Recipient ADAMTS13 Single-Nucleotide Polymorphism Predicts Relapse after Unrelated Bone Marrow Transplantation for Hematologic Malignancy
Abstract
1. Introduction
2. Methods
2.1. Patients
2.2. Genotyping
2.3. Data Management and Statistical Analyses
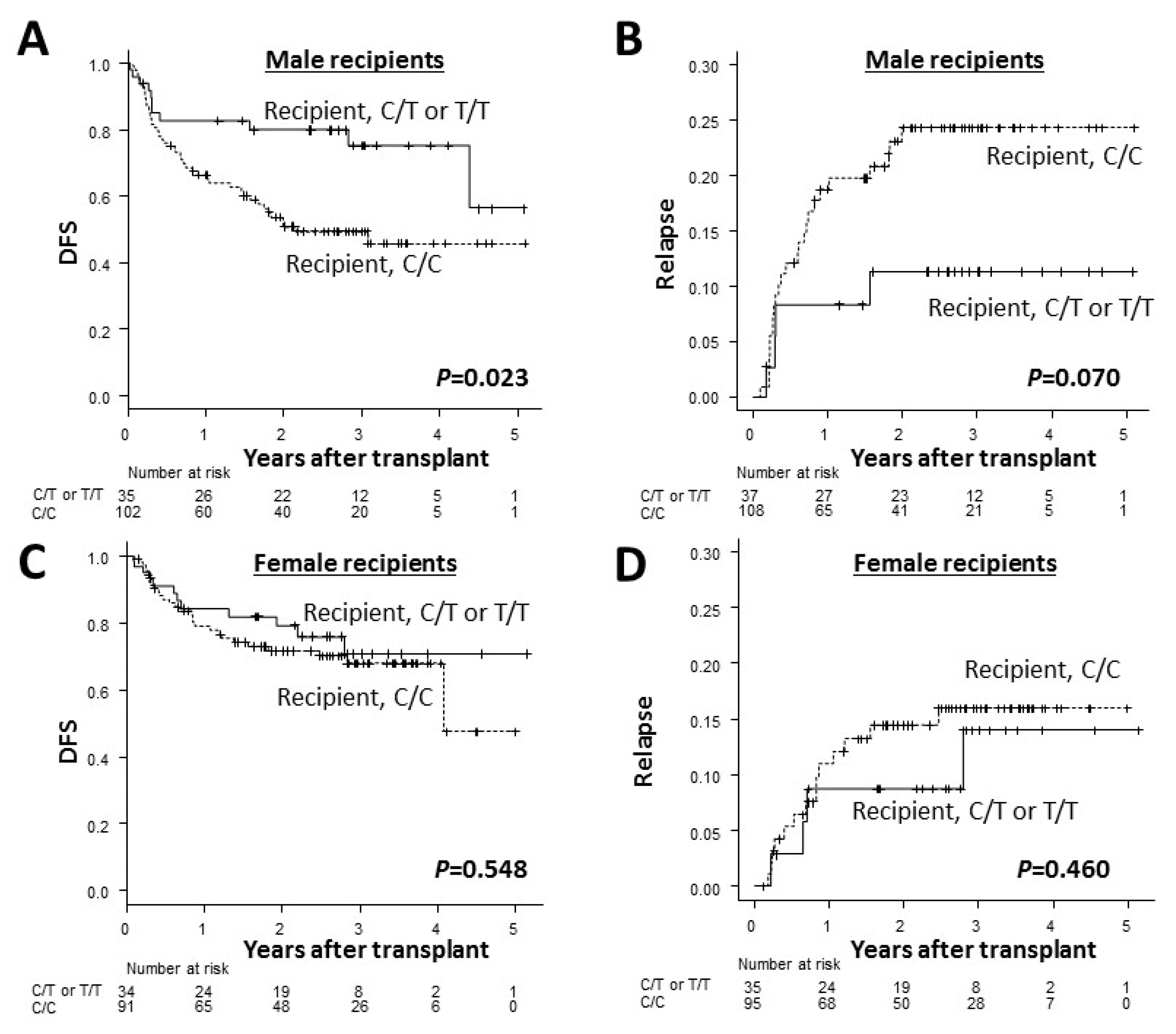
3. Results
3.1. Frequencies of ADAMTS13 Genotypes
3.2. Transplant Outcomes According to ADAMTS13 Genotypes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schriber, J.; Agovi, M.A.; Ho, V.; Ballen, K.K.; Bacigalupo, A.; Lazarus, H.M.; Bredeson, C.N.; Gupta, V.; Maziarz, R.T.; Hale, G.A.; et al. Second unrelated donor hematopoietic cell transplantation for primary graft failure. Biol. Blood Marrow Transplant. 2010, 16, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Takami, A.; Yano, S.; Yokoyama, H.; Kuwatsuka, Y.; Yamaguchi, T.; Kanda, Y.; Morishima, Y.; Fukuda, T.; Miyazaki, Y.; Nakamae, H.; et al. Donor lymphocyte infusion for the treatment of relapsed acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation: A retrospective analysis by the Adult Acute Myeloid Leukemia Working Group of the Japan Society for Hematopoietic Cell Transplantation. Biol. Blood Marrow Transplant. 2014, 20, 1785–1790. [Google Scholar] [PubMed]
- Schmid, C.; Labopin, M.; Nagler, A.; Niederwieser, D.; Castagna, L.; Tabrizi, R.; Stadler, M.; Kuball, J.; Cornelissen, J.; Vorlicek, J.; et al. Treatment, risk factors, and outcome of adults with relapsed AML after reduced intensity conditioning for allogeneic stem cell transplantation. Blood 2012, 119, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Ayuk, F.; Beelen, D.W.; Bornhauser, M.; Stelljes, M.; Zabelina, T.; Finke, J.; Kobbe, G.; Wolff, D.; Wagner, E.M.; Christopeit, M.; et al. Relative Impact of HLA Matching and Non-HLA Donor Characteristics on Outcomes of Allogeneic Stem Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndrome. Biol. Blood Marrow Transplant. 2018, 24, 2558–2567. [Google Scholar] [CrossRef] [PubMed]
- Nakasone, H.; Remberger, M.; Tian, L.; Brodin, P.; Sahaf, B.; Wu, F.; Mattsson, J.; Lowsky, R.; Negrin, R.; Miklos, D.B.; et al. Risks and benefits of sex-mismatched hematopoietic cell transplantation differ according to conditioning strategy. Haematologica 2015, 100, 1477–1485. [Google Scholar] [CrossRef]
- Kim, H.T.; Zhang, M.J.; Woolfrey, A.E.; St Martin, A.; Chen, J.; Saber, W.; Perales, M.A.; Armand, P.; Eapen, M. Donor and recipient sex in allogeneic stem cell transplantation: What really matters. Haematologica 2016, 101, 1260–1266. [Google Scholar] [CrossRef]
- Espinoza, J.L.; Takami, A.; Onizuka, M.; Sao, H.; Akiyama, H.; Miyamura, K.; Okamoto, S.; Inoue, M.; Kanda, Y.; Ohtake, S.; et al. NKG2D gene polymorphism has a significant impact on transplant outcomes after HLA-fully-matched unrelated bone marrow transplantation for standard risk hematologic malignancies. Haematologica 2009, 94, 1427–1434. [Google Scholar] [CrossRef]
