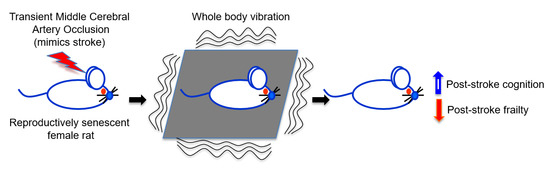
Whole Body Vibration Therapy after Ischemia Reduces Brain Damage in Reproductively Senescent Female Rats
Abstract
:1. Introduction
2. Results
2.1. Post-Ischemic WBV Reduced Infarct Volume in Middle-Aged Female Rats
2.2. Post-Ischemic WBV Improved Neuro-Deficit Score and Motor Function in Middle-Aged Female Rats
2.3. Post-Ischemic WBV Decreased Inflammasome Activation in the Brain of Middle-Aged Female Rats
2.4. Post-Ischemic WBV Increased Brain-Derived Growth Factor (BDNF) and Trk-B Protein Levels in the Peri-Infarct Area
3. Discussion
4. Materials and Methods
4.1. Neurodeficit Sscoring and Motor Deficit Test
4.2. Immunoblot Analysis
4.3. Statistical Analysis
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Xue, Q.L. The frailty syndrome: Definition and natural history. Clin. Geriatr. Med. 2011, 27, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Deplanque, D.; Masse, I.; Lefebvre, C.; Libersa, C.; Leys, D.; Bordet, R. Prior TIA, lipid-lowering drug use, and physical activity decrease ischemic stroke severity. Neurology 2006, 67, 1403–1410. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Li, J.; Luan, X.; Ding, Y.H.; Lai, Q.; Rafols, J.A.; Phillis, J.W.; Clark, J.C.; Diaz, F.G. Exercise pre-conditioning reduces brain damage in ischemic rats that may be associated with regional angiogenesis and cellular overexpression of neurotrophin. Neuroscience 2004, 124, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.H.; Young, C.N.; Luan, X.; Li, J.; Rafols, J.A.; Clark, J.C.; James, P.; McAllister, J.P., II; Ding, Y. Exercise preconditioning ameliorates inflammatory injury in ischemic rats during reperfusion. Acta Neuropathol. 2005, 109, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Krarup, L.H.; Truelsen, T.; Gluud, C.; Andersen, G.; Zeng, X.; Korv, J.; Oskedra, A.; Boysen, G.; ExStroke Pilot Trial Group. Prestroke physical activity is associated with severity and long-term outcome from first-ever stroke. Neurology 2008, 71, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Rist, P.M.; Lee, I.M.; Kase, C.S.; Gaziano, J.M.; Kurth, T. Physical activity and functional outcomes from cerebral vascular events in men. Stroke 2011, 42, 3352–3356. [Google Scholar] [CrossRef] [PubMed]
- Rees, S.S.; Murphy, A.J.; Watsford, M.L. Effects of whole body vibration on postural steadiness in an older population. J. Sci. Med. Sport 2009, 12, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Lee, G. Does whole-body vibration training in the horizontal direction have effects on motor function and balance of chronic stroke survivors? A preliminary study. J. Phys. Ther. Sci. 2015, 27, 1133–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrero, A.J.; Menendez, H.; Gil, L.; Martin, J.; Martin, T.; Garcia-Lopez, D.; Gil-Agudo, Á.; Marin, P.J. Effects of whole-body vibration on blood flow and neuromuscular activity in spinal cord injury. Spinal Cord 2011, 49, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Bovenzi, M. The hand-arm vibration syndrome: (II). The diagnostic aspects and fitness criteria. Med. Lav. 1999, 90, 643–649. [Google Scholar] [PubMed]
- Kalbe, E.; Calabrese, P.; Kohn, N.; Hilker, R.; Riedel, O.; Wittchen, H.U.; Dodel, R.; Otto, J.; Ebersbach, G.; Kessler, J. Screening for cognitive deficits in Parkinson’s disease with the Parkinson neuropsychometric dementia assessment (PANDA) instrument. Parkinsonism Relat. Disord. 2008, 14, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Bautmans, I.; Van Hees, E.; Lemper, J.C.; Mets, T. The feasibility of Whole Body Vibration in institutionalised elderly persons and its influence on muscle performance, balance and mobility: A randomised controlled trial [ISRCTN62535013]. BMC Geriatr. 2005, 5, 17. [Google Scholar] [CrossRef] [PubMed]
- Roelants, M.; Delecluse, C.; Goris, M.; Verschueren, S. Effects of 24 weeks of whole body vibration training on body composition and muscle strength in untrained females. Int. J. Sports Med. 2004, 25, 1–5. [Google Scholar] [PubMed]
