Perivascular Adipose Tissue-Enhanced Vasodilation in Metabolic Syndrome Rats by Apelin and N-Acetyl–l-Cysteine-Sensitive Factor(s)
Abstract
:1. Introduction
2. Results
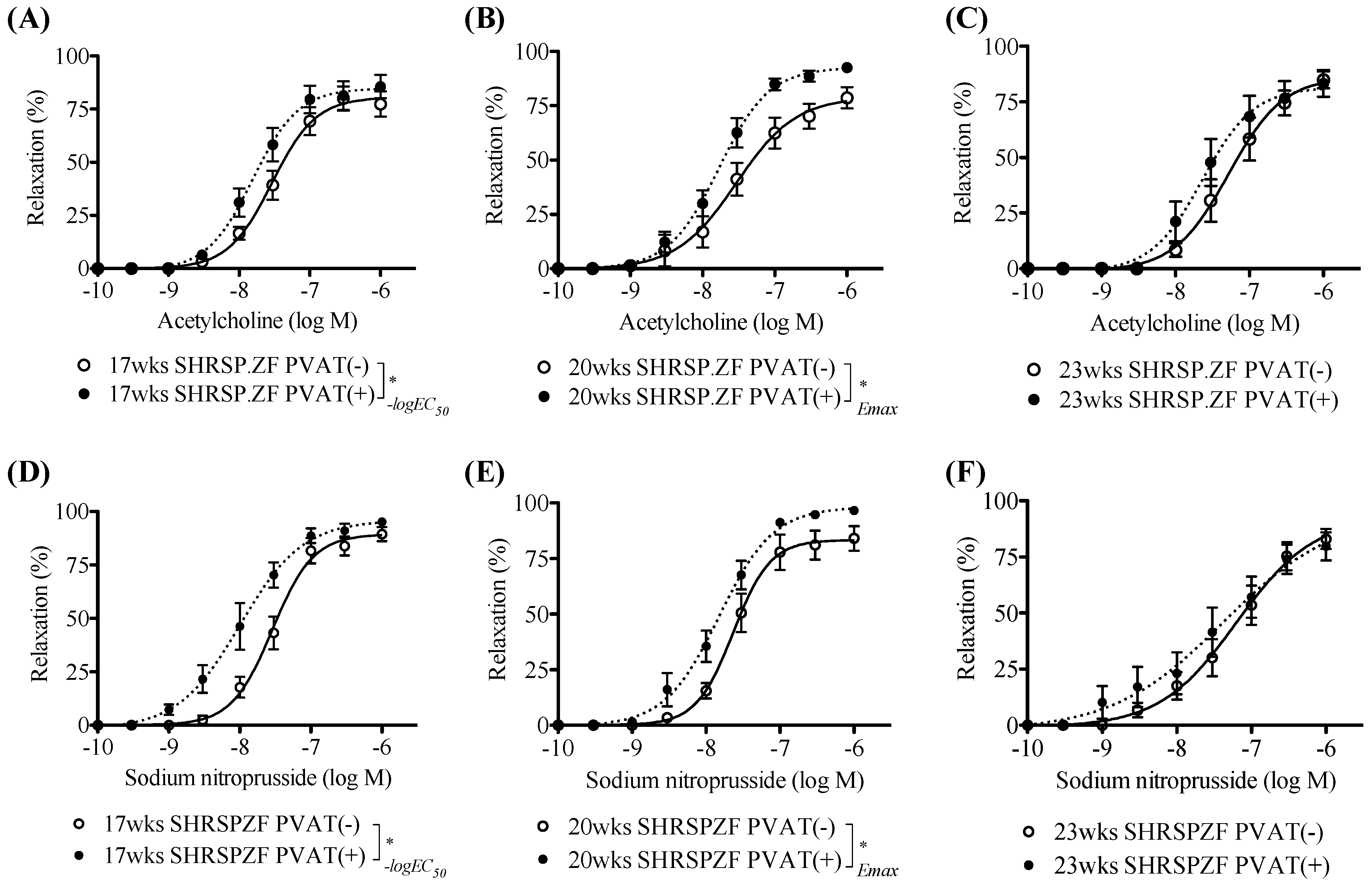
2.1. Changes in PVAT Effects on Relaxation in Superior Mesenteric Arteries from SHRSP.ZF with Aging
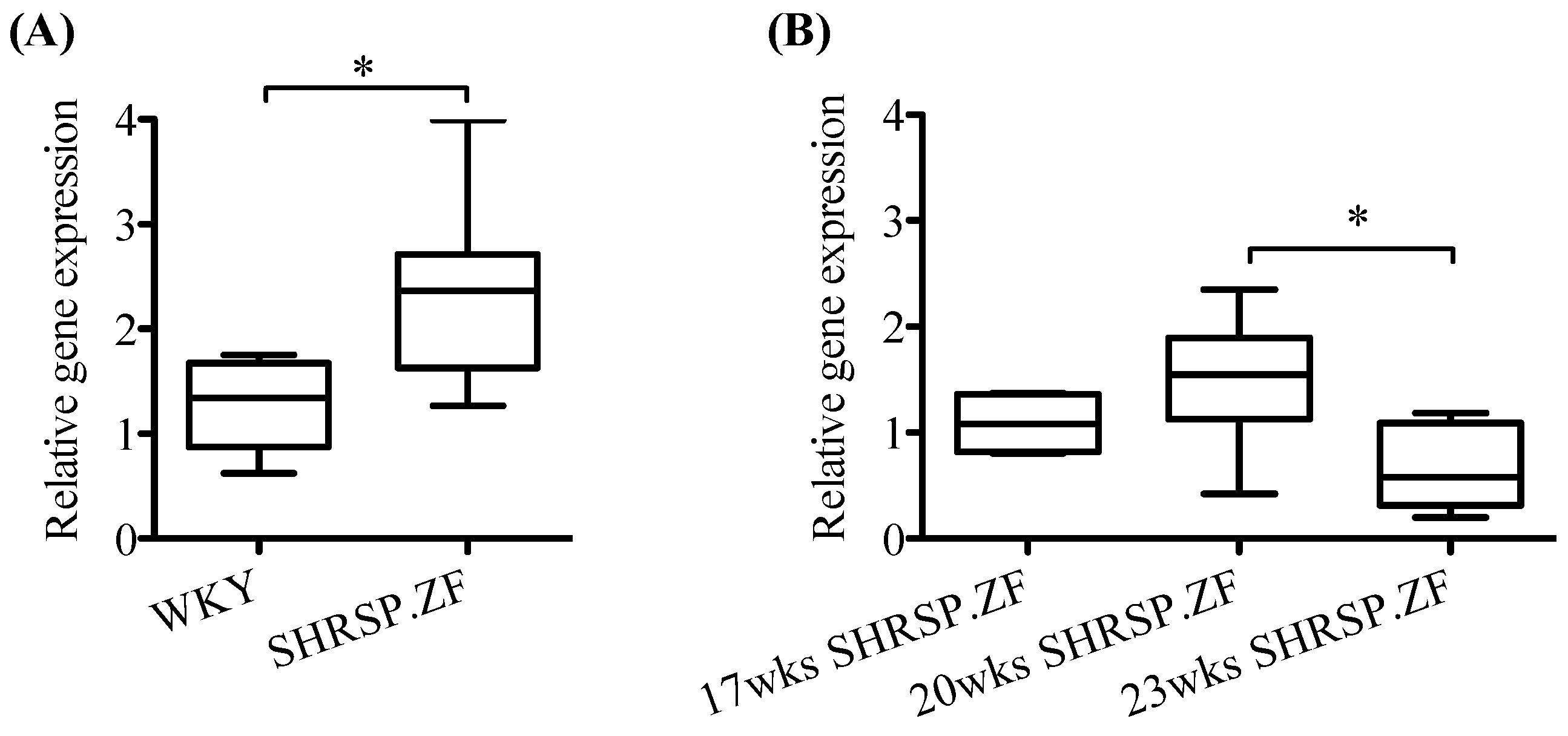
2.2. Changes in mRNA Levels of AT1 Receptor, ATRAP, and Angiotensinogen in Mesenteric Arterial PVAT of SHRSP.ZF with Aging
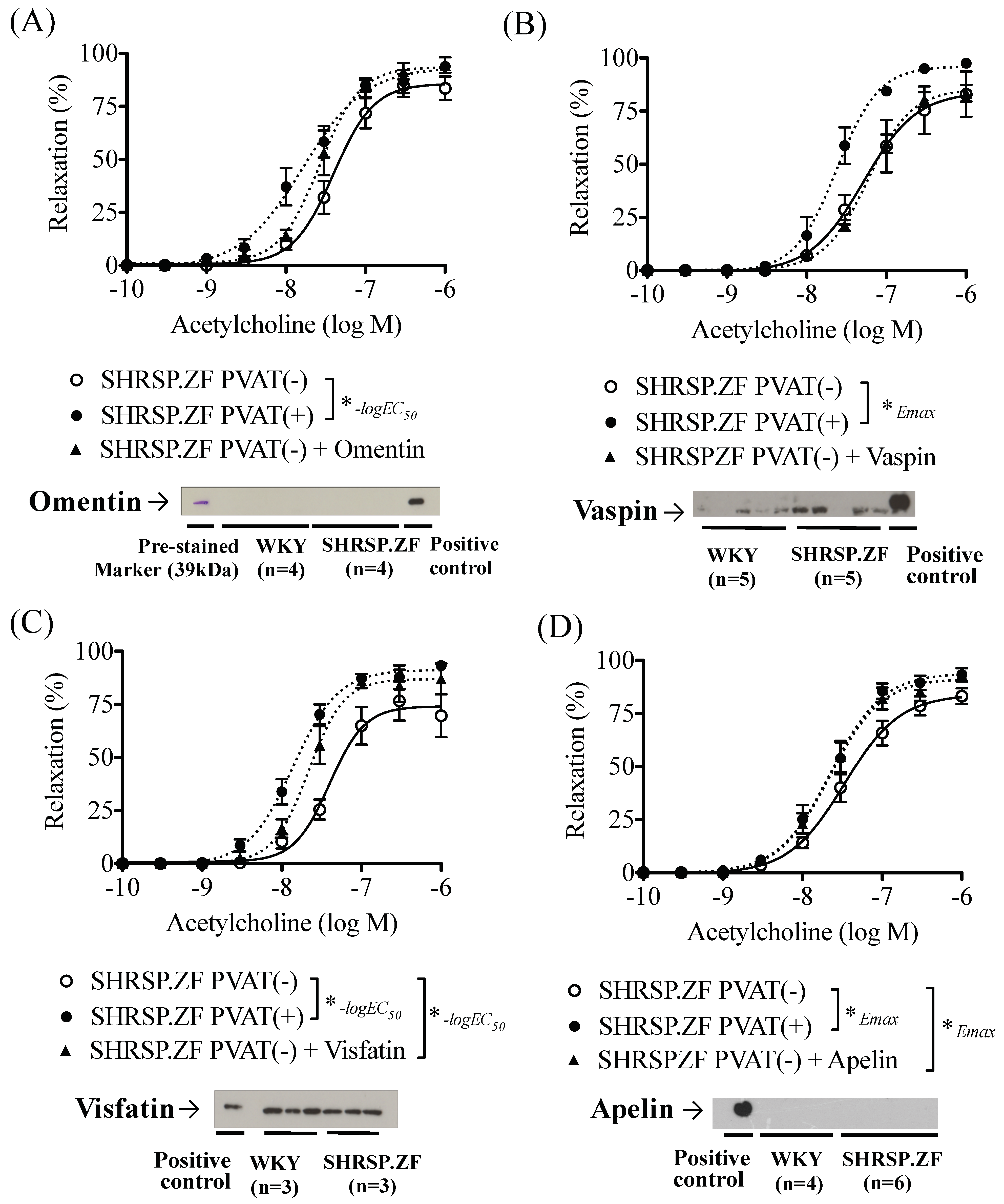
2.3. Effects of Adipokines on the Relaxation in SHRSP.ZF Mesenteric Arteries without PVAT
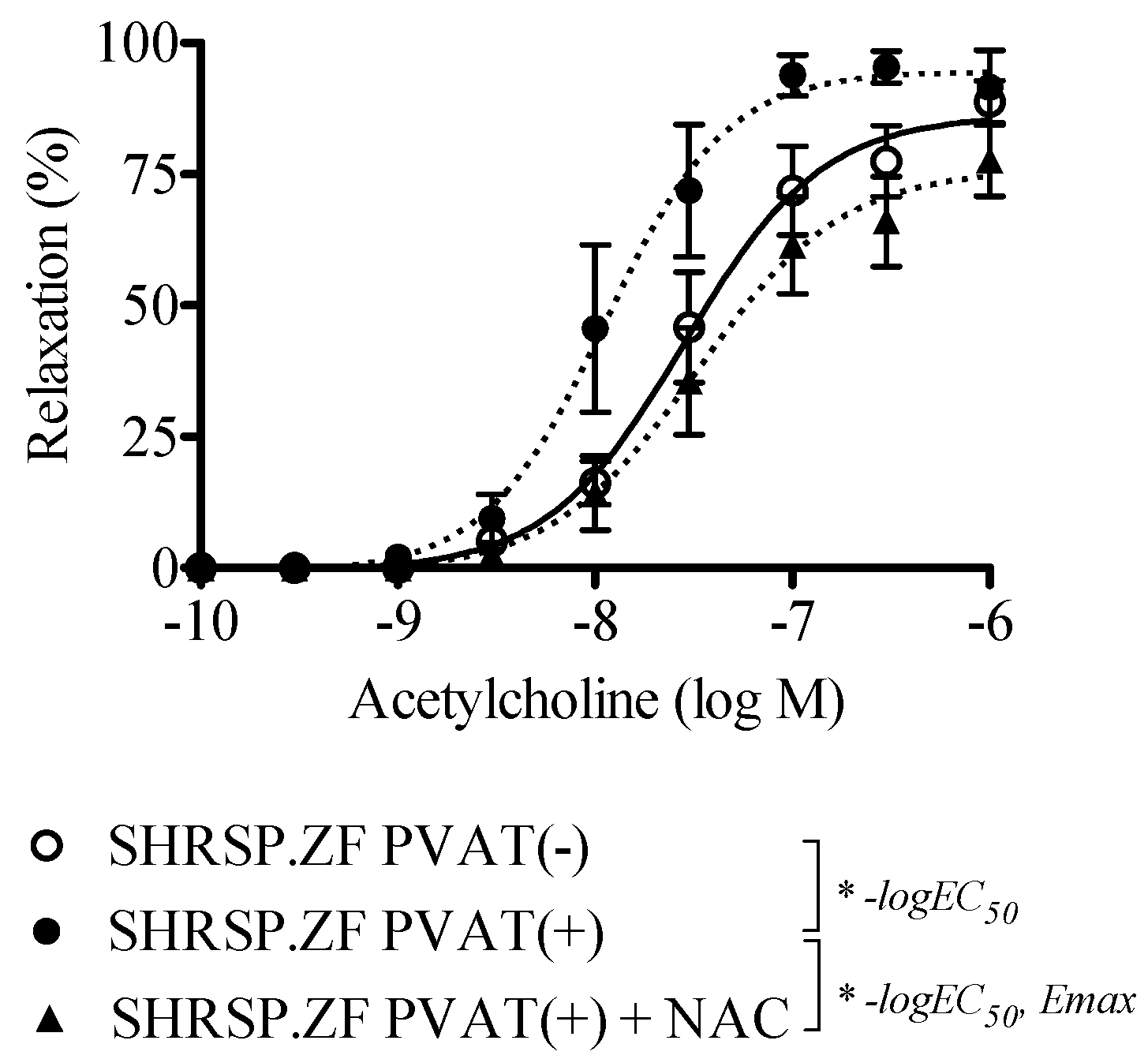
2.4. Effects of NAC on the Relaxation in SHRSP.ZF Mesenteric Arteries with PVAT
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Chemicals
4.3. Determination of Metabolic Parameters
4.4. Determination of Vasodilation
4.5. Determination of mRNA and Protein Expression
4.6. Data Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lian, X.; Gollasch, M. A clinical perspective: Contribution of dysfunctional perivascular adipose tissue (PVAT) to cardiovascular risk. Curr. Hypertens. Rep. 2016, 18, 82. [Google Scholar] [CrossRef] [PubMed]
- Nosalski, R.; Guzik, T.J. Perivascular adipose tissue inflammation in vascular disease. Br. J. Pharmacol. 2017, 174, 3496–3513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hiraoka-Yamamoto, J.; Nara, Y.; Yasui, N.; Onobayashi, Y.; Tsuchikura, S.; Ikeda, K. Establishment of a new animal model of metabolic syndrome: SHRSP fatty (fa/fa) rats. Clin. Exp. Pharmacol. Physiol. 2004, 31, 107–109. [Google Scholar] [CrossRef] [PubMed]
- Kagota, S.; Iwata, S.; Maruyama, K.; McGuire, J.J.; Shinozuka, K. Time-dependent differences in the influence of perivascular adipose tissue on vasomotor functions in metabolic syndrome. Metab. Syndr. Relat. Disord. 2017, 15, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.G.; O’Malley, E.J.; Ho, W.S.V. Pro-contractile effects of perivascular fat in health and disease. Br. J. Pharmacol. 2017, 174, 3482–3495. [Google Scholar] [CrossRef] [Green Version]
