MT-Feeding-Induced Impermanent Sex Reversal in the Orange-Spotted Grouper during Sex Differentiation
Abstract
:1. Introduction
2. Result
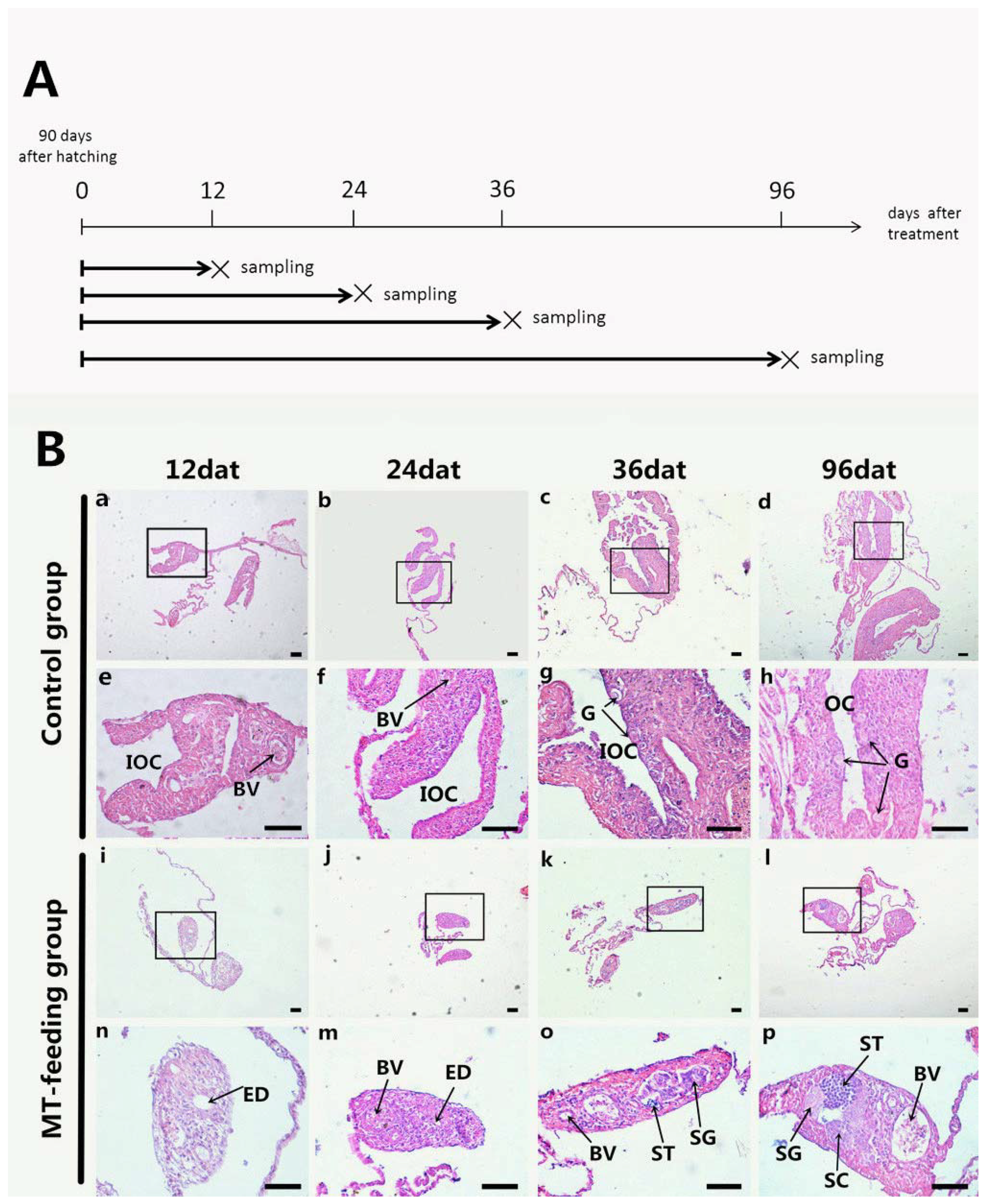
2.1. Gonadal Histology during MT Feeding
2.2. Gene Expression Profiles and Serum Steroid Hormone Levels during MT-Feeding-Induced Sex Reversal
2.3. Dmrt1 Expression, TUNEL Staining, and Immunohistochemistry Analysis of Germ Cells during MT-Feeding-Induced Sex Reversal
2.4. Gonadal Histology after MT-Feeding Withdrawal
2.5. Gene Expression Profiles and Serum Steroid Hormone Levels after MT-Feeding Withdrawal
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. MT-Feeding-Induced and MT-Feeding-Withdrawal-Induced Sex Reversal
4.3. Gonadal Histology
4.4. Serum Estradiol-17β (E2) and 11-Ketotestosterone (11-KT) Assays
4.5. RNA Isolation, Reverse Transcription and Quantitative Real-Time PCR
4.6. Immunohistochemistry and TUNEL Staining
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Smith, C.L. The Patterns of Sexuality and the Classification of Serranid Fishes; American Museum Novitates; American Museum of Natural History: New York, NY, USA, 1965. [Google Scholar]
- Tan, S.M.; Tan, K.S. Biology of the tropical grouper, Epinephelus tauvina (Forskal), I: A preliminary study on hermaphroditism in E. tauvina. Sing. J. Prim. Ind. 1974, 2, 123–133. [Google Scholar]
- Shapiro, D.Y. Differentiation and evolution of sex change in fishes. Bioscience 1987, 37, 490–496. [Google Scholar] [CrossRef]
- Brusle’-Sicard, S.; Debas, L.; Fourcault, B.; Fuchs, J. Ultrastructural study of sex inversion in a protogynous hermaphrodite, Epinephelus microdon (Teleostei, Serranidae). Reprod. Nutr. Dev. 1992, 32, 393–406. [Google Scholar] [CrossRef]
- Shapiro, D.Y.; Sadovy, Y.; McGehee, M.A. Periodicity of sex change and reproduction in the red hind, Epinephelus guttatus, a protogynous grouper. Bull. Mar. Sci. 1993, 53, 1151–1162. [Google Scholar]
- Sadovy, Y.; Colin, P.L. Sexual development and sexuality in the Nassau grouper. J. Fish Biol. 1995, 46, 961–976. [Google Scholar] [CrossRef]
- Bhandari, R.K.; Komuro, H.; Nakamura, S.; Higa, M.; Nakamura, M. Gonadal restricting and correlative steroid hormone profiles during natural sex change in protogynous honeycomb grouper (Epinephelus merra). Zool. Sci. 2003, 20, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, Y.; Li, S.S.; Liu, Q.; Lu, D.Q.; Liu, M.; Meng, Z.N.; Cheng, C.H.; Liu, X.C.; Lin, H.R. Molecular identification of the Kiss2/Kiss1ra system and its potential function during 17α-methyltestosterone-induced sex reversal in the orange-spotted grouper, Epinephelus coioides. Biol. Reprod. 