Cytokine-Modulated Natural Killer Cells Differentially Regulate the Activity of the Hepatitis C Virus
Abstract
:1. Introduction
2. Results
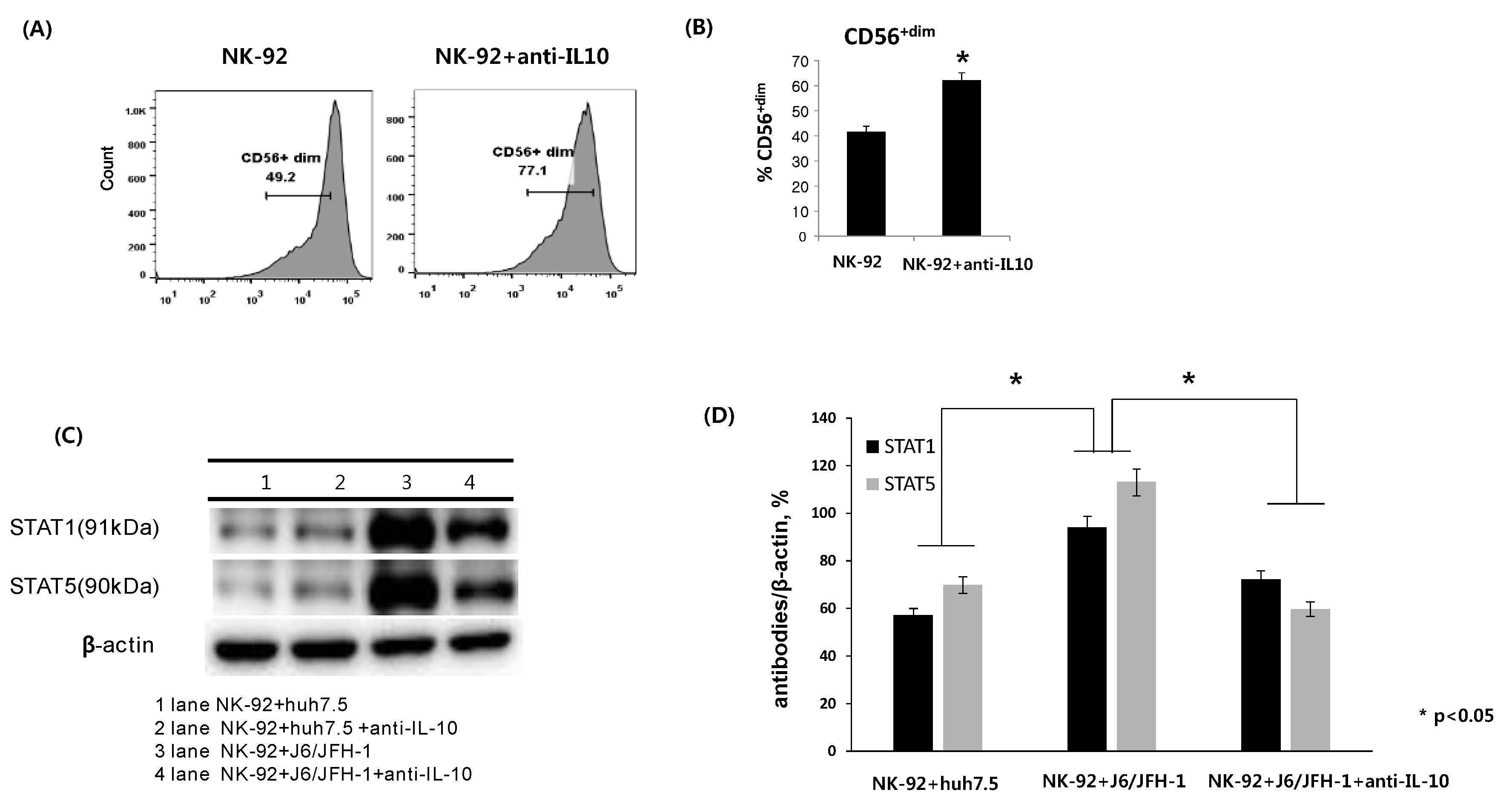
2.1. Effect of Anti-IL-10 on Expression of CD56, and STAT Proteins in NK-92 Cells
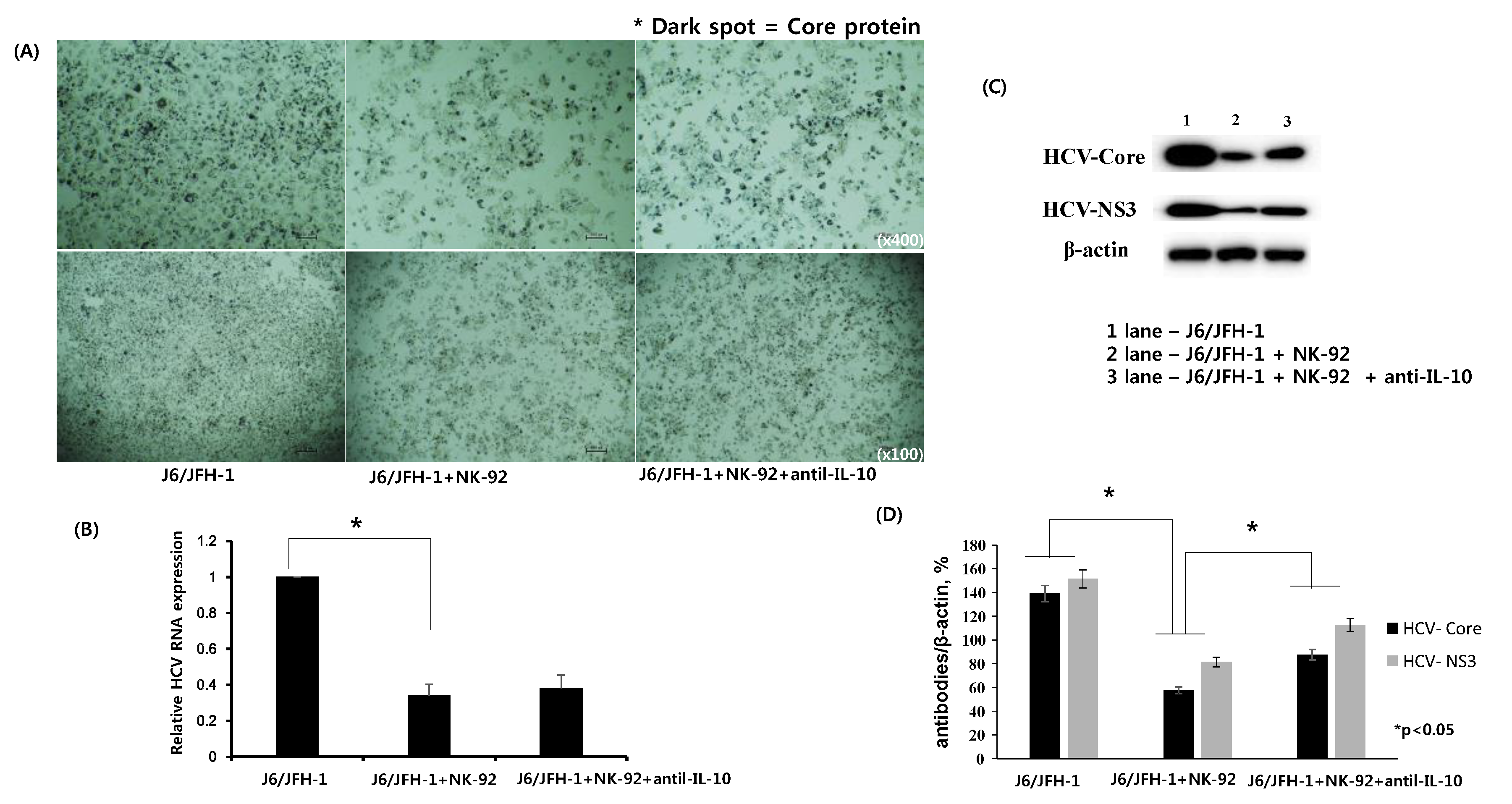
2.2. Effect of Anti-IL-10 on Expression of HCV-Core and HCV-NS3 Proteins in Coculture with NK-92 Cells
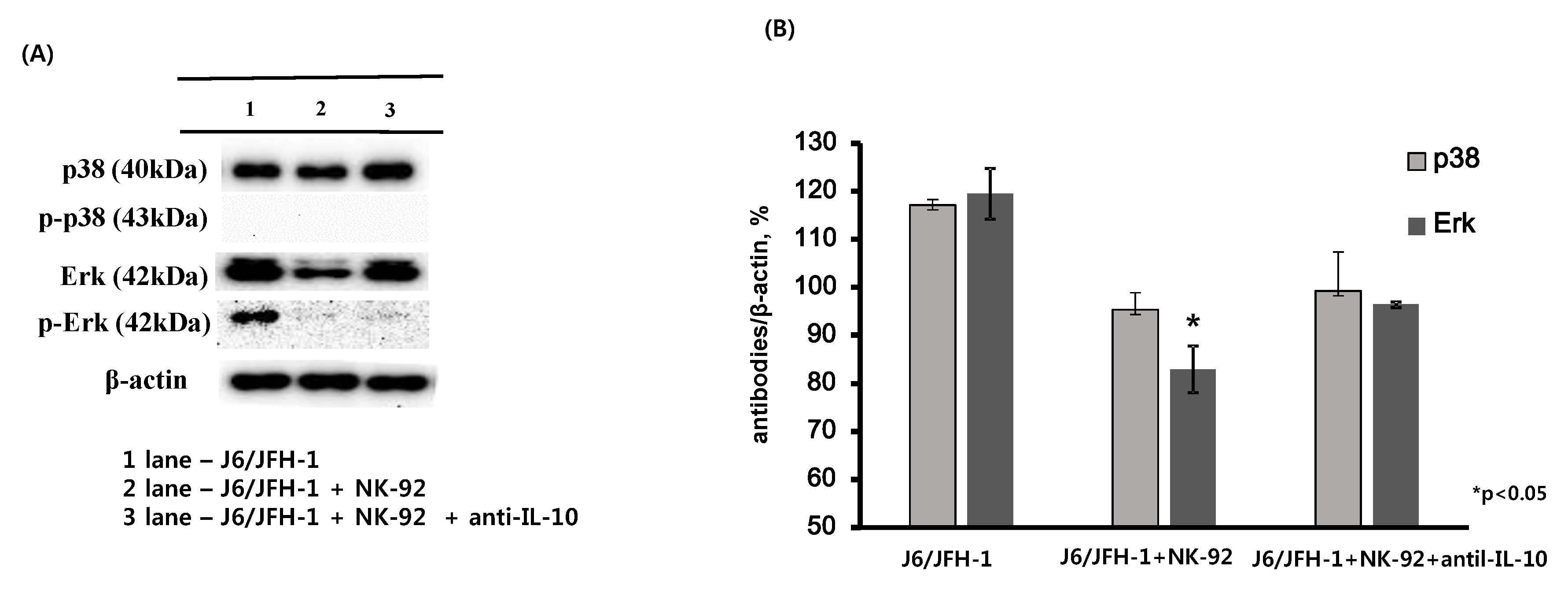
2.3. Effect of Anti-IL-10 on Expression of p38 and Erk in J6/JFH-1-huh 7.5 Cocultured with NK-92 Cells
2.4. Effect of Recombinant IL-21 on Expression of CD56, STAT Proteins, and IFN-γ in NK-92 Cells
2.5. Effect of Recombinant IL-21 on Expression of HCV-Core and -NS3 Proteins in Coculture with NK-92 Cells
3. Discussion
4. Materials and Methods
4.1. Establishment of Infectious HCV (J6/JFH1) Cell Culture System and NK-92 Cells
4.2. Reagents
4.3. Flow Cytometry Analysis
4.4. Immunohistochemistry Staining
4.5. ELISA Assay
4.6. Western Blot Analysis
4.7. Quantitative RT-PCR
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lindenbach, B.D.; Evans, M.J.; Syder, A.J.; Wolk, B.; Tellinghuisen, T.L.; Liu, C.C.; Maruyama, T.; Hynes, R.O.; Burton, D.R.; McKeating, J.A.; et al. Complete replication of hepatitis C virus in cell culture. Science 2005, 309, 623–626. [Google Scholar] [CrossRef] [PubMed]
- Wakita, T.; Pietschmann, T.; Kato, T.; Date, T.; Miyamoto, M.; Zhao, Z.; Murthy, K.; Habermann, A.; Krausslich, H.G.; Mizokami, M.; et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 2005, 11, 791–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blight, K.J.; McKeating, J.A.; Rice, C.M. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J. Virol. 2002, 76, 13001–13014. [Google Scholar] [CrossRef] [PubMed]
- Doherty, D.G.; O’Farrelly, C. Innate and adaptive lymphoid cells in the human liver. Immunol. Rev. 2000, 174, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Kang, H.; Cho, H. Natural killer cells and tumor metastasis. Arch. Pharm. Res 2017, 40, 1037–1049. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A.; Fehniger, T.A.; Caligiuri, M.A. The biology of human natural killer-cell subsets. Trends Immunol. 2001, 22, 633–640. [Google Scholar] [CrossRef]