- Gloeckl, S.; Tyndall, J.D.; Stansfield, S.H.; Timms, P.; Huston, W.M. The active site residue V266 of Chlamydial HtrA is critical for substrate binding during both in vitro and in vivo conditions. J. Mol. Microbiol. Biotechnol. 2012, 22, 10–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, K.J.; Merriman, H.L.; Vanderburgh, P.M.; Brahler, C.J. Acute effects of whole-body vibration on lower extremity muscle performance in persons with multiple sclerosis. J. Neurol. Phys. Ther. 2008, 32, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Verschueren, S.M.; Roelants, M.; Delecluse, C.; Swinnen, S.; Vanderschueren, D.; Boonen, S. Effect of 6-month whole body vibration training on hip density, muscle strength, and postural control in postmenopausal women: A randomized controlled pilot study. J. Bone Miner. Res. 2004, 19, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Semler, O.; Fricke, O.; Vezyroglou, K.; Stark, C.; Schoenau, E. Preliminary results on the mobility after whole body vibration in immobilized children and adolescents. J. Musculoskelet. Neuronal Interact. 2007, 7, 77–81. [Google Scholar] [PubMed]
- Bramlett, H.M.; Dietrich, W.D.; Marcillo, A.; Mawhinney, L.J.; Furones-Alonso, O.; Bregy, A.; Peng, Y.; Wu, Y.; Pan, J.; Wang, J. Effects of low intensity vibration on bone and muscle in rats with spinal cord injury. Osteoporos. Int. 2014, 25, 2209–2219. [Google Scholar] [CrossRef] [PubMed]
- Rubin, C.; Turner, A.S.; Bain, S.; Mallinckrodt, C.; McLeod, K. Anabolism: Low mechanical signals strengthen long bones. Nature 2001, 412, 603–604. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Rubin, C.; Judex, S. Enhancement of the adolescent murine musculoskeletal system using low-level mechanical vibrations. J. Appl. Physiol. 2008, 104, 1056–1062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Nes, I.J.; Latour, I.J.; Schils, H.; Meijer, F.; van Kuijk, R.A.; Geurts, A.C. Long-term effects of 6-week whole-body vibration on balance recovery and activities of daily living in the postacute phase of stroke: A randomized, controlled trial. Stroke 2006, 37, 2331–2335. [Google Scholar] [CrossRef] [PubMed]
- Tihanyi, T.K.; Horváth, M.; Fazekas, G.; Hortobágyi, T.; Tihanyi, J. One session of whole body vibration increases voluntary muscle strength transiently in patients with stroke. Clin. Rehabil. 2007, 21, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Del Pozo-Cruz, B.; Hernández Mocholí, M.A.; Adsuar, J.C.; Parraca, J.A.; Muro, I.; Gusi, N. Effects of whole body vibration therapy on main outcome measures for chronic non-specific low back pain: A single-blind randomized controlled trial. J. Rehabil. Med. 2011, 43, 689–694. [Google Scholar] [PubMed] [Green Version]
- Simpson, R.J.; Kunz, H.; Agha, N.; Graff, R. Exercise and the Regulation of Immune Functions. Prog. Mol. Biol. Transl. Sci. 2015, 135, 355–380. [Google Scholar] [PubMed]
- Nieman, D.C. Exercise immunology: Practical applications. Int. J. Sports Med. 1997, 18, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Mackinnon, L.T. Immunity in athletes. Int. J. Sports Med. 1997, 18, S62–S68. [Google Scholar] [CrossRef] [PubMed]
- Gertz, K.; Kronenberg, G.; Kälin, R.E.; Baldinger, T.; Werner, C.; Balkaya, M.; Eom, G.D.; Regen, J.H.; Kröber, J.; Miller, K.R. Essential role of interleukin-6 in post-stroke angiogenesis. Brain 2012, 135, 1964–1980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamel, H.; Iadecola, C. Brain-immune interactions and ischemic stroke: Clinical implications. Arch. Neurol. 2012, 69, 576–581. [Google Scholar] [PubMed]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion--from mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, N.J.; Ross, J.; Rothwell, N.J.; Loddick, S.A. Delayed administration of interleukin-1 receptor antagonist protects against transient cerebral ischaemia in the rat. Br. J. Pharmacol. 2003, 140, 471–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lotocki, G.; de Rivero Vaccari, J.P.; Perez, E.R.; Sanchez-Molano, J.; Furones-Alonso, O.; Bramlett, H.M.; Dietrich, W.D. Alterations in blood-brain barrier permeability to large and small molecules and leukocyte accumulation after traumatic brain injury: Effects of post-traumatic hypothermia. J. Neurotrauma 2009, 26, 1123–1134. [Google Scholar] [CrossRef] [PubMed]
