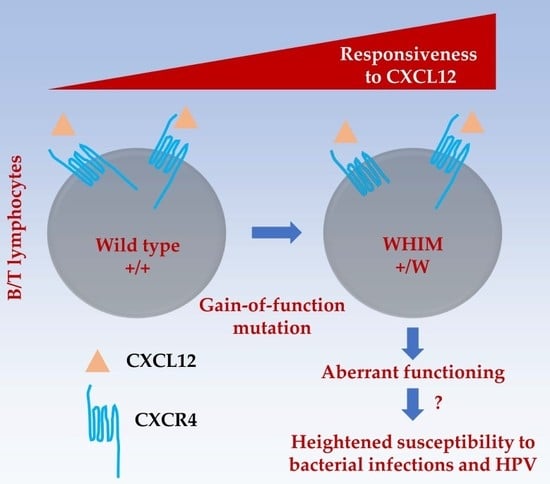
Adaptive Immunodeficiency in WHIM Syndrome
Abstract
:1. Introduction
2. Lymphoid Organs
2.1. Bone Marrow
2.2. Thymus
2.3. Secondary Lymphoid Organs
3. Lymphocytes
3.1. B Cells
3.2. T Cells
3.3. Natural Killer (NK) Cells
4. The Question of HPV Susceptibility
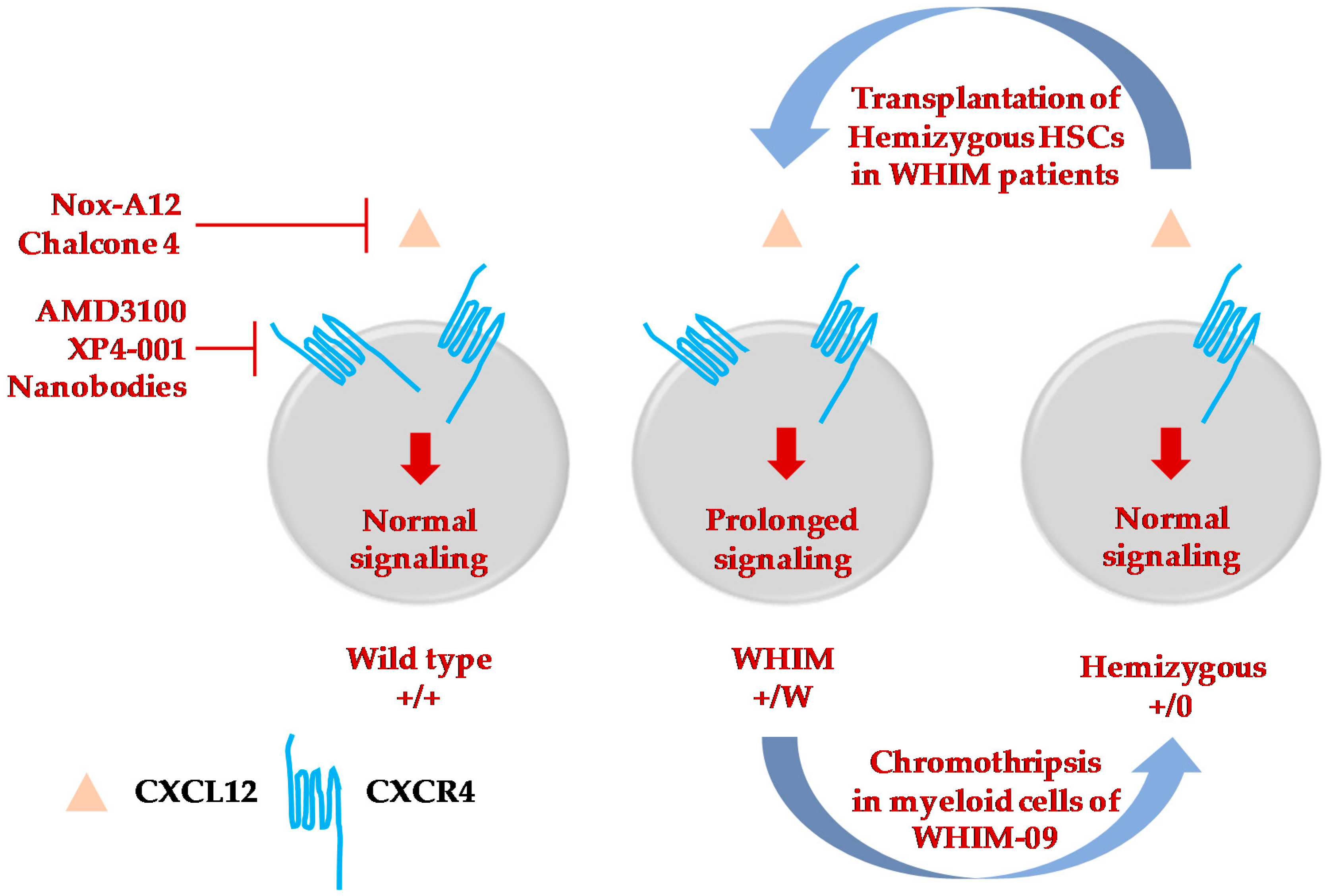
5. Therapeutic Considerations
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BCR | B-cell receptor |
| CLP | Common lymphoid progenitor |
| CMJ | Corticomedullary junction |
| CMP | Common myeloid progenitor |
| DN | Double negative |
| DP | Double positive |
| G-CSF | Granulocyte colony-stimulating factor |
| GVHD | Graft-versus-host disease |
| HSC | Hematopoietic stem cell |
| HSCT | Hematopoietic stem cell transplantation |
| Ig | Immunoglobulin |
| LN | Lymph node |
| NK | Natural killer |
| PCs | Plasma cells |
| pDCs | Plasmacytoid dendritic cells |
| SCN | Severe congenital neutropenia |
| SP | Single positive |
| TCR | T-cell receptor |
| WHIM | Warts, hypogammaglobulinemia, infections, and myelokathexis |
References
- Beaussant Cohen, S.; Fenneteau, O.; Plouvier, E.; Rohrlich, P.-S.; Daltroff, G.; Plantier, I.; Dupuy, A.; Kerob, D.; Beaupain, B.; Bordigoni, P.; et al. Description and outcome of a cohort of 8 patients with WHIM syndrome from the French Severe Chronic Neutropenia Registry. Orphanet J. Rare Dis. 2012, 7, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rath, A.; Wakap, S.N. Valérie Lanneau Prevalence of Rare Diseases: Bibliographic Data. Orphanet Report Series, Rare Diseases collection, June 2018, Number 1: Diseases Listed in Alphabetical Order. Available online: http://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_diseases.pdf (accessed on 18 December 2018).
