Andrographolide Protects PC12 Cells Against β-Amyloid-Induced Autophagy-Associated Cell Death Through Activation of the Nrf2-Mediated p62 Signaling Pathway
Abstract
:1. Introduction
2. Results
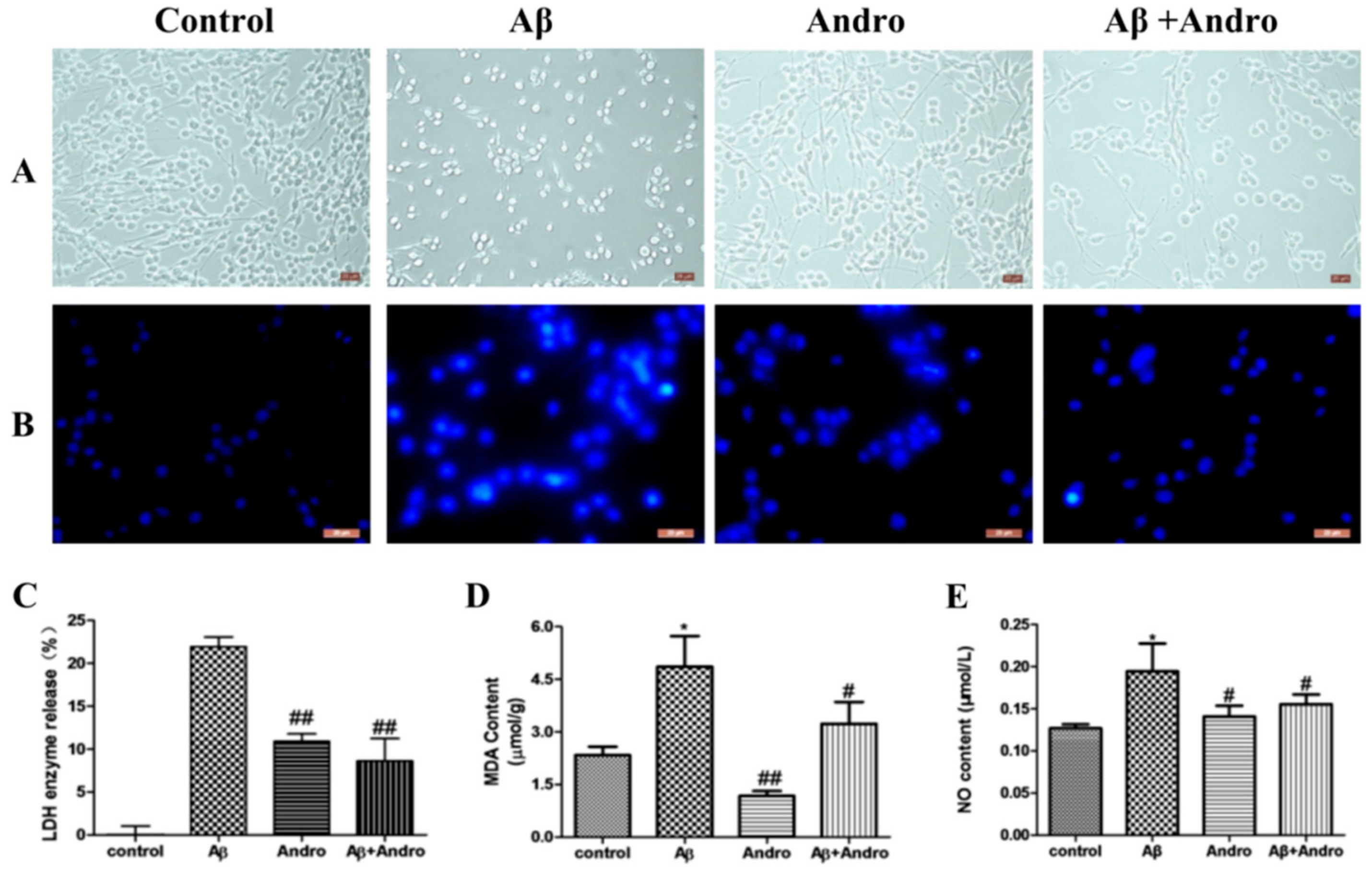
2.1. Andro Protected PC12 Cells from Aβ1–42 Neurotoxicity
2.2. Andro Attenuated the Productions of LDH, MDA, and NO in Aβ1–42-Stimulated PC12 Cells
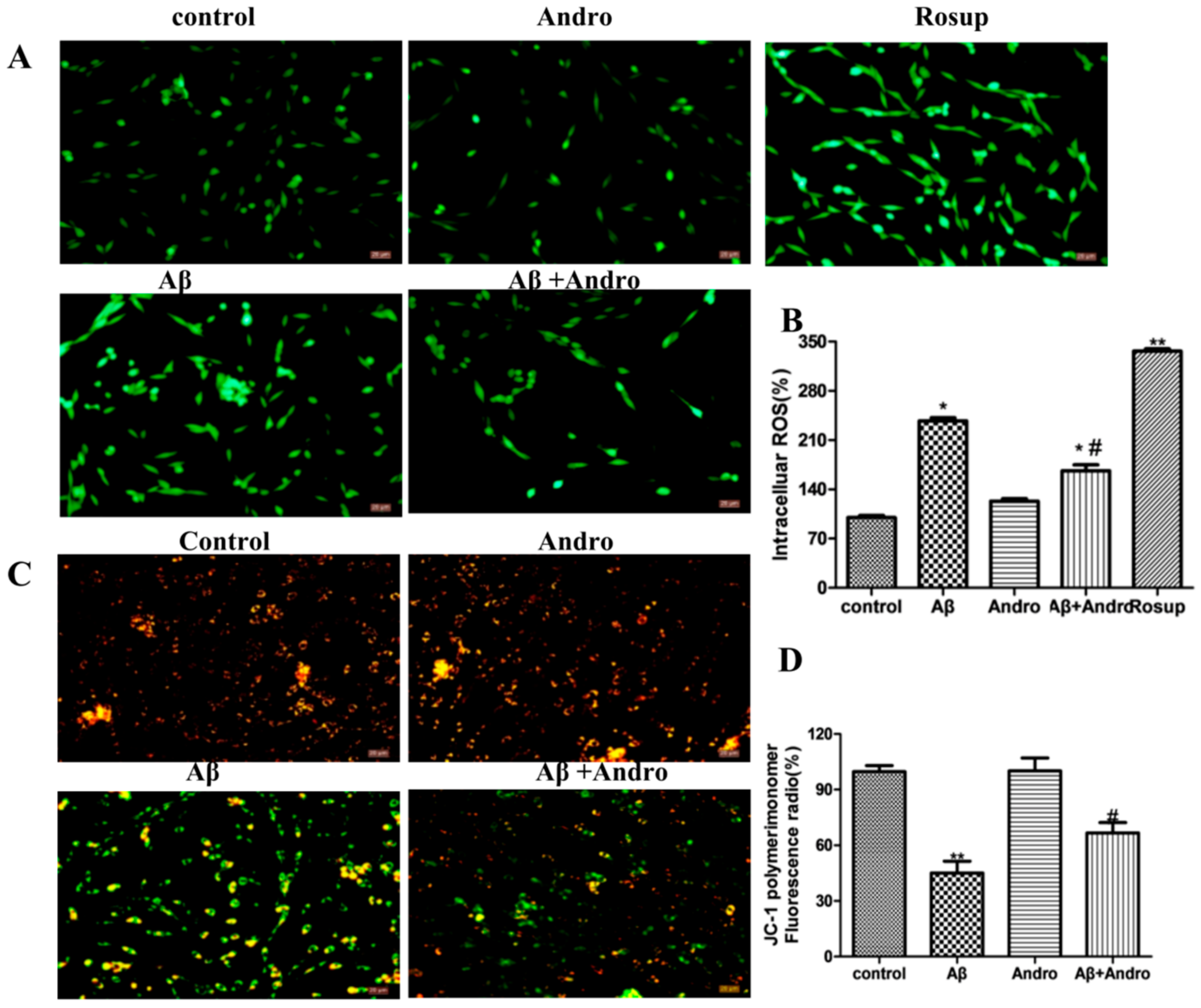
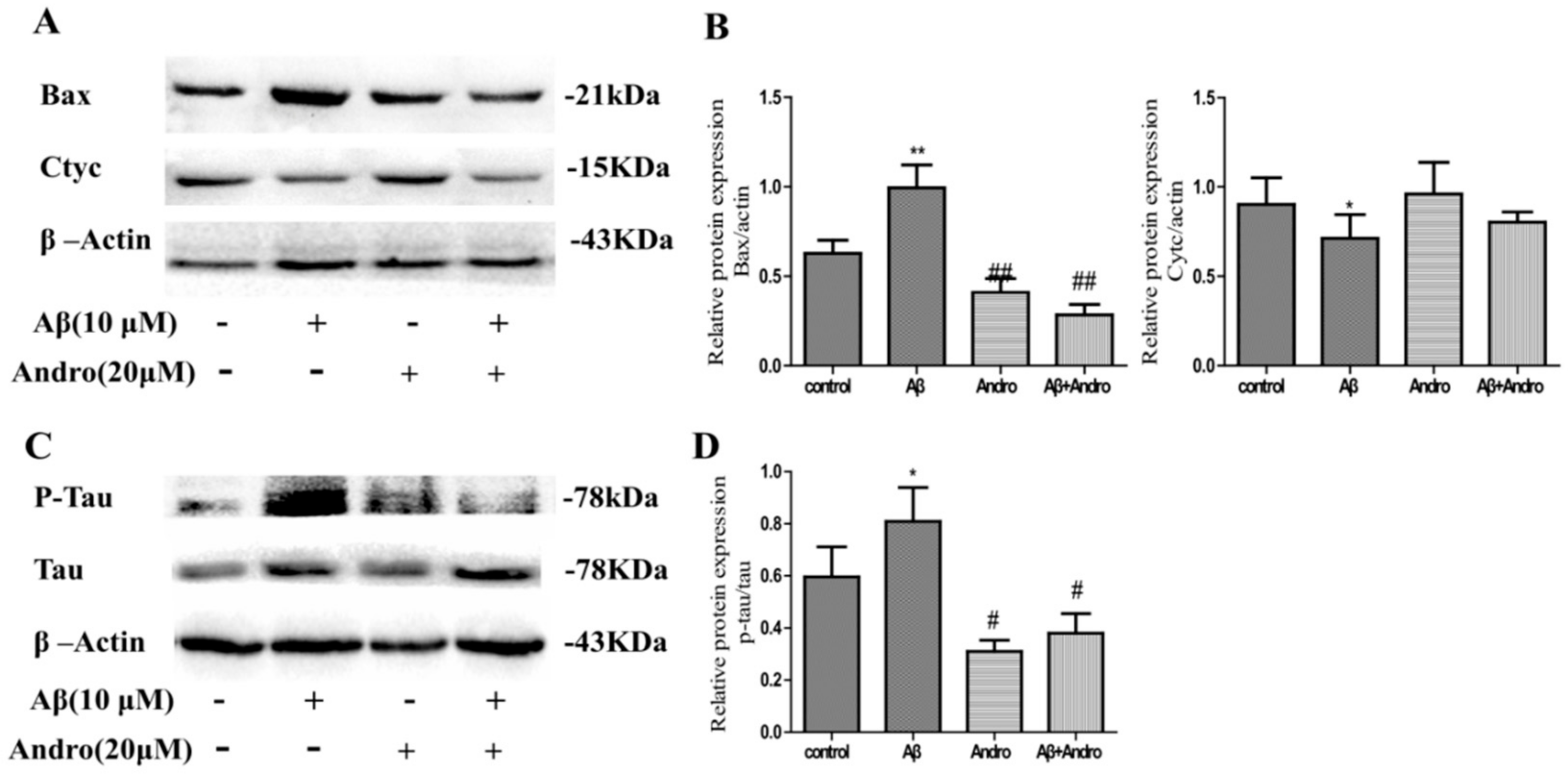
2.3. Andro Reduced the Levels of ROS, MMP, Cytc, and Bax in Aβ1–42-Stimulated PC12 Cells
2.4. Andro Lowered the Protein Levels of Phospho-Tau/Tau in Aβ1–42-Stimulated PC12 Cells
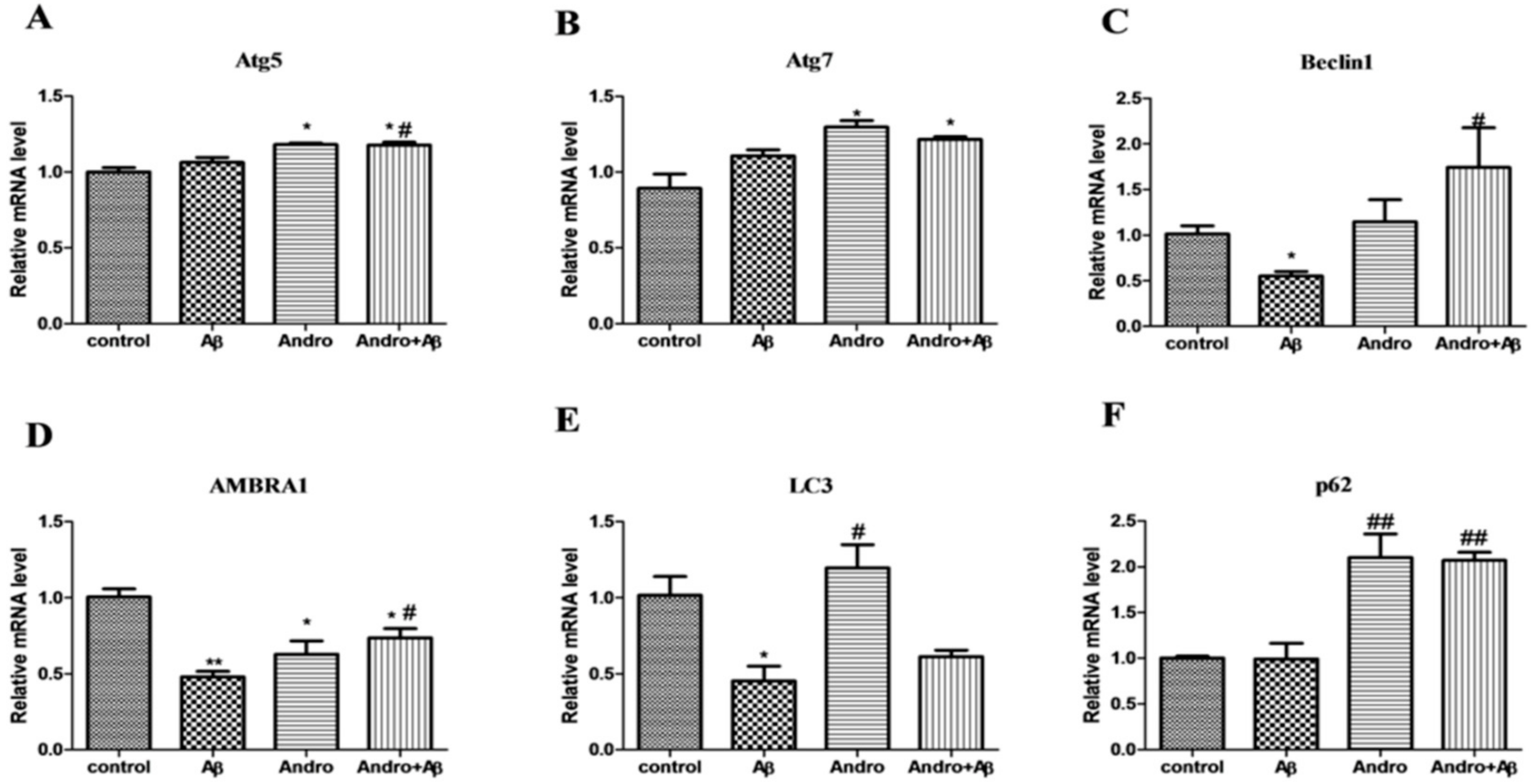
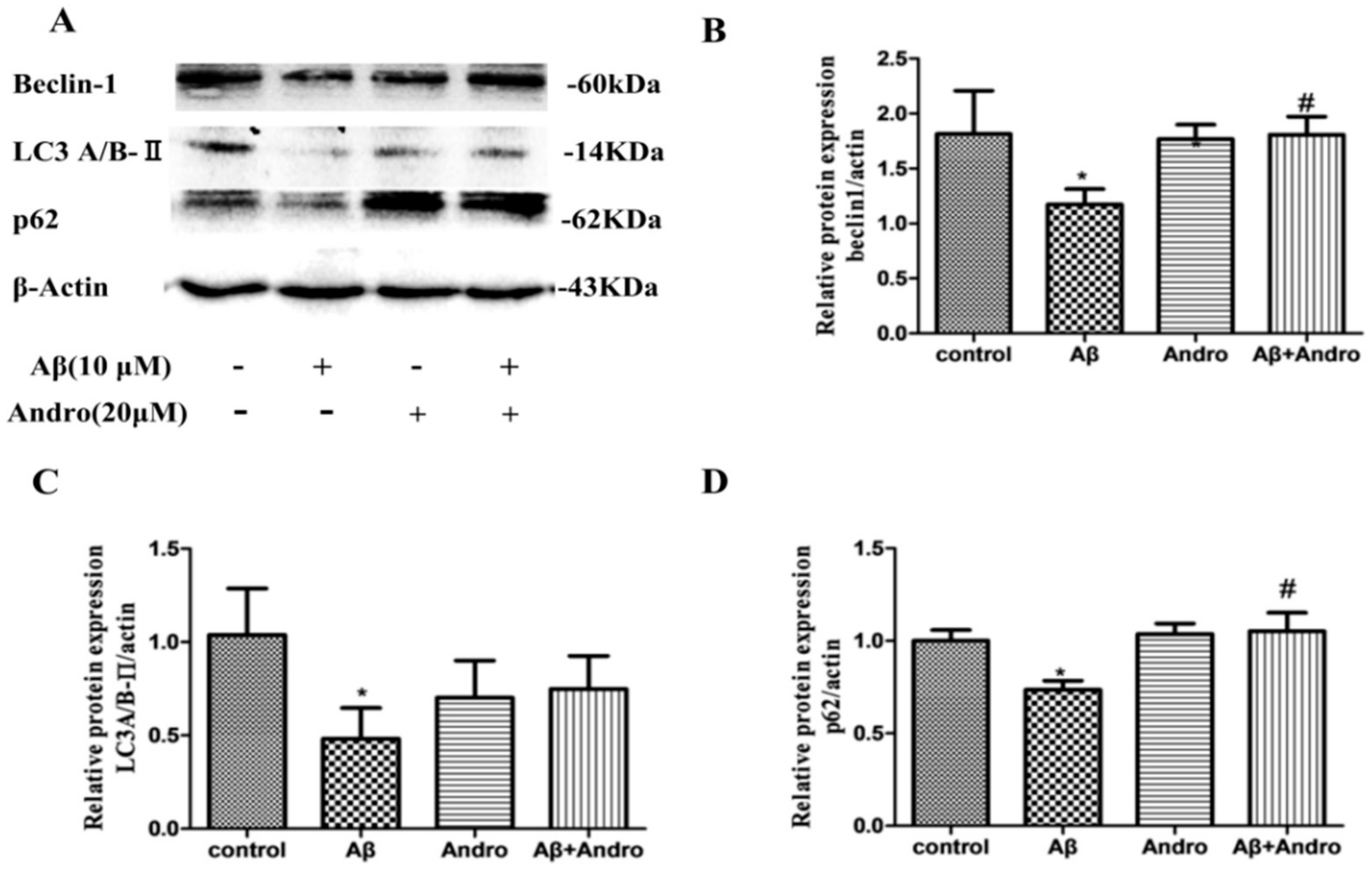
2.5. Andro Altered the mRNA and Protein Levels of Autophagy Markers in Aβ1–42-Stimulated PC12 Cells
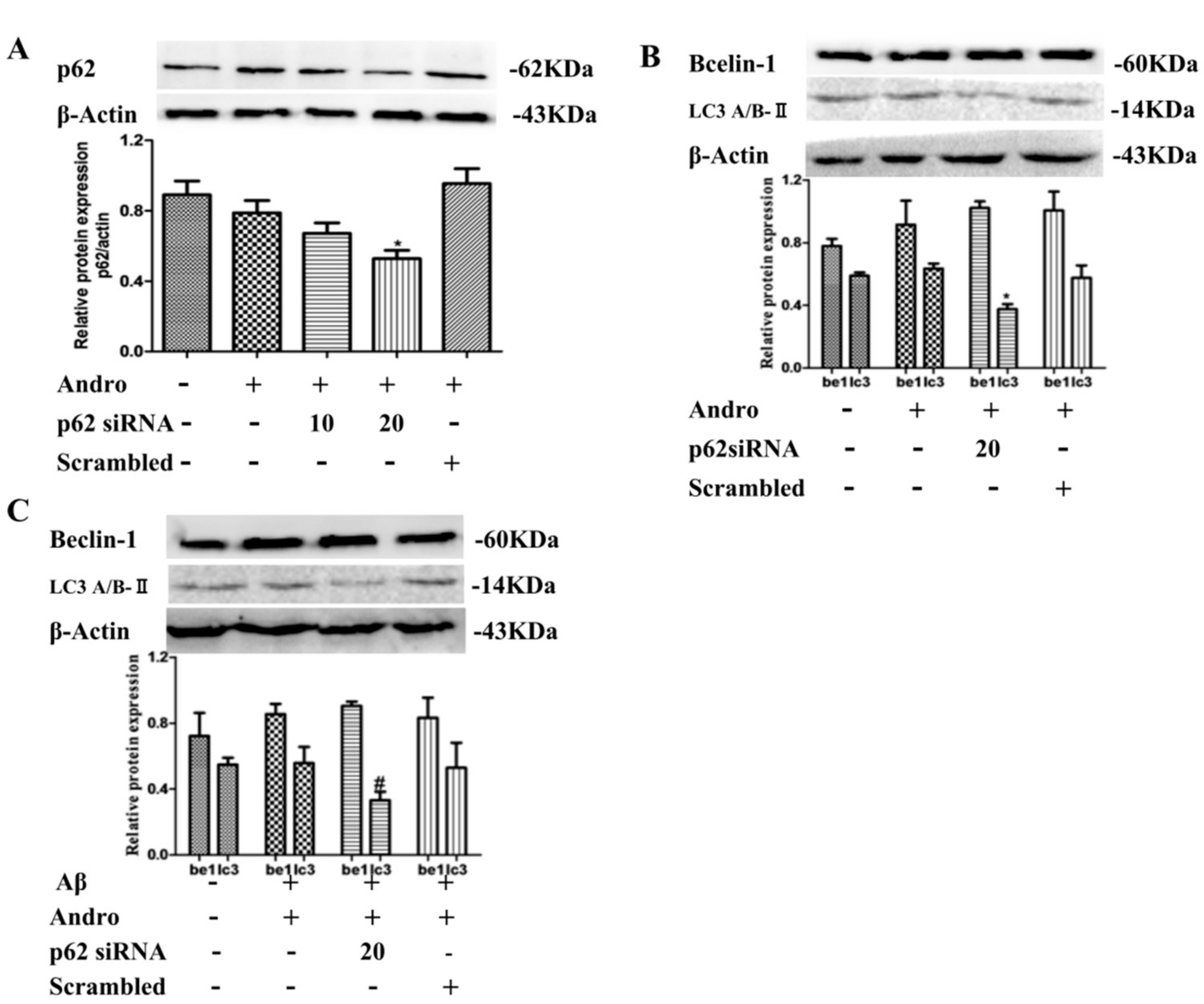
2.6. P62 Induced by Andro Played an Important Role in Preventing Cell Toxicity in Aβ1–42-Stimulated PC12 Cells via Autophagy
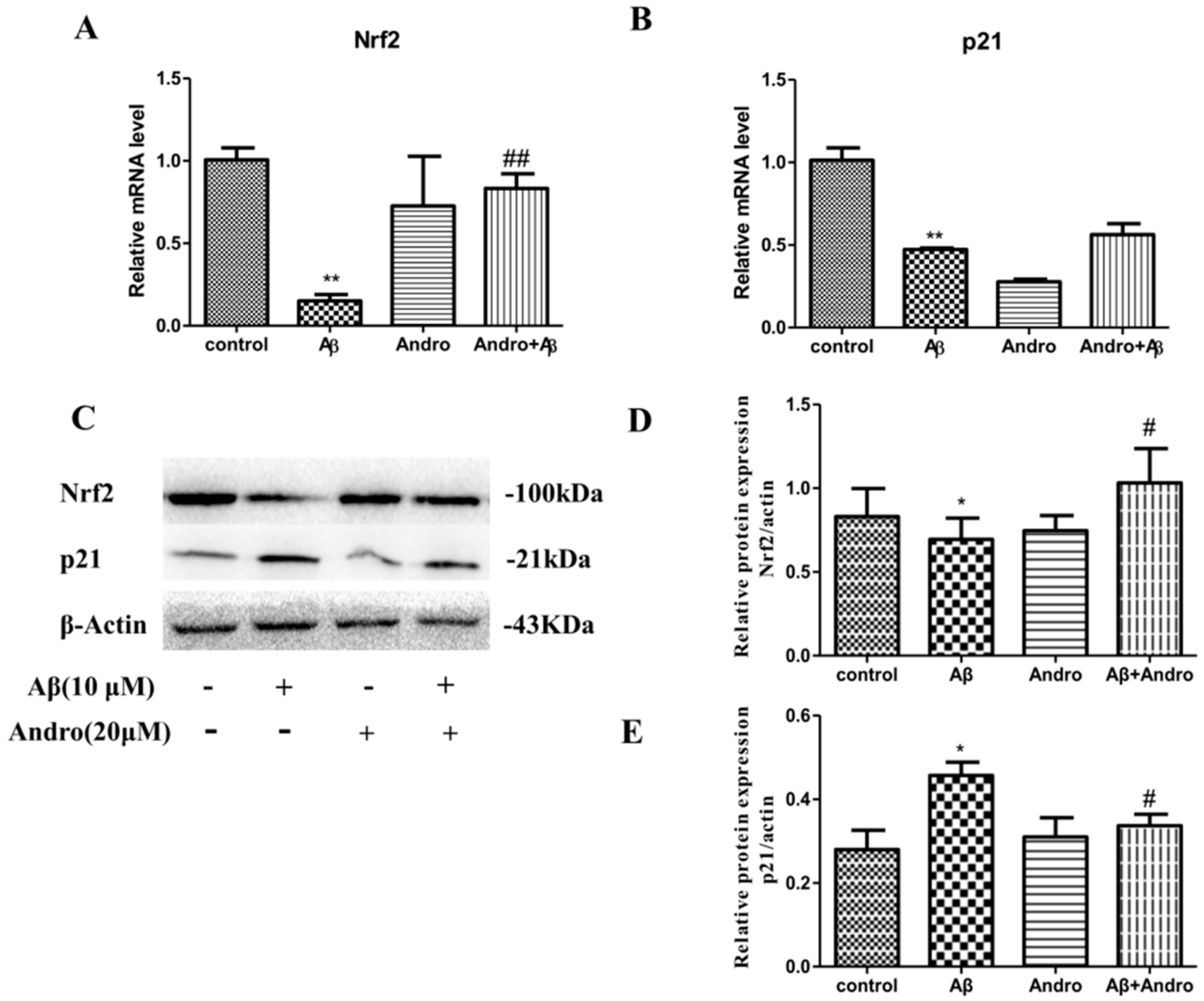
2.7. Andro Enhanced the Nrf2 Activation and Weakened the p21 Expression in Aβ1–42-Stimulated PC12 Cells
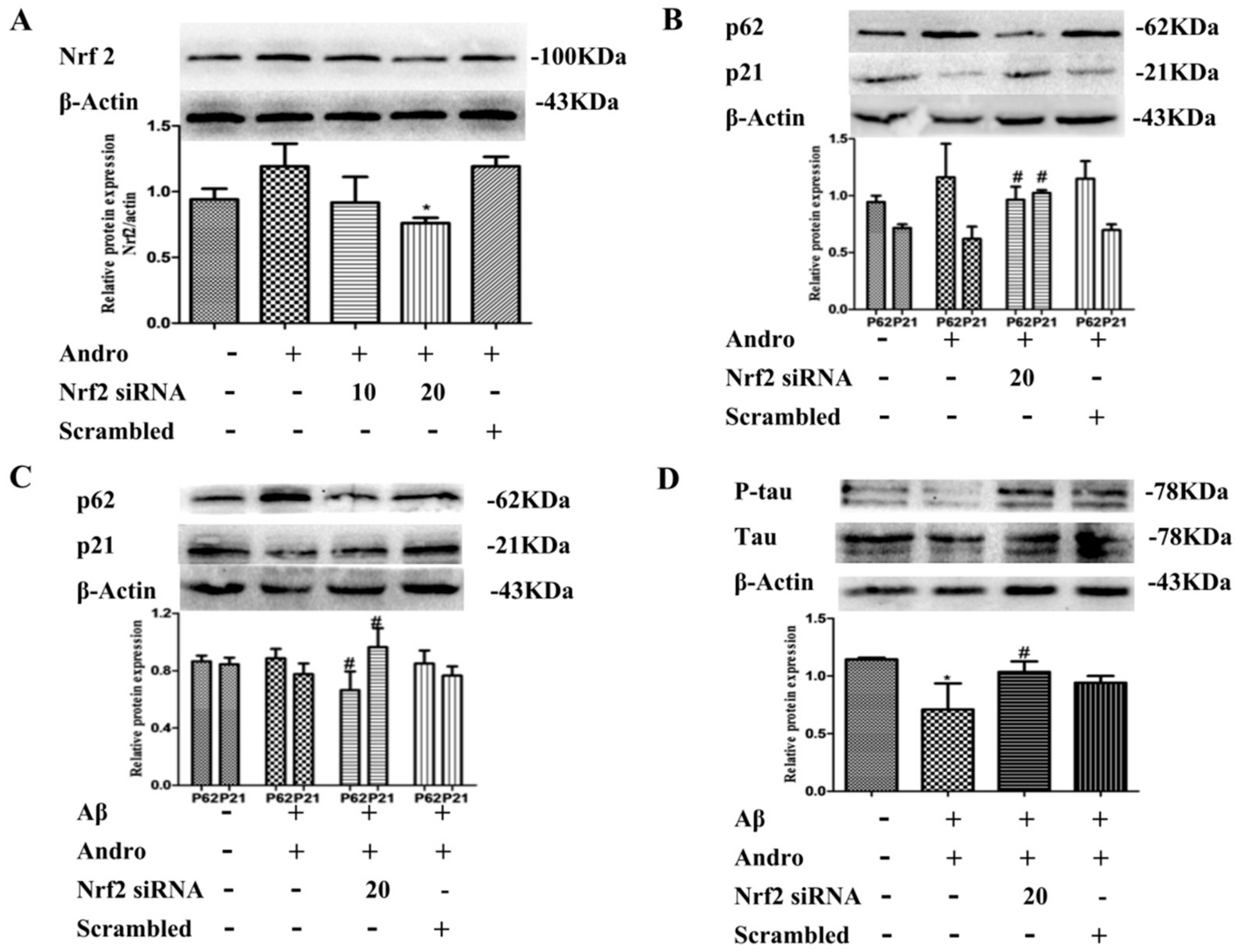
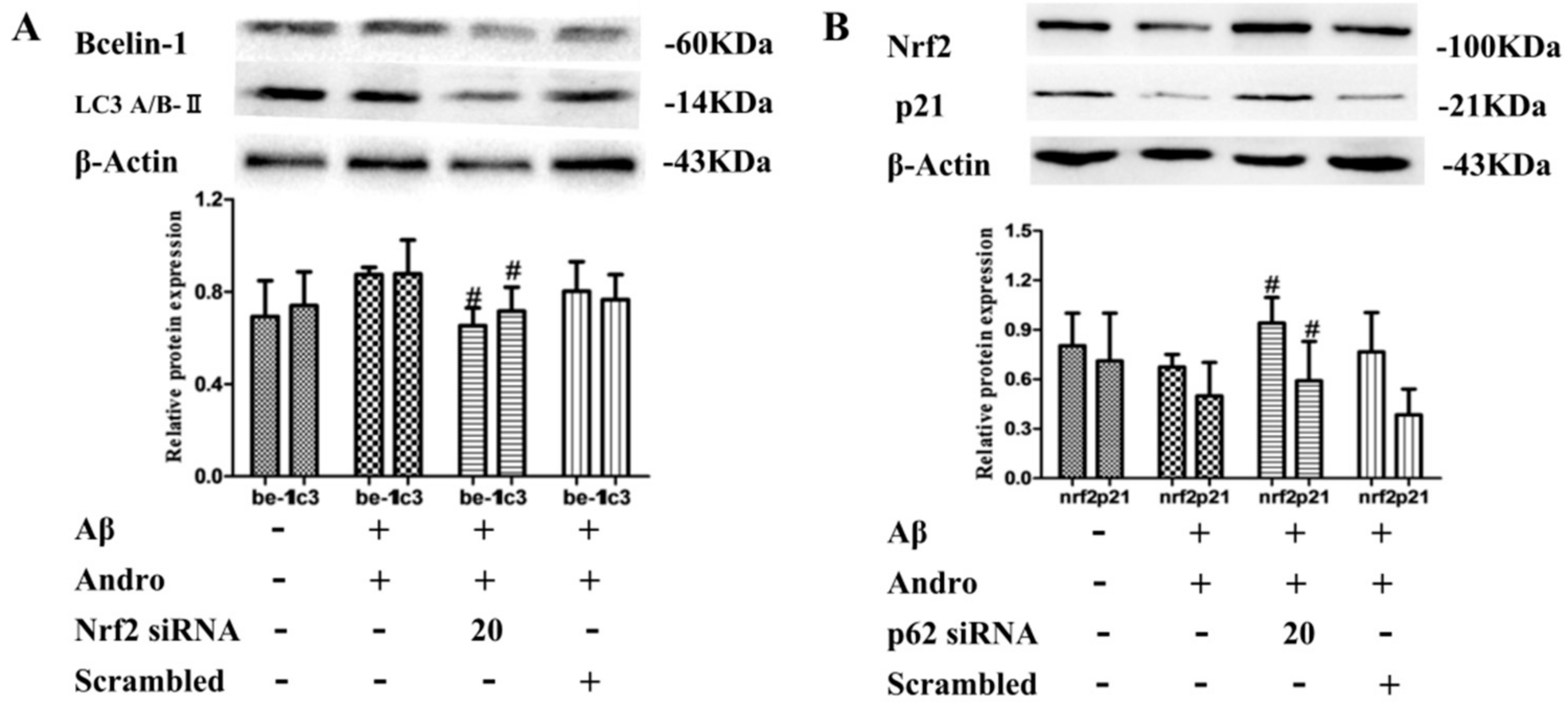