- Meyer-Olson, D.; Shoukry, N.H.; Brady, K.W.; Kim, H.; Olson, D.P.; Hartman, K.; Shintani, A.K.; Walker, C.M.; Kalams, S.A. Limited T cell receptor diversity of HCV-specific T cell responses is associated with CTL escape. J. Exp. Med. 2004, 200, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Jinushi, M.; Takehara, T.; Tatsumi, T.; Kanto, T.; Miyagi, T.; Suzuki, T.; Kanazawa, Y.; Hiramatsu, N.; Hayashi, N. Negative regulation of NK cell activities by inhibitory receptor CD94/NKG2A leads to altered NK cell-induced modulation of dendritic cell functions in chronic hepatitis C virus infection. J. Immunol. 2004, 173, 6072–6081. [Google Scholar] [CrossRef] [PubMed]
- Castriconi, R.; Cantoni, C.; Della Chiesa, M.; Vitale, M.; Marcenaro, E.; Conte, R.; Biassoni, R.; Bottino, C.; Moretta, L.; Moretta, A. Transforming growth factor β1 inhibits expression of NKp30 and NKG2D receptors: Consequences for the NK-mediated killing of dendritic cells. Proc. Natl. Acad. Sci. USA 2003, 100, 4120–4125. [Google Scholar] [CrossRef] [PubMed]
- Rehermann, B. Hepatitis C virus versus innate and adaptive immune responses: A tale of coevolution and coexistence. J. Clin. Investig. 2009, 119, 1745–1754. [Google Scholar] [CrossRef] [PubMed]
- Rehermann, B. Pathogenesis of chronic viral hepatitis: Differential roles of T cells and NK cells. Nat. Med. 2013, 19, 859–868. [Google Scholar] [CrossRef] [PubMed]
- Ahlenstiel, G. The natural killer cell response to HCV infection. Immun. Netw. 2013, 13, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Tatsumi, T.; Takehara, T. Impact of natural killer cells on chronic hepatitis C and hepatocellular carcinoma. Hepatol. Res. 2016, 46, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.C.; Lim, J.B.; Park, J.H.; Lee, J.M. Cell-to-cell contact with hepatitis C virus-infected cells reduces functional capacity of natural killer cells. J. Virol. 