. Acta Neuropathol. 2011, 122, 495–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gensert, J.M.; Goldman, J.E. Heterogeneity of cycling glial progenitors in the adult mammalian cortex and white matter. J. Neurobiol. 2001, 48, 75–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uhrbom, L.; Hesselager, G.; Ostman, A.; Nistér, M.; Westermark, B. Dependence of autocrine growth factor stimulation in platelet-derived growth factor-B-induced mouse brain tumor cells. Int. J. Cancer. 2000, 85, 398–406. [Google Scholar] [CrossRef] [Green Version]
- Yokoo, H.; Nobusawa, S.; Takebayashi, H.; Ikenaka, K.; Isoda, K.; Kamiya, M.; Sasaki, A.; Hirato, J.; Nakazato, Y. Anti-human Olig2 antibody as a useful immunohistochemical marker of normal oligodendrocytes and gliomas. Am. J. Pathol. 2004, 164, 1717–1725. [Google Scholar] [CrossRef]
- Riemenschneider, M.J.; Koy, T.H.; Reifenberger, G. Expression of oligodendrocyte lineage genes in oligodendroglial and astrocytic gliomas. Acta Neuropathol. 2004, 107, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Bannykh, S.I.; Stolt, C.C.; Kim, J.; Perry, A.; Wegner, M. Oligodendroglial-specific transcriptional factor SOX10 is ubiquitously expressed in human gliomas. J. Neurooncol. 2006, 76, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Chekenya, M.; Krakstad, C.; Svendsen, A.; Netland, I.A.; Staalesen, V.; Tysnes, B.B.; Selheim, F.; Wang, J.; Sakariassen, P.Ø.; Sandal, T.; et al. The progenitor cell marker NG2/MPG promotes chemoresistance by activation of integrin–dependent PI3K/Akt signaling. Oncogene 2008, 27, 5182–5194. [Google Scholar] [CrossRef] [PubMed]
- Wade, A.; Robinson, A.E.; Engler, J.R.; Petritsch, C.; James, C.D.; Phillips, J.J. Proteoglycans and their roles in brain cancer. FEBS J. 2013, 280, 2399–2417. [Google Scholar] [CrossRef] [PubMed]
- Burg, M.A.; Nishiyama, A.; Stallcup, W.B. A central segment of the NG2 proteoglycan is critical for the ability of glioma cells to bind and migrate toward type VI collagen. Exp. Cell. Res. 1997, 235, 254–264. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, P.; Schlingemann, R.O.; Rietveld, F.J.; Link, M.; Burger, P.C.; Ruiter, D.J. Early and extensive contribution of pericytes/vascular smooth muscle cells to microvascular proliferation in glioblastoma multiforme: An immune-light and immune-electron microscopic study. J. Neuropathol. Exp. Neurol. 1995, 54, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Pouly, S.; Prat, A.; Blain, M.; Olivier, A.; Antel, J. NG2 immunoreactivity on human brain endothelial cells. Acta Neuropathol. 2001, 102, 313–320. [Google Scholar] [PubMed]
- Ozerdem, U.; Grako, K.A.; Dahlin-Huppe, K.; Monosov, E.; Stallcup, W.B. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev. Dyn. 2001, 222, 218–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozerdem, U.; Stallcup, W.B. Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis 2003, 6, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Virgintino, D.; Girolamo, F.; Errede, M.; Capobianco, C.; Robertson, D.; Stallcup, W.B.; Perris, R.; Roncali, L. An intimate interplay between precocious, migrating pericytes and endothelial cells governs human fetal brain angiogenesis. Angiogenesis 2007, 10, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Girolamo, F.; Dallatomasina, A.; Rizzi, M.; Errede, M.; Wälchli, T.; Mucignat, M.T.; Frei, K.; Roncali, L.; Perris, R.; Virgintino, D. Diversified expression of NG2/CSPG4 isoforms in glioblastoma and human foetal brain identifies pericyte subsets. PLoS ONE 2013, 8, e84883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birbrair, A.; Zhang, T.; Wang, Z.M.; Messi, M.L.; Olson, J.D.; Mintz, A.; Delbono, O. Type-2 pericytes participate in normal and tumoral angiogenesis. Am. J. Physiol. Cell Physiol. 2014, 307, C25–C38. [Google Scholar] [CrossRef] [PubMed]