2010, 83, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Qi, X.; Guo, Y.; Li, S.S.; Zhang, Y.; Liu, X.C.; Lin, H.R. Molecular identification of GnIH/GnIHR signal and its reproductive function in protogynous hermaphroditic orange-spotted grouper (Epinephelus coioides). Gen. Comp. Endocrinol. 2015, 216, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Liu, X.C.; Zhang, H.F.; Zhang, Y.; Li, S.S.; Sang, Q.; Wang, Q.; Luo, W.N.; Liu, Q.; Lu, D.Q.; et al. Expression profiles of gonadotropins and their receptors during 17α-methyltestosterone implantation-induced sex change in the orange-spotted grouper. Mol. Reprod. Dev. 2011, 78, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.M.; Zhang, Y.; Zhang, L.H.; Zhao, H.H.; Li, X.; Huang, H.; Lin, H.R. The mRNA expression of p450 aromatase, gonadotropin beta-subunits and FTZ-F1 in the orange-spotted grouper (Epinephelus coioides) during 17α-methyltestosterone-induced precocious sex change. Mol. Reprod. Dev. 2007, 74, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Li, G.L.; Liu, X.C.; Zhang, Y.; Lin, H.R. Gonadal development, aromatase activity and p450 aromatase gene expression during sex inversion of protogynous red-spotted grouper Epinephelus akaara, (temminck and schlegel) after implantation of the aromatase inhibitor, fadrozole. Aquac. Res. 2006, 37, 484–491. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, W.M.; Zhang, L.H.; Zhu, T.Y.; Tian, J.; Li, X.; Lin, H.R. Two distinct cytochrome p450 aromatases in the orange-spotted grouper (Epinephelus coioides): cDNA cloning and differential mRNA expression. J. Steroid. Biochem. 2004, 92, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.Y.; Chow, M.; Chao, T.M.; Lim, R. Artificial spawning and larval rearing of the grouper, Epinephelus tauvina (Forskal) in Singapore. Sing. J. Prim. Ind. 1977, 5, 1–21. [Google Scholar]
- Kuo, C.M.; Ting, Y.Y.; Yeh, S.L. Induced sex reversal and spawning of blues potted grouper, Epinephelus fario. Aquaculture 1988, 74, 113–126. [Google Scholar] [CrossRef]
- Chao, T.M.; Chow, M. Effect of methyltestosterone on gonadal development of Epinephelus tauvina (Forskal). Sing. J. Prim. Ind. 1990, 18, 1–14. [Google Scholar]
- Chao, T.M.; Lim, L.C. Recent development in the breeding of grouper (Epinephelus) in Singapore. Sing. J. Prim. Ind. 1991, 19, 78–93. [Google Scholar]
- Fang, Y.; Lin, Q.; Qi, X.; Hong, G. Effects of 17a-methyltestosterone on sex reversal in Epinephelus akaara. J. Fish China 1992, 16, 171–174. [Google Scholar]
- Tan-Fermin, J.D.; Garcia, L.M.B.; Castillo, A.R. Induction of sex inversion in juvenile grouper, Epinephelus suillus, (Valenciennes) by injections of 17a-methyltestosterone. Jpn. J. Ichthyol. 1994, 40, 413–420. [Google Scholar]