- Sawicka, M.; Janowska, J.; Chudek, J. Potential beneficial effect of some adipokines positively correlated with the adipose tissue content on the cardiovascular system. Int. J. Cardiol. 2016, 222, 581–589. [Google Scholar] [CrossRef]
- Kagota, S.; Fukushima, K.; Umetani, K.; Tada, Y.; Nejime, N.; Nakamura, K.; Mori, H.; Sugimura, K.; Kunitomo, M.; Shinozuka, K. Coronary vascular dysfunction promoted by oxidative-nitrative stress in SHRSP.Z-Lepr(fa) /IzmDmcr rats with metabolic syndrome. Clin. Exp. Pharmacol. Physiol. 2010, 37, 1035–1043. [Google Scholar] [CrossRef]
- Kagota, S.; Tada, Y.; Nejime, N.; Nakamura, K.; Kunitomo, M.; Shinozuka, K. Telmisartan provides protection against development of impaired vasodilation independently of metabolic effects in SHRSP.Z-Leprfa/IzmDmcr rats with metabolic syndrome. Can. J. Physiol. Pharmacol. 2011, 89, 355–364. [Google Scholar] [CrossRef]
- Daviet, L.; Lehtonen, J.Y.; Tamura, K.; Griese, D.P.; Horiuchi, M.; Dzau, V.J. Cloning and characterization of ATRAP, a novel protein that interacts with the angiotensin II type 1 receptor. J. Biol. Chem. 1999, 274, 17058–17062. [Google Scholar] [CrossRef]
- Lopez-Ilasaca, M.; Liu, X.; Tamura, K.; Dzau, V.J. The angiotensin II type I receptor-associated protein, ATRAP, is a transmembrane protein and a modulator of angiotensin II signaling. Mol. Biol. Cell 2003, 14, 5038–5050. [Google Scholar] [CrossRef]
- Maeda, A.; Tamura, K.; Wakui, H.; Dejima, T.; Ohsawa, M.; Azushima, K.; Kanaoka, T.; Uneda, K.; Matsuda, M.; Yamashita, A.; et al. Angiotensin receptor-binding protein ATRAP/Agtrap inhibits metabolic dysfunction with visceral obesity. J. Am. Heart Assoc. 2013, 2, e000312. [Google Scholar] [CrossRef] [PubMed]