- Silver, D.J.; Siebzehnrubl, F.A.; Schildts, M.J.; Yachnis, A.T.; Smith, G.M.; Smith, A.A.; Scheffler, B.; Reynolds, B.A.; Silver, J.; Steindler, D.A. Chondroitin sulphate proteoglycans potently inhibit invasion and serve as a central organizer of the brain tumor microenvironment. J. Neurosci. 2013, 33, 15603–15617. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Huang, Z.; Zhou, W.; Wu, Q.; Donnola, S.; Liu, J.K.; Fang, X.; et al. Glioblastoma stem cells generate vascular pericytes to support vessel function and tumor growth. Cell 2013, 153, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, D.; Mellai, M.; Bovio, E.; Bisogno, I.; Casalone, C.; Annovazzi, L. Glioblastoma niches: From the concept to the phenotypical reality. Neurol. Sci. 2018, 39, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Al-Mayhani, M.T.; Grenfell, R.; Narita, M.; Piccirillo, S.; Kenney-Herbert, E.; Fawcett, J.W.; Collins, V.P.; Ichimura, K.; Watts, C. NG2 expression in glioblastoma identifies an actively proliferating population with an aggressive molecular signature. Neuro Oncol. 2011, 13, 830–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schrappe, M.; Bumol, T.F.; Apelgren, L.D.; Briggs, S.L.; Koppel, G.A.; Markowitz, D.D.; Mueller, B.M.; Reisfeld, R.A. Long-term growth suppression of human glioma xenografts by chemoimmunoconjugates of 4-desacetylvinblastine-3-carboxyhydrazide and monoclonal antibody 9.2.27. Cancer Res. 1992, 52, 3838–3844. [Google Scholar] [PubMed]
- Poli, A.; Wang, J.; Domingues, O.; Planagumà, J.; Yan, T.; Rygh, C.B.; Skaftnesmo, K.O.; Thorsen, F.; McCormack, E.; Hentges, F.; et al. Targeting glioblastoma with NK cells and mAb against NG2/CSPG4 prolongs animal survival. Oncotarget 2013, 4, 1527–1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kmiecik, J.; Gras Navarro, A.; Poli, A.; Planagumà, J.P.; Zimmer, J.; Chekenya, M. Combining NK cells and mAb9.2.27 to combat NG2–dependent and anti-inflammatory signals in glioblastoma. Oncoimmunology 2014, 3, e27185. [Google Scholar] [CrossRef] [PubMed]
- Higgins, S.C.; Fillmore, H.L.; Ashkan, K.; Butt, A.M.; Pilkington, G.J. Dual targeting NG2 and GD3A using Mab-Zap immunotoxin results in reduced glioma cell viability in vitro. Anticancer Res. 2015, 35, 77–84. [Google Scholar] [PubMed]
- Rygh, C.B.; Wang, J.; Thuen, M.; Gras Navarro, A.; Huuse, E.M.; Thorsen, F.; Poli, A.; Zimmer, J.; Haraldseth, O.; Lie, S.A.; et al. Dynamic contrast enhanced MRI detects early response to adoptive NK cellular immunotherapy targeting the NG2 proteoglycan in a rat model of glioblastoma. PLoS ONE 2014, 9, e108414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.; She, Z.G.; Sakimura, K.; Roberts, A.; Kucharova, K.; Rowitch, D.H.; Stallcup, W.B. Ablation of NG2 proteoglycan leads to deficits in brown fat function and to adult onset obesity. PLoS ONE 2012, 7, e30637. [Google Scholar] [CrossRef] [PubMed]