- Yeh, S.L.; Kuo, C.M.; Ting, Y.Y.; Chang, C.F. Androgens stimulate sex change in protogynous grouper, Epinephelus coioides: Spawning performance in sex-changed males. Comp. Biochem. Physiol. C 2003, 135, 357–382. [Google Scholar] [CrossRef]
- Bhandari, R.K.; Higa, M.; Nakamura, S.; Nakamura, M. Aromatase inhibitor induces complete sex change in the protogynous honeycomb grouper (Epinephelus merra). Mol. Reprod. Dev. 2004, 67, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.K.; Alam, M.A.; Soyano, K.; Nakamura, M. Induction of female-to-male sex change in the honeycomb grouper (Epinephelus merra) by 11-ketotestosterone treatments. Zool. Sci. 2006, 23, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Sarter, K.; Papadaki, M.; Zanuy, S.; Mylonas, C.C. Permanent sex inversion in 1-year-old juveniles of the protogynous dusky grouper (Epinephelus marginatus) using controlled-release 17a-methyltestosterone implants. Aquaculture 2006, 256, 443–456. [Google Scholar] [CrossRef]
- Nakamura, M.; Kobayashi, T.; Chang, X.T.; Nagahama, Y. Gonadal sex differentiation in teleost fish. J. Exp. Zool. 1998, 281, 362–372. [Google Scholar] [CrossRef]
- Bhandari, R.K.; Nakamura, M.; Kobayashi, T.; Nagahama, Y. Suppression of steroidogenic enzyme expression during androgen-induced sex reversal in Niletilapia (Oreochromis niloticus). Gen. Comp. Endocrinol. 2006, 145, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Bhandari, R.K.; Kobayashi, Y.; Soyano, K.; Nakamura, M. Changes in androgen-producing cell size and circulating 11-ketotestosterone levels during female-to-male sex change in honeycomb grouper (Epinephelus merra). Mol. Reprod. Dev. 2006, 73, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhou, L.; Li, Z.; Gui, J.F. Expression pattern, cellular localization and promoter activity analysis of ovarian aromatase (Cyp19a1a) in protogynous hermaphrodite red-spotted grouper. Mol. Cell. Endocrinol. 2009, 307, 224–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Q.; Liu, Y.; Peng, C.; Wang, X.; Xiao, L.; Wang, D.; Chen, J.; Zhang, H.; Zhao, H.; Li, S.S.; et al. Molecular regulation of sex change induced by methyltestosterone-feeding and methyltestosterone-feeding withdrawal in the protogynous orange-spotted grouper. Biol. Reprod. 2017, 97, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Afonso, L.O.; Wassermann, G.J.; Terezinha de Oliveira, R. Sex reversal in Nile tilapia (Oreochromis niloticus) using a nonsteroidal aromatase inhibitor. J. Exp. Zool. 2001, 290, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.N.; Jiang, X.L.; Xie, Q.P.; Yuan, J.; Huang, B.F.; Tao, W.J.; Zhou, L.Y.; Nagahama, Y.; Wang, D.S. Transdifferentiation of differentiated ovary into functional testis by long-term treatment of aromatase inhibitor in Nile tilapia. Endocrinology 2014, 55, 1476–1488. [Google Scholar] [CrossRef] [PubMed]