- Espinoza, J.L.; Takami, A.; Onizuka, M.; Morishima, Y.; Fukuda, T.; Kodera, Y.; Akiyama, H.; Miyamura, K.; Mori, T.; Nakao, S.; et al. Recipient PTPN22-1123 C/C genotype predicts acute graft-versus-host disease after HLA fully matched unrelated bone marrow transplantation for hematologic malignancies. Biol. Blood Marrow Transplant. 2013, 19, 240–246. [Google Scholar] [CrossRef]
- Fujimura, Y.; Matsumoto, M. Registry of 919 patients with thrombotic microangiopathies across Japan: Database of Nara Medical University during 1998–2008. Intern. Med. 2010, 49, 7–15. [Google Scholar] [CrossRef]
- Oleksowicz, L.; Bhagwati, N.; DeLeon-Fernandez, M. Deficient Activity of von Willebrand’s Factor-cleaving Protease in Patients with Disseminated Malignancies. Cancer Res. 1999, 59, 2244–2250. [Google Scholar]
- Liu, C.; Zhao, L.; Zhao, J.; Xu, Q.; Song, Y.; Wang, H. Reduced ADAMTS-13 level negatively correlates with inflammation factors in plasma of acute myeloid leukemia patients. Leuk. Res. 2017, 53, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhao, L.; Zhao, J.; Xu, Q.; Song, Y.; Wang, H. Decreased ADAMTS-13 level is related to inflammation factors and risk stratification of acute lymphoblastic leukemia patients. Medicine 2017, 96, e6136. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Han, M.; Zhao, L.; Zhu, M.; Xu, Q.; Song, Y.; Wang, H. ADAMTS-13 activity reduction in plasma of acute myeloid leukemia predicts poor prognosis after bone marrow transplantation. Hematology (Amsterdam, Netherlands) 2019, 24, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Stoll, M.; Ruhle, F.; Witten, A.; Barysenka, A.; Arning, A.; Strauss, C.; Nowak-Gottl, U. Rare Variants in the ADAMTS13 Von Willebrand Factor-Binding Domain Contribute to Pediatric Stroke. Circ. Cardiovasc. Genet. 2016, 9, 357–367. [Google Scholar] [CrossRef] [PubMed]
- Giralt, S.; Ballen, K.; Rizzo, D.; Bacigalupo, A.; Horowitz, M.; Pasquini, M.; Sandmaier, B. Reduced-intensity conditioning regimen workshop: Defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol. Blood Marrow Transplant. 2009, 15, 367–369. [Google Scholar] [CrossRef] [PubMed]
- Storb, R.; Deeg, H.J.; Whitehead, J.; Appelbaum, F.; Beatty, P.; Bensinger, W.; Buckner, C.D.; Clift, R.; Doney, K.; Farewell, V.; et al. Methotrexate and cyclosporine compared with cyclosporine alone for prophylaxis of acute graft versus host disease after marrow transplantation for leukemia. N. Engl. J. Med. 1986, 314, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Nash, R.A.; Antin, J.H.; Karanes, C.; Fay, J.W.; Avalos, B.R.; Yeager, A.M.; Przepiorka, D.; Davies, S.; Petersen, F.B.; Bartels, P.; et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood 2000, 96, 2062–2068. [Google Scholar]