2.8. Nrf2 Signaling Pathway Mediated by Andro Was Involved in p62 Expression and Autophagy Induction in Aβ1–42-Stimulated PC12 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. MTT Assay
4.3. Lactate Dehydrogenase (LDH) Release Assay
4.4. Hoechst 33258 Staining
4.5. Measurement of Intracellular ROS Levels
4.6. MDA and NO Content Detection
4.7. Measurement of Mitochondrial Membrane Potential (MMP)
4.8. Western Blotting
4.9. Reverse Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
4.10. Transient Gene Silencing by Small Interfering RNAs (siRNAs)
4.11. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Tan, W.S.D.; Liao, W.; Zhou, S.; Wong, W.S.F. Is there a future for andrographolide to be an anti-inflammatory drug? Deciphering its major mechanisms of action. Biochem. Pharmacol. 2017, 139, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.Y.; Hsieh, P.L.; Wang, T.H.; Yu, C.C.; Lu, M.Y.; Liao, Y.W.; Lee, T.H.; Peng, C.Y. Andrographolide impedes cancer stemness and enhances radio-sensitivity in oral carcinomas via miR-218 activation. Oncotarget 2017, 8, 4196–4207. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, J.; Dong, S.F.; Liu, C.H.; Italiani, P.; Sun, S.H.; Xu, J.; Boraschi, D.; Ma, S.P.; Qu, D. Immunomodulatory activity of andrographolide on macrophage activation and specific antibody response. Acta Pharmacol. Sin. 2010, 31, 