2011, 85, 12557–12569. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.K.; Dore, G.J.; Hellard, M.; Yeung, B.; Rawlinson, W.D.; White, P.A.; Kaldor, J.M.; Lloyd, A.R.; Ffrench, R.A.; Group, A.S. Early IL-10 predominant responses are associated with progression to chronic hepatitis C virus infection in injecting drug users. J. Viral. Hepat. 2011, 18, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Rigopoulou, E.I.; Stefanidis, I.; Liaskos, C.; Zervou, E.K.; Rizos, C.; Mina, P.; Zachou, K.; Syrganis, C.; Patsidis, E.; Kyriakopoulos, G.; et al. HCV-RNA qualitative assay based on transcription mediated amplification improves the detection of hepatitis C virus infection in patients on hemodialysis: Results from five hemodialysis units in central Greece. J. Clin. Virol. 2005, 34, 81–85. [Google Scholar] [CrossRef] [PubMed]
- De Maria, A.; Fogli, M.; Mazza, S.; Basso, M.; Picciotto, A.; Costa, P.; Congia, S.; Mingari, M.C.; Moretta, L. Increased natural cytotoxicity receptor expression and relevant IL-10 production in NK cells from chronically infected viremic HCV patients. Eur. J. Immunol. 2007, 37, 445–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MacParland, S.A.; Fadel, S.M.; Mihajlovic, V.; Fawaz, A.; Kim, C.; Rahman, A.K.; Liu, J.; Kaul, R.; Kovacs, C.; Grebely, J.; et al. HCV specific IL-21 producing T cells but not IL-17A producing T cells are associated with HCV viral control in HIV/HCV coinfection. PLoS ONE 2016, 11, e0154433. [Google Scholar] [CrossRef] [PubMed]
- Feng, G.; Zhang, J.Y.; Zeng, Q.L.; Jin, L.; Fu, J.; Yang, B.; Sun, Y.; Jiang, T.; Xu, X.; Zhang, Z.; et al. HCV-specific interleukin-21+CD4+ T cells responses associated with viral control through the modulation of HCV-specific CD8+ T cells function in chronic hepatitis C patients. Mol. Cells 2013, 36, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Ragab, H.M.; El-Maksoud, N.A.; Amin, M.A.; Halim, M.H.; Abdulla, N.A.; Kamel, A.; Moussa, S.M. IL-21 as a Predictor of Sustained Virologic Response in Patients with Chronic Hepatitis C Virus Infection. Appl. Biochem. Biotechnol. 2017, 185, 484–493. [Google Scholar] [CrossRef] [PubMed]