- Przepiorka, D.; Weisdorf, D.; Martin, P.; Klingemann, H.G.; Beatty, P.; Hows, J.; Thomas, E.D. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995, 15, 825–828. [Google Scholar]
- Shulman, H.M.; Sullivan, K.M.; Weiden, P.L.; McDonald, G.B.; Striker, G.E.; Sale, G.E.; Hackman, R.; Tsoi, M.S.; Storb, R.; Thomas, E.D. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am. J. Med. 1980, 69, 204–217. [Google Scholar] [CrossRef]
- Kanda, Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Gooley, T.A.; Leisenring, W.; Crowley, J.; Storer, B.E. Estimation of failure probabilities in the presence of competing risks: New representations of old estimators. Stat. Med. 1999, 18, 695–706. [Google Scholar] [CrossRef]
- Scrucca, L.; Santucci, A.; Aversa, F. Competing risk analysis using R: An easy guide for clinicians. Bone Marrow Transplant. 2007, 40, 381–387. [Google Scholar] [CrossRef]
- Hatzipantelis, E.S.; Athanassiou-Metaxa, M.; Gombakis, N.; Tzimouli, V.; Taparkou, A.; Sidi-Fragandrea, V.; Garipidou, V.; Papageorgiou, T.; Kleta, D.; Koliouskas, D.E.; et al. Thrombomodulin and von Willebrand factor: Relation to endothelial dysfunction and disease outcome in children with acute lymphoblastic leukemia. Acta Haematol. 2011, 125, 130–135. [Google Scholar] [CrossRef]
- Athale, U.; Moghrabi, A.; Nayiager, T.; Delva, Y.L.; Thabane, L.; Chan, A.K. von Willebrand factor and thrombin activation in children with newly diagnosed acute lymphoblastic leukemia: An impact of peripheral blasts. Pediatr. Blood Cancer 2010, 54, 963–969. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Dou, M.; Du, X.; Ma, L.; Sun, P.; Cao, H.; Ye, S.; Jiang, P.; Liu, F.; Lin, F.; et al. Influences of ABO blood group, age and gender on plasma coagulation factor VIII, fibrinogen, von Willebrand factor and ADAMTS13 levels in a Chinese population. PeerJ 2017, 5, e3156. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.D.; Yoshioka, A.; Kawa, K.; Ishizashi, H.; Yagi, H.; Yamamoto, Y.; Matsumoto, M.; Fujimura, Y. Impaired activity of plasma von Willebrand factor-cleaving protease may predict the occurrence of hepatic veno-occlusive disease after stem cell transplantation. Bone Marrow Transplant. 2002, 29, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Kentouche, K.; Zintl, F.; Angerhaus, D.; Fuchs, D.; Hermann, J.; Schneppenheim, R.; Budde, U. von Willebrand factor-cleaving protease (ADAMTS13) in the course of stem cell transplantation. Semin. Thromb. Hemost. 2006, 32, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Bari, R.; Rujkijyanont, P.; Sullivan, E.; Kang, G.; Turner, V.; Gan, K.; Leung, W. Effect of donor KIR2DL1 allelic polymorphism on the outcome of pediatric allogeneic hematopoietic stem-cell transplantation. J. Clin. Oncol. 2013, 31, 3782–3790. [Google Scholar] [CrossRef]