191–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panossian, A.; Hovhannisyan, A.; Mamikonyan, G.; Abrahamian, H.; Hambardzumyan, E.; Gabrielian, E.; Goukasova, G.; Wikman, G.; Wagner, H. Pharmacokinetic and oral bioavailability of andrographolide from Andrographis paniculata fixed combination Kan Jang in rats and human. Phytomedicine 2000, 7, 351–364. [Google Scholar] [CrossRef]
- Li, J. Effects of andrographolide on platelet-activating factor and amyloid-beta protein in rats with Alzheimer’s disease. J. Community Med. 2014, 12, 16–19. [Google Scholar]
- Serrano, F.G.; Tapia-Rojas, C.; Carvajal, F.J.; Hancke, J.; Cerpa, W.; Inestrosa, N.C. Andrographolide reduces cognitive impairment in young and mature AbetaPPswe/PS-1 mice. Mol. Neurodegener. 2014, 9, 61. [Google Scholar] [CrossRef] [PubMed]
- Rivera, D.S.; Lindsay, C.; Codocedo, J.F.; Morel, I.; Pinto, C.; Cisternas, P.; Bozinovic, F.; Inestrosa, N.C. Andrographolide recovers cognitive impairment in a natural model of Alzheimer’s disease (Octodon degus). Neurobiol. Aging 2016, 46, 204–220. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Mishra, K.; Ganju, L.; Singh, S. Andrographolide—A promising therapeutic agent, negatively regulates glial cell derived neurodegeneration of prefrontal cortex, hippocampus and working memory impairment. J. Neuroimmunol. 