- Azushima, K.; Ohki, K.; Wakui, H.; Uneda, K.; Haku, S.; Kobayashi, R.; Haruhara, K.; Kinguchi, S.; Matsuda, M.; Maeda, A.; et al. Adipocyte-specific enhancement of angiotensin II type 1 receptor-associated protein ameliorates diet-induced visceral obesity and insulin resistance. J. Am. Heart Assoc. 2017, 6, e004488. [Google Scholar] [CrossRef] [PubMed]
- Tatemoto, K.; Takayama, K.; Zou, M.X.; Kumaki, I.; Zhang, W.; Kumano, K.; Fujimiya, M. The novel peptide apelin lowers blood pressure via a nitric oxide-dependent mechanism. Regul. Pept. 2001, 99, 87–92. [Google Scholar] [CrossRef]
- Japp, A.G.; Cruden, N.L.; Amer, D.A.; Li, V.K.; Goudie, E.B.; Johnston, N.R.; Sharma, S.; Neilson, I.; Webb, D.J.; Megson, I.L.; et al. Vascular effects of apelin in vivo in man. J. Am. Coll. Cardiol. 2008, 52, 908–913. [Google Scholar] [CrossRef]
- Yamawaki, H.; Hara, N.; Okada, M.; Hara, Y. Visfatin causes endothelium-dependent relaxation in isolated blood vessels. Biochem. Biophys. Res. Commun. 2009, 383, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Kameshima, S.; Sakamoto, Y.; Okada, M.; Yamawaki, H. Vaspin prevents elevation of blood pressure through inhibition of peripheral vascular remodelling in spontaneously hypertensive rats. Acta Physiol. 2016, 217, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Krist, J.; Wieder, K.; Kloting, N.; Oberbach, A.; Kralisch, S.; Wiesner, T.; Schon, M.R.; Gartner, D.; Dietrich, A.; Shang, E.; et al. Effects of weight loss and exercise on apelin serum concentrations and adipose tissue expression in human obesity. Obes. Facts 2013, 6, 57–69. [Google Scholar] [CrossRef]
- Fukuhara, A.; Matsuda, M.; Nishizawa, M.; Segawa, K.; Tanaka, M.; Kishimoto, K.; Matsuki, Y.; Murakami, M.; Ichisaka, T.; Murakami, H.; et al. Visfatin: A protein secreted by visceral fat that mimics the effects of insulin. Science 2005, 307, 426–430. [Google Scholar] [CrossRef]