- Shaffer, B.C.; Hsu, K.C. How important is NK alloreactivity and KIR in allogeneic transplantation? Best Pract. Res. Clin. Haematol. 2016, 29, 351–358. [Google Scholar] [CrossRef]
- Fleischhauer, K.; Beelen, D.W. HLA mismatching as a strategy to reduce relapse after alternative donor transplantation. Semin. Hematol. 2016, 53, 57–64. [Google Scholar] [CrossRef]



| Recipient ADAMTS13 Genotype, n (%) | ||||
|---|---|---|---|---|
| Variable | Total | C/C, 206 (73.3%) | C/T, 70 (24.9%) T/T, 5 (1.8%) | P |
| Number of cases | 281 | 206 | 75 | |
| Recipient age, years, median (range) | 47 (1–66) | 47 (2–66) | 46 (1–65) | 0.874 |
| Donor age, years, median (range) | 34 (20–66) | 34 (20–66) | 34 (21–55) | 0.582 |
| Year of HSCT, median (range) | 2008 (2006–2009) | 2008 (2006–2009) | 2008 (2006–2009) | 0.787 |
| Donor ADAMTS13 genotype, n (%) | ||||
| C/C | 203 (72.2) | 152 (73.8) | 51 (68.0) | 0.447 |
| C/T | 71 (25.3) | 50 (24.3) | 21 (28.0) | |
| T/T | 7 (2.5) | 4 (1.9) | 3 (4.0) | |
| Patient sex, n (%) | ||||
| Male | 150 (53.4) | 111 (53.9) | 39 (52.0) | 0.789 |
| Female | 131 (46.6) | 95 (46.1) | 36 (48.0) | |
| Donor sex, n (%) | ||||
| Male | 193 (68.7) | 138 (67.0) | 55 (73.3) | 0.383 |
| Female | 88 (31.3) | 68 (33.0) | 20 (26.7) | |
| Recipient/Donor sex match, n (%) | ||||
| Sex-matched | 158 (56.2) | 119 (57.8) | 39 (52.0) | 0.416 |
| Not sex-matched | 123 (43.8) | 87 (42.2) | 36 (48.0) | |
| Disease, n (%) | ||||
| AML | 165 (55.9) | 124 (60.2) | 41 (54.7) | 0.509 |
| ALL | 65 (23.1) | 44 (21.4) | 21 (28.0) | |
| MDS | 51 (18.1) | 38 18.4) | 13 (17.3) | |
| Disease stage, n (%) | ||||
| Standard risk | 198 (70.5) | 148 (71.8) | 50 (66.7) | 0.460 |
| High risk | 83 (29.5) | 58 (28.2) | 25 (33.3) | |
| ABO matching, n (%) | ||||
| ABO-matched | 157 (55.9) | 112 (54.4) | 45 (60.0) | 0.641 |
| Major mismatch | 74 (26.3) | 53 (25.7) | 21 (28.0) | 0.756 |
| Minor mismatch | 69 (24.6) | 55 (26.7) | 14 (18.7) | 0.155 |
| Bidirectional | 19 (6.8) | 14 (6.8) | 5 (6.7) | 1.000 |
| Conditioning regimen, n (%) | 69 (25.1) | 57 (28.1) | 12 (16.7) | |
| Reduced intensity | 209 (74.4) | 147 (71.4) | 62 (82.7) | 0.064 |
| Myeloablative | 72 (25.6) | 59 (28.6) | 13 (17.3) | |
| GVHD prophylaxis, n (%) | ||||
| Cyclosporine | 66 (23.5) | 54 (26.2) | 12 (16.0) | 0.082 |
| Tacrolimus | 215 (76.5) | 152 (73.8) | 63 (84.0) | |
| PS at transplant, n (%) | ||||
| 2–4 | 17 (6.0) | 12 (5.8) | 5 (6.7) | 0.748 |
| Pretransplantation CMV serostatus, n (%) | ||||
| CMV-positive recipient | 222 (82.5) | 162 (78.6) | 60 (80.0) | 0.854 |
| Missing | 12 (4.3) | 10 (4.9) | 2 (2.7) | |
| TNC, ×108/kg, median (range) | 2.77 (0.54–8.83) | 2.66 (0.77–6.29) | 2.91 (0.54–8.83) | 0.502 |