2017, 313, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Pyo, E.; An, J.P.; Kim, J.; Sung, S.H.; Oh, W.K. Andrographolide Activates Keap1/Nrf2/ARE/HO-1 Pathway in HT22 Cells and Suppresses Microglial Activation by Aβ42 through Nrf2-Related Inflammatory Response. Mediat. Inflamm. 2017, 2017, 5906189. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Liu, S.; Zhou, J.; Bu, S.; Zhang, J. Andrographolide attenuates microglia-mediated Aβ neurotoxicity partially through inhibiting NF-κB and JNK-MAPK signaling pathway. Immunopharmacol. Immunotoxicol. 2017, 39, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Liu, Y.; Sun, M. Autophagy and Alzheimer’s Disease. Cell. Mol. Neurobiol. 2017, 37, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Ntsapi, C.; Lumkwana, D.; Swart, C.; du Toit, A.; Loos, B. New Insights Into Autophagy Dysfunction Related to Amyloid Beta Toxicity and Neuropathology in Alzheimer’s Disease. Int. Rev. Cell Mol. Biol. 2018, 336, 321–361. [Google Scholar] [PubMed]
- Yuwen, D.; Mi, S.; Ma, Y.; Guo, W.; Xu, Q.; Shen, Y.; Shu, Y. Andrographolide enhances cisplatin-mediated anticancer effects in lung cancer cells through blockade of autophagy. Anti-Cancer Drugs 2017, 28, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Mi, S.; Xiang, G.; Yuwen, D.; Gao, J.; Guo, W.; Wu, X.; Wu, X.; Sun, Y.; Su, Y.; Shen, Y.; et