- Kloting, N.; Berndt, J.; Kralisch, S.; Kovacs, P.; Fasshauer, M.; Schon, M.R.; Stumvoll, M.; Bluher, M. Vaspin gene expression in human adipose tissue: Association with obesity and type 2 diabetes. Biochem. Biophys. Res. Commun. 2006, 339, 430–436. [Google Scholar] [CrossRef]
- de Souza Batista, C.M.; Yang, R.Z.; Lee, M.J.; Glynn, N.M.; Yu, D.Z.; Pray, J.; Ndubuizu, K.; Patil, S.; Schwartz, A.; Kligman, M.; et al. Omentin plasma levels and gene expression are decreased in obesity. Diabetes 2007, 56, 1655–1661. [Google Scholar] [CrossRef]
- Yamawaki, H.; Tsubaki, N.; Mukohda, M.; Okada, M.; Hara, Y. Omentin, a novel adipokine, induces vasodilation in rat isolated blood vessels. Biochem. Biophys. Res. Commun. 2010, 393, 668–672. [Google Scholar] [PubMed]
- Fang, L.; Zhao, J.; Chen, Y.; Ma, T.; Xu, G.; Tang, C.; Liu, X.; Geng, B. Hydrogen sulfide derived from periadventitial adipose tissue is a vasodilator. J. Hypertens. 2009, 27, 2174–2185. [Google Scholar] [PubMed]
- Materazzi, S.; Zagli, G.; Nassini, R.; Bartolini, I.; Romagnoli, S.; Chelazzi, C.; Benemei, S.; Coratti, A.; De Gaudio, A.R.; Patacchini, R. Vasodilator activity of hydrogen sulfide (H2S) in human mesenteric arteries. Microvasc. Res. 2017, 109, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Berenyiova, A.; Grman, M.; Mijuskovic, A.; Stasko, A.; Misak, A.; Nagy, P.; Ondriasova, E.; Cacanyiova, S.; Brezova, V.; Feelisch, M.; et al. The reaction products of sulfide and S-nitrosoglutathione are potent vasorelaxants. Nitric Oxide 2015, 46, 123–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellis, A.; Li, C.G.; Rand, M.J. Differential actions of l-cysteine on responses to nitric oxide, nitroxyl anions and EDRF in the rat aorta. Br. J. Pharmacol. 