| Variable | n | Adjusted 3-y DFS | HR (95% CI) | P | Adjusted 3-y OS | HR (95% CI) | P | Adjusted 3-y Relapse | HR (95% CI) | P |
| Overall | 281 | 61% | 63% | 18% | ||||||
| Recipient ADAMTS13, C/C | 206 | 58% | 1.64 (1.01–2.67) | 0.045 | 61% | 1.40 (0.86–2.29) | 0.180 | 21% | 3.12 (1.25–7.77) | 0.015 |
| Recipient ADAMTS13, C/T or T/T | 75 | 71% | 71% | 13% | ||||||
| Donor ADAMTS13, C/C | 203 | 60% | 1.18 (0.74–1.89) | 0.489 | 62% | 1.23 (0.76–2.01) | 0.403 | 20% | 1.26 (0.60–2.66) | 0.550 |
| Donor ADAMTS13, C/T or T/T | 78 | 66% | 67% | 14% | ||||||
| Variable | n | Adjusted 3-y NRM | HR (95% CI) | P | Adjusted grades 2–4 acute GVHD | HR (95% CI) | P | Adjusted chronic GVHD | HR (95% CI) | P |
| Overall | 281 | 23% | 34% | 28% | ||||||
| Recipient ADAMTS13, C/C | 206 | 23% | 1.03 (0.55–1.94) | 0.920 | 35% | 1.08 (0.64–1.81) | 0.770 | 28% | 1.00 (0.57–1.73) | 0.990 |
| Recipient ADAMTS13, C/T or T/T | 75 | 25% | 32% | 27% | ||||||
| Donor ADAMTS13, C/C | 203 | 24% | 1.03 (0.55–1.91) | 0.930 | 34% | 0.93 (0.58–1.49) | 0.750 | 27% | 0.74 (0.46–1.22) | 0.240 |
| Donor ADAMTS13, C/T or T/T | 78 | 22% | 34% | 33% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nomoto, H.; Takami, A.; Espinoza, J.L.; Onizuka, M.; Kashiwase, K.; Morishima, Y.; Fukuda, T.; Kodera, Y.; Doki, N.; Miyamura, K.; et al. Recipient ADAMTS13 Single-Nucleotide Polymorphism Predicts Relapse after Unrelated Bone Marrow Transplantation for Hematologic Malignancy. Int. J. Mol. Sci. 2019, 20, 214. https://doi.org/10.3390/ijms20010214
Nomoto H, Takami A, Espinoza JL, Onizuka M, Kashiwase K, Morishima Y, Fukuda T, Kodera Y, Doki N, Miyamura K, et al. Recipient ADAMTS13 Single-Nucleotide Polymorphism Predicts Relapse after Unrelated Bone Marrow Transplantation for Hematologic Malignancy. International Journal of Molecular Sciences. 2019; 20(1):214. https://doi.org/10.3390/ijms20010214
Chicago/Turabian StyleNomoto, Haruka, Akiyoshi Takami, J. Luis Espinoza, Makoto Onizuka, Koichi Kashiwase, Yasuo Morishima, Takahiro Fukuda, Yoshihisa Kodera, Noriko Doki, Koichi Miyamura, and et al. 2019. "Recipient ADAMTS13 Single-Nucleotide Polymorphism Predicts Relapse after Unrelated Bone Marrow Transplantation for Hematologic Malignancy" International Journal of Molecular Sciences 20, no. 1: 214. https://doi.org/10.3390/ijms20010214
APA StyleNomoto, H., Takami, A., Espinoza, J. L., Onizuka, M., Kashiwase, K., Morishima, Y., Fukuda, T., Kodera, Y., Doki, N., Miyamura, K., Mori, T., Nakao, S., & Morishita, E. (2019). Recipient ADAMTS13 Single-Nucleotide Polymorphism Predicts Relapse after Unrelated Bone Marrow Transplantation for Hematologic Malignancy. International Journal of Molecular Sciences, 20(1), 214. https://doi.org/10.3390/ijms20010214