al. Inhibition of autophagy by andrographolide resensitizes cisplatin-resistant non-small cell lung carcinoma cells via activation of the Akt/mTOR pathway. Toxicol. Appl. Pharmacol. 2016, 310, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Zou, J.; Yan, L.; Yu, X.; Lu, P.; Wu, X.; Li, Q.; Gu, R.; Zhu, D. Andrographolide Induces Autophagic Cell Death and Inhibits Invasion and Metastasis of Human Osteosarcoma Cells in An Autophagy-Dependent Manner. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2017, 44, 1396–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Qiu, X. Andrographolide radiosensitizes human ovarian cancer SKOV3 xenografts due to an enhanced apoptosis and autophagy. Tumour Biol. J. Int. Soc. Oncodev. Biol. Med. 2015, 36, 8359–8365. [Google Scholar] [CrossRef] [PubMed]
- Han, X.J.; Hu, Y.Y.; Yang, Z.J.; Jiang, L.P.; Shi, S.L.; Li, Y.R.; Guo, M.Y.; Wu, H.L.; Wan, Y.Y. Amyloid beta-42 induces neuronal apoptosis by targeting mitochondria. Mol. Med. Rep. 2017, 16, 4521–4528. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mandal, A.K.; Son, Y.O.; Pratheeshkumar, P.; Wise, J.T.F.; Wang, L.; Zhang, Z.; Shi, X.; Chen, Z. Roles of ROS, Nrf2, and autophagy in cadmium-carcinogenesis and its prevention by sulforaphane. Toxicol. Appl. Pharmacol. 