- Mawhinney, L.J.; de Rivero Vaccari, J.P.; Dale, G.A.; Keane, R.W.; Bramlett, H.M. Heightened inflammasome activation is linked to age-related cognitive impairment in Fischer 344 rats. BMC Neurosci. 2011, 12, 123. [Google Scholar] [CrossRef] [PubMed]
- De Rivero Vaccari, J.P.; Patel, H.H.; Brand, F.J., III; Perez-Pinzon, M.A.; Bramlett, H.M.; Raval, A.P. Estrogen receptor β signaling alters cellular inflammasomes activity after global cerebral ischemia in reproductively senescence female rats. J. Neurochem. 2016, 136, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Sarvari, M.; Kalló, I.; Hrabovszky, E.; Solymosi, N.; Liposits, Z. Ovariectomy and subsequent treatment with estrogen receptor agonists tune the innate immune system of the hippocampus in middle-aged female rats. PLoS ONE 2014, 9, e88540. [Google Scholar] [CrossRef] [PubMed]
- Colson, B.A.; Petersen, K.J.; Collins, B.C.; Lowe, D.A.; Thomas, D.D. The myosin super-relaxed state is disrupted by estradiol deficiency. Biochem. Biophys. Res. Commun. 2015, 456, 151–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nedergaard, A.; Henriksen, K.; Karsdal, M.A.; Christiansen, C. Menopause, estrogens and frailty. Gynecol. Endocrinol. 2013, 29, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C.; Anrather, J. The immunology of stroke: From mechanisms to translation. Nat. Med. 2011, 17, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Barbacid, M. Neurotrophic factors and their receptors. Curr. Opin. Cell Biol. 1995, 7, 148–155. [Google Scholar] [CrossRef]
- Thoenen, H. Neurotrophins and neuronal plasticity. Science 1995, 270, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, M.E.; Xu, B.; Lu, B.; Hempstead, B.L. New insights in the biology of BDNF synthesis and release: Implications in CNS function. J. Neurosci. 2009, 29, 12764–12767. [Google Scholar] [CrossRef] [PubMed]
- Binder, D.K.; Scharfman, H.E. Brain-derived neurotrophic factor. Growth Factors 2004, 22, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O.; Kokaia, Z.; Bengzon, J.; Elme, E.; Kokaia, M. Neurotrophins and brain insults. Trends Neurosci. 1994, 17, 490–496. [Google Scholar] [CrossRef]
- Kramar, E.A.; Lin, B.; Lin, C.Y.; Arai, A.C.; Gall, C.M.; Lynch, G. A novel mechanism for the facilitation of theta-induced long-term potentiation by brain-derived neurotrophic factor. J. Neurosci. 2004, 24, 5151–5161. [Google Scholar] [CrossRef] [PubMed]
- Rex, C.S.; Lin, C.Y.; Kramár, E.A.; Chen, L.Y.; Gall, C.M.; Lynch, G. Brain-derived neurotrophic factor promotes long-term potentiation-related cytoskeletal changes in adult hippocampus. J. Neurosci. 2007, 27, 3017–3029. [Google Scholar] [CrossRef] [PubMed]
- Bramham, C.R.; Messaoudi, E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 2005, 76, 99–125. [Google Scholar] [CrossRef] [PubMed]
- Bath, K.G.; Akins, M.R.; Lee, F.S. BDNF control of adult SVZ neurogenesis. Dev. Psychobiol. 2012, 54, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Beck, T.; Lindholm, D.; Castren, E.; Wree, A. Brain-derived neurotrophic factor protects against ischemic cell damage in rat hippocampus. J. Cereb. Blood Flow Metab. 1994, 14, 689–692. [Google Scholar] [CrossRef] [PubMed]
- Schabitz, W.R.; Schwab, S.; Spranger, M.; Hacke, W. Intraventricular brain-derived neurotrophic factor reduces infarct size after focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 1997, 17, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood-brain barrier delivery. Drug Discov. Today 2007, 12, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M.; Wu, D.; Sakane, T. Combined use of carboxyl-directed protein pegylation and vector-mediated blood-brain barrier drug delivery system optimizes brain uptake of brain-derived neurotrophic factor following intravenous administration. Pharm. Res. 1998, 15, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.F.; Wang, Q.Q.; Yu, Z.J.; Yu, Y.; Xiao, C.L.; Kang, C.S.; Ge, G.; Linghu, Y.; Zhu, J.D.; Li, Y.M. Exercise Prevents Memory Impairment Induced by Arsenic Exposure in Mice: Implication of Hippocampal BDNF and CREB. PLoS ONE 2015, 10, e0137810. [Google Scholar] [CrossRef] [PubMed]