- Yotsumoto, F.; You, W.K.; Cejudo-Martin, P.; Kucharova, K.; Sakimura, K.; Stallcup, W.B. NG2 proteoglycan-dependent recruitment of tumor macrophages promotes pericyte-endothelial cell interactions required for brain tumor vascularization. Oncoimmunology 2015, 4, e1001204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordaan, S.; Chetty, S.; Mungra, N.; Koopmans, I.; van Bommel, P.E.; Helfrich, W.; Barth, S. CSPG4: A Target for Selective Delivery of Human Cytolytic Fusion Proteins and TRAIL. Biomedicines 2017, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Geldres, C.; Ferrone, S.; Dotti, G. Chondroitin sulfate proteoglycan 4 as a target for chimeric antigen receptor-based T-cell immunotherapy of solid tumors. Exp. Opin. Ther. Targets 2015, 19, 1339–1350. [Google Scholar] [CrossRef] [PubMed]
- Beard, R.E.; Zheng, Z.; Lagisetty, K.H.; Burns, W.R.; Tran, E.; Hewitt, S.M.; Abate-Daga, D.; Rosati, S.F.; Fine, H.A.; Ferrone, S.; et al. Multiple chimeric antigen receptors successfully target chondroitin sulfate proteoglycan 4 in several different cancer histologies and cancer stem cells. J. Immunother. Cancer 2014, 2, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pituch, K.C.; Miska, J.; Krenciute, G.; Panek, W.K.; Li, G.; Rodriguez-Cruz, T.; Wu, M.; Han, Y.; Lesniak, M.S.; Gottschalk, S.; et al. Adoptive Transfer of IL13Rα2-Specific Chimeric Antigen Receptor T Cells Creates a Pro-inflammatory Environment in Glioblastoma. Mol. Ther. 2018, 26, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Hide, T.; Komohara, Y.; Miyasato, Y.; Nakamura, H.; Makino, K.; Takeya, M.; Kuratsu, J.I.; Mukasa, A.; Yano, S. Oligodendrocyte Progenitor Cells and Macrophages/Microglia Produce Glioma Stem Cell Niches at the Tumor Border. EBioMedicine 2018, 30, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Schiffer, D.; Annovazzi, L.; Mazzucco, M.; Mellai, M. The Microenvironment in Gliomas: Phenotypic Expressions. Cancers 2015, 7, 2352–2359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmitt, B.M.; Larschke, M.W.; Rössler, O.G.; Huang, W.; Scheller, A.; Menger, M.D.; Ampofo, E. Nerve/glial antigen NG2 is a crucial regulator of intercellular adhesion molecules (ICAM)-1 expression. Biochim. Biophys. Acta 2018, 1865, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Birey, F.; Kloc, M.; Chavali, M.; Hussein, I.; Wilson, M.; Christoffel, D.J.; Chen, T.; Frohman, M.A.; Robinson, J.K.; Russo, S.J.; et al. Genetic and Stress-Induced Loss of NG2 Glia Triggers Emergence of Depressive-like Behaviors through Reduced Secretion of FGF2. Neuron 2015, 88, 941–956. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schiffer, D.; Mellai, M.; Boldorini, R.; Bisogno, I.; Grifoni, S.; Corona, C.; Bertero, L.; Cassoni, P.; Casalone, C.; Annovazzi, L. The Significance of Chondroitin Sulfate Proteoglycan 4 (CSPG4) in Human Gliomas. Int. J. Mol. Sci. 2018, 19, 2724. https://doi.org/10.3390/ijms19092724
Schiffer D, Mellai M, Boldorini R, Bisogno I, Grifoni S, Corona C, Bertero L, Cassoni P, Casalone C, Annovazzi L. The Significance of Chondroitin Sulfate Proteoglycan 4 (CSPG4) in Human Gliomas. International Journal of Molecular Sciences. 2018; 19(9):2724. https://doi.org/10.3390/ijms19092724
Chicago/Turabian StyleSchiffer, Davide, Marta Mellai, Renzo Boldorini, Ilaria Bisogno, Silvia Grifoni, Cristiano Corona, Luca Bertero, Paola Cassoni, Cristina Casalone, and Laura Annovazzi. 2018. "The Significance of Chondroitin Sulfate Proteoglycan 4 (CSPG4) in Human Gliomas" International Journal of Molecular Sciences 19, no. 9: 2724. https://doi.org/10.3390/ijms19092724
APA StyleSchiffer, D., Mellai, M., Boldorini, R., Bisogno, I., Grifoni, S., Corona, C., Bertero, L., Cassoni, P., Casalone, C., & Annovazzi, L. (2018). The Significance of Chondroitin Sulfate Proteoglycan 4 (CSPG4) in Human Gliomas. International Journal of Molecular Sciences, 19(9), 2724. https://doi.org/10.3390/ijms19092724