- Murata, R.; Kobayashi, Y.; Karimata, H.; Kishimoto, K.; Kimura, M.; Nakamura, M. Transient Sex Change in the Immature Malabar Grouper, Epinephelus malabaricus, Androgen Treatment. Biol. Reprod. 2014, 91, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Mitcheson, Y.S.D. Gonad development during sexual differentiation in hatchery-produced orange-spotted grouper (Epinephelus coioides) and humpback grouper (Cromileptes altivelis) (Pisces: Serranidae, Epinephelinae). Aquaculture 2009, 287, 191–202. [Google Scholar] [CrossRef]
- Wu, G.C.; Tomy, S.; Lee, M.F.; Lee, Y.H.; Yueh, W.S.; Lin, C.J.; Lau, E.L.; Chang, C.F. Sex differentiation and sex change in the protandrous black porgy, Acanthopagrus schlegeli. Gen. Comp. Endocrinol. 2010, 167, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Hur, S.W.; Na, O.S.; Baek, H.J.; Noh, C.H.; Han, S.H.; Lee, Y.D. Induction of primary male in juvenile red spotted grouper Epinephelus akaara by immersion of 17α-methyltestosterone. Dev. Reprod. 2014, 18, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.A.; Komuro, H.; Bhandari, R.K.; Nakamura, S.; Soyano, K.; Nakamura, M. Immunohistochemical evidence identifying the site of androgen production in the ovary of the protogynous grouper Epinephelus merra. Cell Tissue Res. 2005, 320, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Murata, R.; Karimata, H.; Kobayashi, Y.; Horiguchi, R.; Kishimoto, K.; Kimura, M.; Kobayashi, T.; Soyano, K.; Nakamura, M. Differentiation of steroid-producing cells during ovarian differentiation in the protogynous Malabar grouper, Epinephelus malabaricus. Int. J. Dev. Biol. 2011, 55, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Couse, J.F.; Hewitt, S.C.; Bunch, D.O.; Sar, M.; Walker, V.R.; Davis, B.J.; Korach, K.S. Postnatal sex reversal of the ovaries in mice lacking estrogen receptors α and β. Science 1999, 286, 2328–2331. [Google Scholar] [CrossRef] [PubMed]
- Uhlenhaut, N.H.; Jakob, S.; Anlag, K.; Schütz, G. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 2009, 39, 1130–1142. [Google Scholar] [CrossRef] [PubMed]
- Matson, C.K.; Murphy, M.W.; Sarver, A.L.; Griswold, M.D.; Bardwell, V.J.; Zarkower, D. DMRT1 prevents female reprogramming in the postnatal mammalian testis. Nature 2011, 476, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Paul-Prasanth, B.; Bhandari, R.K.; Kobayashi, T.; Horiguchi, R.; Kobayashi, Y.; Nakamoto, M.; Shibata, Y.; Sakai, F.; Nakamura, M.; Nagahama, Y. Estrogen oversees the maintenance of the female genetic program in terminally differentiated gonochorists. Sci. Rep. 2013, 3, 3738. [Google Scholar] [CrossRef] [PubMed]









