Increased Expression of Maturation Promoting Factor Components Speeds Up Meiosis in Oocytes from Aged Females
Abstract
:1. Introduction
2. Results
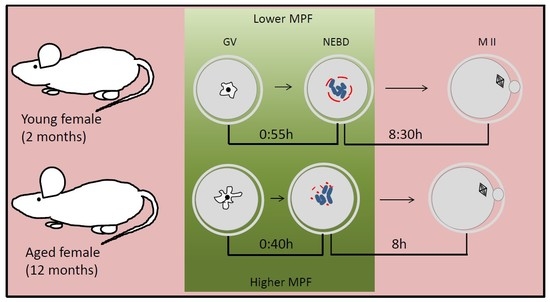
2.1. Meiosis I Is Accelerated in Oocytes from Females of Advanced Age
2.2. Dissociation of Nuclear Envelope Is Accelerated in the Oocytes from Aged Females
2.3. CDK1 Activity Is Responsible for NEBD in Mouse Oocytes
2.4. CDK1 Activity Is Increased in Mouse Oocytes from Aged Females
2.5. Translation of Positive Regulators of Translation Is Increased in the Oocytes from Aged Females
2.6. Elevated CDK1 Activity Is Responsible for Faster Meiosis I in Mouse Oocytes
3. Discussion
4. Material and Methods
4.1. Oocyte Cultivation, Treatment, and Microinjection
4.2. Time-Lapse Microscopy
4.3. Immunoblotting
4.4. Measurement of Overall Protein Synthesis
4.5. Immunocytochemistry and Cold-Stable MT Assay
4.6. Transmission Electron Microscopy
4.7. RNA Isolation and RT-PCR
4.8. Polysome Fractionation and RNA Extraction
4.9. Library Preparation, RNA Sequencing and Data Analysis
4.10. Kinase Assay
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Colegrove-Otero, L.J.; Devaux, A.; Standart, N. The Xenopus ELAV protein ElrB represses Vg1 mRNA translation during oogenesis. Mol. Cell. Biol. 2005, 25, 9028–9039. [Google Scholar] [CrossRef] [PubMed]
- Schuh, M.; Ellenberg, J. Self-organization of MTOCs replaces centrosome function during acentrosomal spindle assembly in live mouse oocytes. Cell 2007, 130, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Hassold, T.; Jacobs, P.; Kline, J.; Stein, Z.; Warburton, D. Effect of maternal age on autosomal trisomies. Ann. Hum. Genet. 1980, 44, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Ma, P.; Zhu, W.; Schultz, R.M. Age-associated increase in aneuploidy and changes in gene expression in mouse eggs. Dev. Biol. 2008, 316, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Sebestova, J.; Danylevska, A.; Novakova, L.; Kubelka, M.; Anger, M. Lack of response to unaligned chromosomes in mammalian female gametes. Cell Cycle 2012, 11, 3011–3018. [Google Scholar] [CrossRef] [PubMed]
- Hassold, T.; Hunt, P. To err (meiotically) is human: The genesis of human aneuploidy. Nat. Rev. Genet. 2001, 2, 280–291. [Google Scholar] [CrossRef] [PubMed]
- Hunt, P. Oocyte Biology: Do the Wheels Fall Off with Age? Curr. Biol. 2017, 27, R266–R269. [Google Scholar] [CrossRef] [PubMed]
- Chiang, T.; Duncan, F.E.; Schindler, K.; Schultz, R.M.; Lampson, M.A. Evidence that weakened centromere cohesion is a leading cause of age-related aneuploidy in oocytes. Curr. Biol. 2010, 20, 1522–1528. [Google Scholar] [CrossRef] [PubMed]
- Merriman, J.A.; Jennings, P.C.; McLaughlin, E.A.; Jones, K.T. Effect of aging on superovulation efficiency, aneuploidy rates, and sister chromatid cohesion in mice aged up to 15 months. Biol. Reprod. 2012, 86. [Google Scholar] [CrossRef] [PubMed]
- Chiang, T.; Schultz, R.M.; Lampson, M.A. Meiotic Origins of Maternal Age-Related Aneuploidy. Biol. Reprod. 2012, 86. [Google Scholar] [CrossRef] [PubMed]
- Dechat, T.; Pfleghaar, K.; Sengupta, K.; Shimi, T.; Shumaker, D.K.; Solimando, L.; Goldman, R.D. Nuclear lamins: Major factors in the structural organization and function of the nucleus and chromatin. Genes Dev. 2008, 22, 832–853. [Google Scholar] [CrossRef] [PubMed]
- Heald, R.; McKeon, F. Mutations of phosphorylation sites in lamin A that prevent nuclear lamina disassembly in mitosis. Cell 1990, 61, 579–589. [Google Scholar] [CrossRef]