- Forti, L.N.; Van Roie, E.; Njemini, R.; Coudyzer, W.; Beyer, I.; Delecluse, C.; Bautmans, I. Dose-and gender-specific effects of resistance training on circulating levels of brain derived neurotrophic factor (BDNF) in community-dwelling older adults. Exp. Gerontol. 2015, 70, 144–149. [Google Scholar] [CrossRef] [PubMed]
- Schabitz, W.R.; Steigleder, T.; Cooper-Kuhn, C.M.; Schwab, S.; Sommer, C.; Schneider, A.; Kuhn, H.G. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke 2007, 38, 2165–2171. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Luikart, B.W.; Birnbaum, S.; Chen, J.; Kwon, C.H.; Kernie, S.G.; Bassel-Dub, R.; Parada, L.F. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron 2008, 59, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Marlatt, M.W.; Potter, M.C.; Lucassen, P.J.; van Praag, H. Running throughout middle-age improves memory function, hippocampal neurogenesis, and BDNF levels in female C57BL/6J mice. Dev. Neurobiol. 2012, 72, 943–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raval, A.P.; Saul, I.; Dave, K.R.; DeFazio, R.A.; Perez-Pinzon, M.A.; Bramlett, H. Pretreatment with a single estradiol-17β bolus activates cyclic-AMP response element binding protein and protects CA1 neurons against global cerebral ischemia. Neuroscience 2009, 160, 307–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvamani, A.; Sohrabji, F. The neurotoxic effects of estrogen on ischemic stroke in older female rats is associated with age-dependent loss of insulin-like growth factor-1. J. Neurosci. 2010, 30, 6852–6861. [Google Scholar] [CrossRef] [PubMed]
- Belayev, L.; Alonso, O.F.; Busto, R.; Zhao, W.; Ginsberg, M.D. Middle cerebral artery occlusion in the rat by intraluminal suture. Neurological and pathological evaluation of an improved model. Stroke 1996, 27, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.W.; Saul, I.; Gresia, V.L.; Neumann, J.T.; Dave, K.R.; Perez-Pinzon, M.A. Fatty acid methyl esters and Solutol HS 15 confer neuroprotection after focal and global cerebral ischemia. Transl. Stroke Res. 2014, 5, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Jacobson, J.M.; Choi, E.S.; Busa, B.; Donahue, L.R.; Miller, L.M.; Rubin, C.T.; Judex, S. Low-level mechanical vibrations can influence bone resorption and bone formation in the growing skeleton. Bone 2006, 39, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, A.; Butler, M.; Shamliyan, T.; Kane, R.L. Whole-Body Vibration Therapy for Osteoporosis. In Proceedings of the Agency for Healthcare Research and Quality, Rockville, MD, USA, 15 November 2011. [Google Scholar]
- Ley, J.J.; Vigdorchik, A.; Belayev, L.; Zhao, W.; Busto, R.; Khoutorova, L.; Becker, D.A.; Ginsberg, M.D. Stilbazulenyl nitrone, a second-generation azulenyl nitrone antioxidant, confers enduring neuroprotection in experimental focal cerebral ischemia in the rat: Neurobehavior, histopathology, and pharmacokinetics. J. Pharmacol. Exp. Ther. 2005, 313, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Abulafia, D.P.; de Rivero Vaccari, J.P.; Lozano, J.D.; Lotocki, G.; Keane, R.W.; Dietrich, W.D. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J. Cereb. Blood Flow Metab. 2009, 29, 534–544. [Google Scholar] [CrossRef] [PubMed]




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raval, A.P.; Schatz, M.; Bhattacharya, P.; D’Adesky, N.; Rundek, T.; Dietrich, W.D.; Bramlett, H.M. Whole Body Vibration Therapy after Ischemia Reduces Brain Damage in Reproductively Senescent Female Rats. Int. J. Mol. Sci. 2018, 19, 2749. https://doi.org/10.3390/ijms19092749
Raval AP, Schatz M, Bhattacharya P, D’Adesky N, Rundek T, Dietrich WD, Bramlett HM. Whole Body Vibration Therapy after Ischemia Reduces Brain Damage in Reproductively Senescent Female Rats. International Journal of Molecular Sciences. 2018; 19(9):2749. https://doi.org/10.3390/ijms19092749
Chicago/Turabian StyleRaval, Ami P., Marc Schatz, Pallab Bhattacharya, Nathan D’Adesky, Tatjana Rundek, W. Dalton Dietrich, and Helen M. Bramlett. 2018. "Whole Body Vibration Therapy after Ischemia Reduces Brain Damage in Reproductively Senescent Female Rats" International Journal of Molecular Sciences 19, no. 9: 2749. https://doi.org/10.3390/ijms19092749