- Stegmann, K.A.; Bjorkstrom, N.K.; Ciesek, S.; Lunemann, S.; Jaroszewicz, J.; Wiegand, J.; Malinski, P.; Dustin, L.B.; Rice, C.M.; Manns, M.P.; et al. Interferon α-stimulated natural killer cells from patients with acute hepatitis C virus (HCV) infection recognize HCV-infected and uninfected hepatoma cells via DNAX accessory molecule-1. J. Infect. Dis. 2012, 205, 1351–1362. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; O’Garra, A.; de Waal Malefyt, R.; Vieira, P.; Mosmann, T.R. Interleukin-10. Annu. Rev. Immunol. 1993, 11, 165–190. [Google Scholar] [CrossRef] [PubMed]
- Tripp, C.S.; Wolf, S.F.; Unanue, E.R. Interleukin 12 and tumor necrosis factor α are costimulators of interferon γ production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc. Natl. Acad. Sci. USA 1993, 90, 3725–3729. [Google Scholar] [CrossRef] [PubMed]
- Scott, M.J.; Hoth, J.J.; Turina, M.; Woods, D.R.; Cheadle, W.G. Interleukin-10 suppresses natural killer cell but not natural killer T cell activation during bacterial infection. Cytokine 2006, 33, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Lee, S.H.; Yoon, S.R.; Park, Y.J.; Jung, H.; Kim, T.D.; Choi, I. IL-15-induced IL-10 increases the cytolytic activity of human natural killer cells. Mol. Cells 2011, 32, 265–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, K.; Ait-Goughoulte, M.; Truscott, S.M.; Meyer, K.; Blazevic, A.; Abate, G.; Ray, R.B.; Hoft, D.F.; Ray, R. Hepatitis C virus inhibits cell surface expression of HLA-DR, prevents dendritic cell maturation, and induces interleukin-10 production. J. Virol. 2008, 82, 3320–3328. [Google Scholar] [CrossRef] [PubMed]