- Susor, A.; Jansova, D.; Cerna, R.; Danylevska, A.; Anger, M.; Toralova, T.; Malik, R.; Supolikova, J.; Cook, M.S.; Oh, J.S.; et al. Temporal and spatial regulation of translation in the mammalian oocyte via the mTOR-eIF4F pathway. Nat. Commun. 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Sanfins, A.; Plancha, C.E.; Overstrom, E.W.; Albertini, D.F. Meiotic spindle morphogenesis in in vivo and in vitro matured mouse oocytes: Insights into the relationship between nuclear and cytoplasmic quality. Hum. Reprod. 2004, 19, 2889–2899. [Google Scholar] [CrossRef] [PubMed]
- Haithcock, E.; Dayani, Y.; Neufeld, E.; Zahand, A.J.; Feinstein, N.; Mattout, A.; Gruenbaum, Y.; Liu, J. Age-related changes of nuclear architecture in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2005, 102, 16690–16695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Righolt, C.H.; van ’t Hoff, M.L.R.; Vermolen, B.J.; Young, I.T.; Raz, V. Robust nuclear lamina-based cell classification of aging and senescent cells. Aging 2011, 3, 1192–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adhikari, D.; Zheng, W.; Shen, Y.; Gorre, N.; Ning, Y.; Halet, G.; Kaldis, P.; Liu, K. Cdk1, but not Cdk2, is the sole Cdk that is essential and sufficient to drive resumption of meiosis in mouse oocytes. Hum. Mol. Genet. 2012, 21, 2476–2484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peter, M.; Nakagawa, J.; Dorée, M.; Labbé, J.C.; Nigg, E.A. In vitro disassembly of the nuclear lamina and M phase-specific phosphorylation of lamins by cdc2 kinase. Cell 1990, 61, 591–602. [Google Scholar] [CrossRef]
- Jones, K.T. Turning it on and off: M-phase promoting factor during meiotic maturation and fertilization. Mol. Hum. Reprod. 2004, 10, 1–5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, D.O. Principles of CDK regulation. Nature 1995, 374, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Ducommun, B.; Brambilla, P.; Félix, M.A.; Franza, B.R.; Karsenti, E.; Draetta, G. cdc2 phosphorylation is required for its interaction with cyclin. EMBO J. 1991, 10, 3311–3319. [Google Scholar] [CrossRef] [PubMed]
- Solomon, M.J. Activation of the various cyclin/cdc2 protein kinases. Curr. Opin. Cell Biol. 1993, 5, 180–186. [Google Scholar] [CrossRef]
- Hampl, A.; Eppig, J.J. Translational regulation of the gradual increase in histone H1 kinase activity in maturing mouse oocytes. Mol. Reprod. Dev. 1995, 40, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Lévesque, J.T.; Sirard, M.A. Resumption of meiosis is initiated by the accumulation of cyclin B in bovine oocytes. Biol. Reprod. 1996, 55, 1427–1436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davydenko, O.; Schultz, R.M.; Lampson, M.A. Increased CDK1 activity determines the timing of kinetochore-microtubule attachments in meiosis I. J. Cell Biol. 2013, 202, 221–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rieder, C.L. The structure of the cold-stable kinetochore fiber in metaphase PtK1 cells. Chromosoma 1981, 84, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Eichenlaub-Ritter, U.; Boll, I. Age-related non-disjunction, spindle formation and progression through maturation of mammalian oocytes. Prog. Clin. Biol. Res. 1989, 318, 259–269. [Google Scholar] [PubMed]
- Zuccotti, M.; Boiani, M.; Garagna, S.; Redi, C.A. Analysis of aneuploidy rate in antral and ovulated mouse oocytes during female aging. Mol. Reprod. Dev. 1998, 50, 305–312. [Google Scholar] [CrossRef]