2000, 129, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Wanstall, J.C.; Jeffery, T.K.; Gambino, A.; Lovren, F.; Triggle, C.R. Vascular smooth muscle relaxation mediated by nitric oxide donors: A comparison with acetylcholine, nitric oxide and nitroxyl ion. Br. J. Pharmacol. 2001, 134, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Favaloro, J.L.; Kemp-Harper, B.K. The nitroxyl anion (HNO) is a potent dilator of rat coronary vasculature. Cardiovasc. Res. 2007, 73, 587–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irvine, J.C.; Favaloro, J.L.; Kemp-Harper, B.K. NO-activates soluble guanylate cyclase and Kv channels to vasodilate resistance arteries. Hypertension 2003, 41, 1301–1307. [Google Scholar] [CrossRef]
- Favaloro, J.L.; Kemp-Harper, B.K. Redox variants of NO (NO{middle dot} and HNO) elicit vasorelaxation of resistance arteries via distinct mechanisms. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1274–H1280. [Google Scholar] [CrossRef]
- Kagota, S.; Maruyama, K.; Iwata, S.; Tada, Y. Impairment of vasodilation and effects of perivascular adipose tissue in metabolic syndrome. Nihon Yakurigaku Zasshi 2015, 145, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Kagota, S.; Iwata, S.; Maruyama, K.; Wakuda, H.; Shinozuka, K. Functional relationship between arterial tissue and perivascular adipose tissue in metabolic syndrome. Yakugaku Zasshi 2016, 136, 693–697. [Google Scholar] [CrossRef] [PubMed]
- Maeda, A.; Tamura, K.; Wakui, H.; Ohsawa, M.; Azushima, K.; Uneda, K.; Kobayashi, R.; Tsurumi-Ikeya, Y.; Kanaoka, T.; Dejima, T.; et al. Effects of Ang II receptor blocker irbesartan on adipose tissue function in mice with metabolic disorders. Int. J. Med. Sci. 2014, 11, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.; Lezama, M.A.; Ontiveros, J.A.; Bravo, G.; Villafana, S.; del-Rio-Navarro, B.E.; Hong, E. Effect of losartan on vascular function in fructose-fed rats: The role of perivascular adipose tissue. Clin. Exp. Hypertens. 