- Dinsmore, C.J.; Soriano, P. MAPK and PI3K signaling: At the crossroads of neural crest development. Dev. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Erhardt, A.; Hassan, M.; Heintges, T.; Haussinger, D. Hepatitis C virus core protein induces cell proliferation and activates ERK, JNK, and p38 MAP kinases together with the MAP kinase phosphatase MKP-1 in a HepG2 Tet-Off cell line. Virology 2002, 292, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.J.; Zhang, X.L.; Zhao, P.; Cao, J.; Cao, M.M.; Zhu, S.Y.; Liu, H.Q.; Qi, Z.T. Up-regulation of ERK and p38 MAPK signaling pathways by hepatitis C virus E2 envelope protein in human T lymphoma cell line. J. Leukoc. Biol. 2006, 80, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Ndjomou, J.; Park, I.W.; Liu, Y.; Mayo, L.D.; He, J.J. Up-regulation of hepatitis C virus replication and production by inhibition of MEK/ERK signaling. PLoS ONE 2009, 16, e7498. [Google Scholar] [CrossRef] [PubMed]
- Skak, K.; Frederiksen, K.S.; Lundsgaard, D. Interleukin-21 activates human natural killer cells and modulates their surface receptor expression. Immunology 2008, 123, 575–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wendt, K.; Wilk, E.; Buyny, S.; Schmidt, R.E.; Jacobs, R. Interleukin-21 differentially affects human natural killer cell subsets. Immunology 2007, 122, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Strengell, M.; Sareneva, T.; Foster, D.; Julkunen, I.; Matikainen, S. IL-21 up-regulates the expression of genes associated with innate immunity and Th1 response. J. Immunol. 2002, 169, 3600–3605. [Google Scholar] [CrossRef] [PubMed]
- Serti, E.; Werner, J.M.; Chattergoon, M.; Cox, A.L.; Lohmann, V.; Rehermann, B. Monocytes activate natural killer cells via inflammasome-induced interleukin 18 in response to hepatitis C virus replication. Gastroenterology 2014, 147, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.K.; Shin, D.J.; Cho, D.; Kim, S.K.; Lee, J.J.; Shin, M.G.; Ryang, D.W.; Lee, J.S.; Park, M.H.; Yoon, J.H.; et al. Interleukin-21 increases direct cytotoxicity and IFN-γ production of ex vivo expanded NK cells towards breast cancer cells. Anticancer Res. 2012, 32, 839–846. [Google Scholar] [PubMed]