| Duration of Administration | Sample No | Body Weight e (g) | Total Length e (cm) | Gonadal Status | |||
|---|---|---|---|---|---|---|---|
| Ovary a | Ovary b | Testis c | Testis d | ||||
| Initial control at 90 day after hatching | 8 | 4.02 ± 0.30 | 6.69 ± 0.16 | 5 | 0 | 0 | 0 |
| Control group | |||||||
| 12 dat | 5 | 5.32 ± 0.48 | 6.78 ± 0.16 | 5 | 0 | 0 | 0 |
| 24 dat | 5 | 6.76 ± 0.52 | 7.84 ± 0.19 | 5 | 0 | 0 | 0 |
| 36 dat | 5 | 13.9 ± 0.65 | 9.64 ± 0.25 | 3 | 2 | 0 | 0 |
| 96 dat | 5 | 22.72 ± 1.74 | 11.84 ± 0.25 | 0 | 5 | 0 | 0 |
| 156 dat | 5 | 55.72 ± 2.80 | 16.06 ± 0.36 | 0 | 5 | 0 | 0 |
| MT treatment group | |||||||
| 12 dat | 5 | 6.77 ± 1.52 | 7.22 ± 0.54 | 0 | 0 | 5 | 0 |
| 24 dat | 5 | 7.16 ± 1.00 | 7.70 ± 0.45 | 0 | 0 | 5 | 0 |
| 36 dat | 5 | 11.77 ± 0.79 | 9.22 ± 0.20 | 0 | 0 | 1 | 4 |
| 96 dat | 5 | 20.56 ± 2.05 | 10.48 ± 0.47 | 0 | 0 | 0 | 5 |
| 156dat | 5 | 50.36 ± 1.99 | 15.84 ± 0.72 | 0 | 0 | 0 | 5 |
| MT termination group | |||||||
| 96 dat | 5 | 21.28 ± 0.92 | 10.30 ± 0.72 | 0 | 5 | 0 | 0 |
| 156 dat | 5 | 58.18 ± 1.50 | 16.1 ±0.65 | 0 | 0 | 0 | 5 |
| Primers | Primers Sequence (from 5′ to 3′) |
|---|---|
| Primers for real-time polymerase chain reaction (PCR) | |
| dmrt1-F | GCTGGAGTAGACTGCTTGTTT |
| dmrt1-R | CGACTGTGCGTCAGTATGAGC |
| cyp11b-F | TGTTGCCGTCTGACATCG |
| cyp11b-R | TCGCCACTCCTCACCGTTC |
| sox9-F | GCAATGCAGGCTCAGAATAG |
| sox9-R | GGTATCAAGGCAGTACCCAG |
| cyp19a1a-F | GGAGACATTGTGAGAGTCTGGATC |
| cyp19a1a-R | TGACAGGTACATCCAGGAAGAGTC |
| foxl2-F | CCACCGTACTCCTATGTCGC |
| foxl2-R | GTCTGATACTGTTCTGCCAAC |
| β-actin-F | ACCATCGGCAATGAGAGGTT |
| β-actin-R | ACATCTGCTGGAAGGTGGAC |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Huang, M.; Peng, C.; Wang, X.; Xiao, L.; Wang, D.; Chen, J.; Zhao, H.; Zhang, H.; Li, S.; et al. MT-Feeding-Induced Impermanent Sex Reversal in the Orange-Spotted Grouper during Sex Differentiation. Int. J. Mol. Sci. 2018, 19, 2828. https://doi.org/10.3390/ijms19092828
Wang Q, Huang M, Peng C, Wang X, Xiao L, Wang D, Chen J, Zhao H, Zhang H, Li S, et al. MT-Feeding-Induced Impermanent Sex Reversal in the Orange-Spotted Grouper during Sex Differentiation. International Journal of Molecular Sciences. 2018; 19(9):2828. https://doi.org/10.3390/ijms19092828
Chicago/Turabian StyleWang, Qing, Minwei Huang, Cheng Peng, Xiang Wang, Ling Xiao, Dengdong Wang, Jiaxing Chen, Huihong Zhao, Haifa Zhang, Shuisheng Li, and et al. 2018. "MT-Feeding-Induced Impermanent Sex Reversal in the Orange-Spotted Grouper during Sex Differentiation" International Journal of Molecular Sciences 19, no. 9: 2828. https://doi.org/10.3390/ijms19092828