2010, 32, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.C.; Yu, X.Y.; Huang, Y.; Yung, L.M.; Lau, C.W.; Lin, S.G. Apelin modulates aortic vascular tone via endothelial nitric oxide synthase phosphorylation pathway in diabetic mice. Cardiovasc. Res. 2007, 74, 388–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beltowski, J. Endogenous hydrogen sulfide in perivascular adipose tissue: Role in the regulation of vascular tone in physiology and pathology. Can. J. Physiol. Pharmacol. 2013, 91, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Cacanyiova, S.; Berenyiova, A.; Balis, P.; Kristek, F.; Grman, M.; Ondrias, K.; Breza, J.; Breza, J., Jr. Nitroso-sulfide coupled signaling triggers specific vasoactive effects in the intrarenal arteries of patients with arterial hypertension. J. Physiol. Pharmacol. 2017, 68, 527–538. [Google Scholar] [PubMed]
- Brancaleone, V.; Roviezzo, F.; Vellecco, V.; De Gruttola, L.; Bucci, M.; Cirino, G. Biosynthesis of H2S is impaired in non-obese diabetic (NOD) mice. Br. J. Pharmacol. 2008, 155, 673–680. [Google Scholar] [CrossRef]
- Beltowski, J.; Guranowski, A.; Jamroz-Wisniewska, A.; Wolski, A.; Halas, K. Hydrogen-sulfide-mediated vasodilatory effect of nucleoside 5′-monophosphorothioates in perivascular adipose tissue. Can. J. Physiol. Pharmacol. 2015, 93, 585–595. [Google Scholar] [CrossRef]
- Wojcicka, G.; Jamroz-Wisniewska, A.; Atanasova, P.; Chaldakov, G.N.; Chylinska-Kula, B.; Beltowski, J. Differential effects of statins on endogenous H2S formation in perivascular adipose tissue. Pharmacol. Res. 2011, 63, 68–76. [Google Scholar] [CrossRef]
- Yang, G.; Wu, L.; Jiang, B.; Yang, W.; Qi, J.; Cao, K.; Meng, Q.; Mustafa, A.K.; Mu, W.; Zhang, S.; et al. H2S as a physiologic vasorelaxant: Hypertension in mice with deletion of cystathionine gamma-lyase. Science 2008, 322, 587–590. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef]