- Pallikkuth, S.; Rogers, K.; Villinger, F.; Dosterii, M.; Vaccari, M.; Franchini, G.; Pahwa, R.; Pahwa, S. Interleukin-21 administration to rhesus macaques chronically infected with simian immunodeficiency virus increases cytotoxic effector molecules in T cells and NK cells and enhances B cell function without increasing immune activation or viral replication. Vaccine 2011, 29, 9229–9238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iannello, A.; Boulassel, M.R.; Samarani, S.; Tremblay, C.; Toma, E.; Routy, J.P.; Ahmad, A. IL-21 enhances NK cell functions and survival in healthy and HIV-infected patients with minimal stimulation of viral replication. J. Leukoc. Biol. 2010, 87, 857–867. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, H.H.; Kim, J.; Jung, J.; Moon, A.; Jeong, C.S.; Kang, H.; Cho, H. Anti-tumor effect of Cordyceps militaris in HCV-infected human hepatocarcinoma 7.5 cells. J. Microbiol. 2015, 53, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Kang, H.; Cho, H. Recovery of NK(CD56+CD3−) cells after one year of tenofovir therapy for chronic hepatitis B infection. J. Microbiol. Biotechnol. 2017, 27, 1204–1308. [Google Scholar] [PubMed]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, Y.J.; Lee, H.H.; Kang, H.; Cho, H. Cytokine-Modulated Natural Killer Cells Differentially Regulate the Activity of the Hepatitis C Virus. Int. J. Mol. Sci. 2018, 19, 2771. https://doi.org/10.3390/ijms19092771
Cho YJ, Lee HH, Kang H, Cho H. Cytokine-Modulated Natural Killer Cells Differentially Regulate the Activity of the Hepatitis C Virus. International Journal of Molecular Sciences. 2018; 19(9):2771. https://doi.org/10.3390/ijms19092771
Chicago/Turabian StyleCho, Yoo Jin, Hwan Hee Lee, Hyojeung Kang, and Hyosun Cho. 2018. "Cytokine-Modulated Natural Killer Cells Differentially Regulate the Activity of the Hepatitis C Virus" International Journal of Molecular Sciences 19, no. 9: 2771. https://doi.org/10.3390/ijms19092771