2018, 353, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Calkins, M.J.; Johnson, D.A.; Townsend, J.A.; Vargas, M.R.; Dowell, J.A.; Williamson, T.P.; Kraft, A.D.; Lee, J.M.; Li, J.; Johnson, J.A. The Nrf2/ARE pathway as a potential therapeutic target in neurodegenerative disease. Antioxid. Redox Signal. 2009, 11, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, Y.; Zhou, X.; Dai, P.; Li, C. Autophagy involved in the activation of the Nrf2-antioxidant system in testes of heat-exposed mice. J. Therm. Biol. 2018, 71, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K.; Haapasalo, A.; Hiltunen, M.; Soininen, H.; Alafuzoff, I. Emerging role of p62/sequestosome-1 in the pathogenesis of Alzheimer’s disease. Prog. Neurobiol. 2012, 96, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, Y.M.; Lee, Y.C.; Cheng, W.T.; Shih, H.N.; Wang, H.C.; Rao, Y.K.; Lee, M.J. Effects of andrographolide and 14-deoxy-11,12-didehydroandrographolide on cultured primary astrocytes and PC12 cells. Life Sci. 2012, 90, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Alzaharna, M.; Alqouqa, I.; Cheung, H.-Y. Taxifolin synergizes Andrographolide-induced cell death by attenuation of autophagy and augmentation of caspase dependent and independent cell death in HeLa cells. PLoS ONE 2017, e0171325. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Feng, L.; Nie, H.; Zheng, X. Andrographolide induces autophagic cell death in human liver cancer cells through cyclophilin D-mediated mitochondrial permeability transition pore. Carcinogenesis 2012, 33, 2190–2198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M.A.; Sooro, M.A.; Zhang, P. Autophagic Regulation of p62 is Critical for Cancer Therapy. Int. J. Mol. Sci. 2018, 19, 1405. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Y.; Wang, X.L.; Peng, Y. Role of Nrf2 in neurodegenerative diseases and recent progress of its activators. Acta Pharm. Sin. 2015, 50, 375–384. [Google Scholar]
- Jo, C.; Gundemir, S.; Pritchard, S.; Jin, Y.N.; Rahman, I.; Johnson, G.V. Nrf2 reduces levels of phosphorylated tau protein by inducing autophagy adaptor protein NDP52. Nat. Commun. 2014, 5, 3496. [Google Scholar] [CrossRef] [PubMed]
- Santarino, I.B.; Viegas, M.S.; Domingues, N.S.; Ribeiro, A.M.; Soares, M.P.; Vieira, O.V. Involvement of the p62/NRF2 signal transduction pathway on erythrophagocytosis. Sci. Rep. 2017, 7, 5812. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, K.; Mimura, J.; Itoh, K.; Satoh, T.; Shimojo, Y.; Kitajima, C.; Maruyama, A.; Yamamoto, M.; Shirasawa, T. Role of Nrf2 and p62/ZIP in the neurite outgrowth by carnosic acid in PC12h cells. J. Biochem. 