- Liu, L.; Keefe, D.L. Ageing-associated aberration in meiosis of oocytes from senescence-accelerated mice. Hum. Reprod. 2002, 17, 2678–2685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cukurcam, S.; Betzendahl, I.; Michel, G.; Vogt, E.; Hegele-Hartung, C.; Lindenthal, B.; Eichenlaub-Ritter, U. Influence of follicular fluid meiosis-activating sterol on aneuploidy rate and precocious chromatid segregation in aged mouse oocytes. Hum. Reprod. 2007, 22, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Chiang, T.; Schultz, R.M.; Lampson, M.A. Age-Dependent Susceptibility of Chromosome Cohesion to Premature Separase Activation in Mouse Oocytes. Biol. Reprod. 2011, 85, 1279–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eichenlaub-Ritter, U.; Chandley, A.C.; Gosden, R.G. The CBA mouse as a model for age-related aneuploidy in man: Studies of oocyte maturation, spindle formation and chromosome alignment during meiosis. Chromosoma 1988, 96, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Eichenlaub-Ritter, U.; Boll, I. Nocodazole sensitivity, age-related aneuploidy, and alterations in the cell cycle during maturation of mouse oocytes. Cytogenet. Genome Res. 1989, 52, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Duncan, F.E.; Chiang, T.; Schultz, R.M.; Lampson, M.A. Evidence That a Defective Spindle Assembly Checkpoint Is Not the Primary Cause of Maternal Age-Associated Aneuploidy in Mouse Eggs1. Biol. Reprod. 2009, 81, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Lister, L.M.; Kouznetsova, A.; Hyslop, L.A.; Kalleas, D.; Pace, S.L.; Barel, J.C.; Nathan, A.; Floros, V.; Adelfalk, C.; Watanabe, Y.; et al. Age-Related Meiotic Segregation Errors in Mammalian Oocytes Are Preceded by Depletion of Cohesin and Sgo2. Curr. Biol. 2010, 20, 1511–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, L.-B.; Zhou, X.-Y.; Zhao, Z.-J.; Li, Q.; Huang, X.-Y.; Sun, F.-Z. The Kunming mouse: As a model for age-related decline in female fertility in human. Zygote 2013, 21, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Gui, L.; Homer, H. Hec1-dependent cyclin B2 stabilization regulates the G2-M transition and early prometaphase in mouse oocytes. Dev. Cell 2013, 25, 43–54. [Google Scholar] [CrossRef] [PubMed]
- De Smedt, V.; Poulhe, R.; Cayla, X.; Dessauge, F.; Karaiskou, A.; Jessus, C.; Ozon, R. Thr-161 phosphorylation of monomeric Cdc2. Regulation by protein phosphatase 2C in Xenopus oocytes. J. Biol. Chem. 2002, 277, 28592–28600. [Google Scholar] [CrossRef] [PubMed]
- Félix, M.A.; Cohen, P.; Karsenti, E. Cdc2 H1 kinase is negatively regulated by a type 2A phosphatase in the Xenopus early embryonic cell cycle: Evidence from the effects of okadaic acid. EMBO J. 1990, 9, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, J.-X.; Cheng, J.-M.; Hu, B.; Wang, Y.-Q.; Aalia, B.; Li, X.-Y.; Jin, C.; Wang, X.-X.; Deng, S.-L.; et al. Cyclin B2 can compensate for Cyclin B1 in oocyte meiosis I. J. Cell Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Duncan, F.E.; Jasti, S.; Paulson, A.; Kelsh, J.M.; Fegley, B.; Gerton, J.L. Age-associated dysregulation of protein metabolism in the mammalian oocyte. Aging Cell 2017, 16, 1381–1393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, S.; Barna, M. Specialized ribosomes: A new frontier in gene regulation and organismal biology. Nat. Rev. Mol. Cell Biol. 2012, 13, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Landis, J.N.; Krisher, R.L.; Duncan, F.E.; Silva, E.; Lonczak, A.; Scott, R.T.; Zhan, Y.; Chu, T.; Scott, R.T.; et al. Mitochondrial DNA content is associated with ploidy status, maternal age, and oocyte maturation methods in mouse blastocysts. J. Assist. Reprod. Genet. 2017, 34, 1587–1594. [Google Scholar] [CrossRef] [PubMed]
- Fragouli, E.; Spath, K.; Alfarawati, S.; Kaper, F.; Craig, A.; Michel, C.-E.; Kokocinski, F.; Cohen, J.; Munne, S.; Wells, D. Altered Levels of Mitochondrial DNA Are Associated with Female Age, Aneuploidy, and Provide an Independent Measure of Embryonic Implantation Potential. PLoS Genet. 2015, 11. [Google Scholar] [CrossRef] [PubMed]