- Baltieri, N.; Guizoni, D.M.; Victorio, J.A.; Davel, A.P. Protective role of perivascular adipose tissue in endothelial dysfunction and insulin-induced vasodilatation of hypercholesterolemic LDL receptor-deficient mice. Front. Physiol. 2018, 9, 229. [Google Scholar] [CrossRef]
- Kong, L.R.; Zhou, Y.P.; Chen, D.R.; Ruan, C.C.; Gao, P.J. Decrease of perivascular adipose tissue browning is associated with vascular dysfunction in spontaneous hypertensive rats during aging. Front. Physiol. 2018, 9, 400. [Google Scholar] [CrossRef]
- Ohki, K.; Wakui, H.; Azushima, K.; Uneda, K.; Haku, S.; Kobayashi, R.; Haruhara, K.; Kinguchi, S.; Matsuda, M.; Ohsawa, M.; et al. ATRAP expression in brown adipose tissue does not influence the development of diet-induced metabolic disorders in mice. Int. J. Mol. Sci. 2017, 18, 676. [Google Scholar] [CrossRef] [PubMed]
- Kubota, Y.; Umegaki, K.; Kagota, S.; Tanaka, N.; Nakamura, K.; Kunitomo, M.; Shinozuka, K. Evaluation of blood pressure measured by tail-cuff methods (without heating) in spontaneously hypertensive rats. Biol. Pharm. Bull. 2006, 29, 1756–1758. [Google Scholar] [CrossRef] [PubMed]
- Kameshima, S.; Yamada, K.; Morita, T.; Okada, M.; Yamawaki, H. Visceral adipose tissue-derived serine protease inhibitor augments acetylcholine-induced relaxation via the inhibition of acetylcholine esterase activity in rat isolated mesenteric artery. Acta Physiol. 2016, 216, 203–210. [Google Scholar] [CrossRef]
- Kagota, S.; Maruyama, K.; Wakuda, H.; McGuire, J.J.; Yoshikawa, N.; Nakamura, K.; Shinozuka, K. Disturbance of vasodilation via protease-activated receptor 2 in SHRSP.Z-Leprfa/IzmDmcr rats with metabolic syndrome. Vascul. Pharmacol. 2014, 63, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Kagota, S.; Tada, Y.; Nejime, N.; Nakamura, K.; Kunitomo, M.; Shinozuka, K. Chronic production of peroxynitrite in the vascular wall impairs vasorelaxation function in SHR/NDmcr-cp rats, an animal model of metabolic syndrome. J. Pharmacol. Sci. 2009, 109, 556–564. [Google Scholar] [CrossRef] [PubMed]