2010, 147, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Pun, T.N.; Park, P.H. Role of p62 in the suppression of inflammatory cytokine production by adiponectin in macrophages: Involvement of autophagy and p21/Nrf2 axis. Sci. Rep. 2017, 7, 393. [Google Scholar] [CrossRef]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gu, L.; Yu, Q.; Li, Q.; Zhang, L.; Lu, H.; Zhang, X. Andrographolide Protects PC12 Cells Against β-Amyloid-Induced Autophagy-Associated Cell Death Through Activation of the Nrf2-Mediated p62 Signaling Pathway. Int. J. Mol. Sci. 2018, 19, 2844. https://doi.org/10.3390/ijms19092844
Gu L, Yu Q, Li Q, Zhang L, Lu H, Zhang X. Andrographolide Protects PC12 Cells Against β-Amyloid-Induced Autophagy-Associated Cell Death Through Activation of the Nrf2-Mediated p62 Signaling Pathway. International Journal of Molecular Sciences. 2018; 19(9):2844. https://doi.org/10.3390/ijms19092844
Chicago/Turabian StyleGu, Lili, Qingqing Yu, Qin Li, Lingxi Zhang, Hong Lu, and Xinyue Zhang. 2018. "Andrographolide Protects PC12 Cells Against β-Amyloid-Induced Autophagy-Associated Cell Death Through Activation of the Nrf2-Mediated p62 Signaling Pathway" International Journal of Molecular Sciences 19, no. 9: 2844. https://doi.org/10.3390/ijms19092844