- Raz, V.; Vermolen, B.J.; Garini, Y.; Onderwater, J.J.M.; Mommaas-Kienhuis, M.A.; Koster, A.J.; Young, I.T.; Tanke, H.; Dirks, R.W. The nuclear lamina promotes telomere aggregation and centromere peripheral localization during senescence of human mesenchymal stem cells. J. Cell. Sci. 2008, 121, 4018–4028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maiato, H.; Hergert, P.J.; Moutinho-Pereira, S.; Dong, Y.; Vandenbeldt, K.J.; Rieder, C.L.; McEwen, B.F. The ultrastructure of the kinetochore and kinetochore fiber in Drosophila somatic cells. Chromosoma 2006, 115, 469–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schweizer, N.; Pawar, N.; Weiss, M.; Maiato, H. An organelle-exclusion envelope assists mitosis and underlies distinct molecular crowding in the spindle region. J. Cell Biol. 2015, 210, 695–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsani, K.R.; Karess, R.E.; Dostatni, N.; Doye, V. In vivo dynamics of Drosophila nuclear envelope components. Mol. Biol. Cell 2008, 19, 3652–3666. [Google Scholar] [CrossRef] [PubMed]
- Tetkova, A.; Hancova, M. Mouse Oocyte Isolation, Cultivation and RNA Microinjection. Bio-Protocol 2016, 6. [Google Scholar] [CrossRef]
- McGuinness, B.E.; Anger, M.; Kouznetsova, A.; Gil-Bernabé, A.M.; Helmhart, W.; Kudo, N.R.; Wuensche, A.; Taylor, S.; Hoog, C.; Novak, B.; et al. Regulation of APC/C activity in oocytes by a Bub1-dependent spindle assembly checkpoint. Curr. Biol. 2009, 19, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Eden, E.; Lipson, D.; Yogev, S.; Yakhini, Z. Discovering Motifs in Ranked Lists of DNA Sequences. PLoS Comput. Biol. 2007, 3, e39. [Google Scholar] [CrossRef] [PubMed]
- Eden, E.; Navon, R.; Steinfeld, I.; Lipson, D.; Yakhini, Z. GOrilla: A tool for discovery and visualization of enriched GO terms in ranked gene lists. BMC Bioinform. 2009, 10, 48. [Google Scholar] [CrossRef] [PubMed]
- Motlík, J.; Sutovský, P.; Kalous, J.; Kubelka, M.; Moos, J.; Schultz, R.M. Co-culture with pig membrana granulosa cells modulates the activity of cdc2 and MAP kinase in maturing cattle oocytes. Zygote 1996, 4, 247–256. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koncicka, M.; Tetkova, A.; Jansova, D.; Del Llano, E.; Gahurova, L.; Kracmarova, J.; Prokesova, S.; Masek, T.; Pospisek, M.; Bruce, A.W.; et al. Increased Expression of Maturation Promoting Factor Components Speeds Up Meiosis in Oocytes from Aged Females. Int. J. Mol. Sci. 2018, 19, 2841. https://doi.org/10.3390/ijms19092841
Koncicka M, Tetkova A, Jansova D, Del Llano E, Gahurova L, Kracmarova J, Prokesova S, Masek T, Pospisek M, Bruce AW, et al. Increased Expression of Maturation Promoting Factor Components Speeds Up Meiosis in Oocytes from Aged Females. International Journal of Molecular Sciences. 2018; 19(9):2841. https://doi.org/10.3390/ijms19092841
Chicago/Turabian StyleKoncicka, Marketa, Anna Tetkova, Denisa Jansova, Edgar Del Llano, Lenka Gahurova, Jana Kracmarova, Sarka Prokesova, Tomas Masek, Martin Pospisek, Alexander W. Bruce, and et al. 2018. "Increased Expression of Maturation Promoting Factor Components Speeds Up Meiosis in Oocytes from Aged Females" International Journal of Molecular Sciences 19, no. 9: 2841. https://doi.org/10.3390/ijms19092841
APA StyleKoncicka, M., Tetkova, A., Jansova, D., Del Llano, E., Gahurova, L., Kracmarova, J., Prokesova, S., Masek, T., Pospisek, M., Bruce, A. W., Kubelka, M., & Susor, A. (2018). Increased Expression of Maturation Promoting Factor Components Speeds Up Meiosis in Oocytes from Aged Females. International Journal of Molecular Sciences, 19(9), 2841. https://doi.org/10.3390/ijms19092841