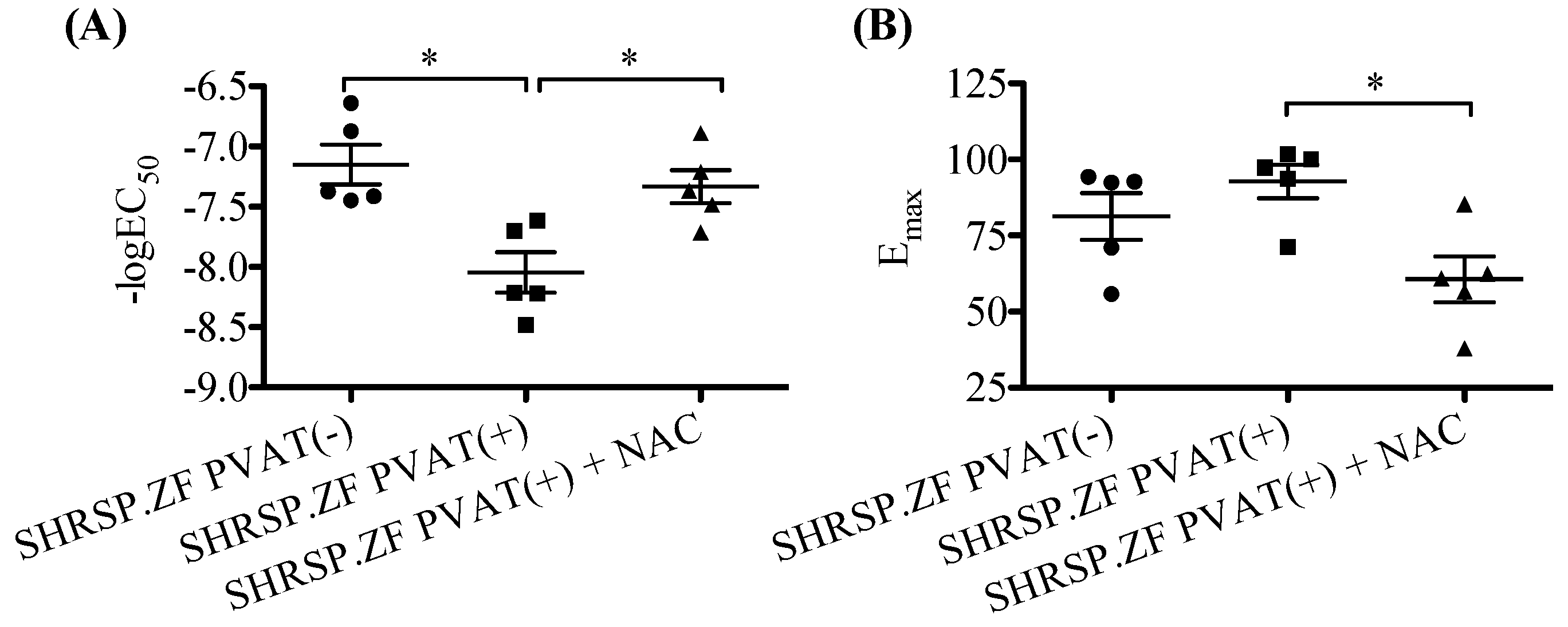
| SHRSP.ZF | |||
|---|---|---|---|
| 17 wks | 20 wks | 23 wks | |
| Body weight (g) | 414 ± 5 | 438 ± 7 | 462 ± 10 * |
| Waist length/body length (cm/cm) | 1.03 ± 0.01 | 1.00 ± 0.01 | 1.03 ± 0.01 |
| Systolic blood pressure (mmHg) | 215 ± 7 | 205 ± 8 | 234 ± 9 # |
| Serum triglycerides (mg/100 mL) | 749 ± 35 | 558 ± 71 | 709 ± 68 |
| Serum glucose (mg/100 mL) | 386 ± 24 | 328 ± 29 | 360 ± 24 |
| Serum insulin (ng/mL) | 49.8 ± 5.5 | 41.3 ± 5.2 | 63.2 ± 5.2 # |
| Serum TBARS (µM) | 14.7 ± 0.9 | 13.1 ± 0.9 | 19.9 ± 1.8 *, # |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kagota, S.; Maruyama-Fumoto, K.; Iwata, S.; Shimari, M.; Koyanagi, S.; Shiokawa, Y.; McGuire, J.J.; Shinozuka, K. Perivascular Adipose Tissue-Enhanced Vasodilation in Metabolic Syndrome Rats by Apelin and N-Acetyl–l-Cysteine-Sensitive Factor(s). Int. J. Mol. Sci. 2019, 20, 106. https://doi.org/10.3390/ijms20010106
Kagota S, Maruyama-Fumoto K, Iwata S, Shimari M, Koyanagi S, Shiokawa Y, McGuire JJ, Shinozuka K. Perivascular Adipose Tissue-Enhanced Vasodilation in Metabolic Syndrome Rats by Apelin and N-Acetyl–l-Cysteine-Sensitive Factor(s). International Journal of Molecular Sciences. 2019; 20(1):106. https://doi.org/10.3390/ijms20010106
Chicago/Turabian StyleKagota, Satomi, Kana Maruyama-Fumoto, Saki Iwata, Miho Shimari, Shiori Koyanagi, Yayoi Shiokawa, John J. McGuire, and Kazumasa Shinozuka. 2019. "Perivascular Adipose Tissue-Enhanced Vasodilation in Metabolic Syndrome Rats by Apelin and N-Acetyl–l-Cysteine-Sensitive Factor(s)" International Journal of Molecular Sciences 20, no. 1: 106. https://doi.org/10.3390/ijms20010106