- Gulino, A.V. Altered leukocyte response to CXCL12 in patients with warts hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome. Blood 2004, 104, 444–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tassone, L.; Notarangelo, L.D.; Bonomi, V.; Savoldi, G.; Sensi, A.; Soresina, A.; Smith, C.I.E.; Porta, F.; Plebani, A.; Notarangelo, L.D.; et al. Clinical and genetic diagnosis of warts, hypogammaglobulinemia, infections, and myelokathexis syndrome in 10 patients. J. Allergy Clin. Immunol. 2009, 123, 1170–1173. [Google Scholar] [CrossRef] [PubMed]
- Gorlin, R.J.; Gelb, B.; Diaz, G.A.; Lofsness, K.G.; Pittelkow, M.R.; Fenyk, J.R. WHIM syndrome, an autosomal dominant disorder: Clinical, hematological, and molecular studies. Am. J. Med. Genet. 2000, 91, 368–376. [Google Scholar] [CrossRef]
- Balabanian, K. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood 2005, 105, 2449–2457. [Google Scholar] [CrossRef] [Green Version]
- Heusinkveld, L.E.; Yim, E.; Yang, A.; Azani, A.B.; Liu, Q.; Gao, J.-L.; McDermott, D.H.; Murphy, P.M. Pathogenesis, diagnosis and therapeutic strategies in WHIM syndrome immunodeficiency. Expert Opin. Orphan Drugs 2017, 5, 813–825. [Google Scholar] [CrossRef]
- Hernandez, P.A.; Gorlin, R.J.; Lukens, J.N.; Taniuchi, S.; Bohinjec, J.; Francois, F.; Klotman, M.E.; Diaz, G.A. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat. Genet. 2003, 34, 70–74. [Google Scholar] [CrossRef]
- Lagane, B.; Chow, K.Y.C.; Balabanian, K.; Levoye, A.; Harriague, J.; Planchenault, T.; Baleux, F.; Gunera-Saad, N.; Arenzana-Seisdedos, F.; Bachelerie, F. CXCR4 dimerization and -arrestin-mediated signaling account for the enhanced chemotaxis to CXCL12 in WHIM syndrome. Blood 2008, 112, 34–44. [Google Scholar] [CrossRef] [Green Version]
- Kawai, T.; Choi, U.; Whiting-Theobald, N.L.; Linton, G.F.; Brenner, S.; Sechler, J.M.G.; Murphy, P.M.; Malech, H.L. Enhanced function with decreased internalization of carboxy-terminus truncated CXCR4 responsible for WHIM syndrome. Exp. Hematol. 2005, 33, 460–468. [Google Scholar] [CrossRef]
- Scala, S. Molecular Pathways: Targeting the CXCR4-CXCL12 Axis--Untapped Potential in the Tumor Microenvironment. Clin. Cancer Res. 2015, 21, 4278–4285. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, Z.; Ma, L.; Pei, G. β-Arrestin2 Is Critically Involved in CXCR4-mediated Chemotaxis, and This Is Mediated by Its Enhancement of p38 MAPK Activation. J. Biol. Chem. 2002, 277, 49212–49219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krill, C.E.; Smith, H.D.; Mauer, A.M. Chronic Idiopathic Granulocytopenia. N. Engl. J. Med. 1964, 270, 973–979. [Google Scholar] [CrossRef] [PubMed]
- Zuelzer, W.W. “Myelokathexis”—A New Form of Chronic Granulocytopenia. Report of A Case. N. Engl. J. Med. 1964, 270, 699–704. [Google Scholar] [CrossRef]
- McDermott, D.H.; Gao, J.-L.; Liu, Q.; Siwicki, M.; Martens, C.; Jacobs, P.; Velez, D.; Yim, E.; Bryke, C.R.; Hsu, N.; et al. Chromothriptic Cure of WHIM Syndrome. Cell 2015, 160, 686–699. [Google Scholar] [CrossRef] [Green Version]
- Liu, Q.; Li, Z.; Yang, A.Y.; Gao, J.-L.; Velez, D.S.; Cho, E.J.; McDermott, D.H.; Murphy, P.M. Mechanisms of Sustained Neutrophilia in Patient WHIM-09, Cured of WHIM Syndrome by Chromothripsis. J. Clin. Immunol. 2018, 38, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Taniuchi, S.; Yamamoto, A.; Fujiwara, T.; Hasui, M.; Tsuji, S.; Kobayashi, Y. Dizygotic twin sisters with myelokathexis: Mechanism of its neutropenia. Am. J. Hematol. 1999, 62, 106–111. [Google Scholar] [CrossRef] [Green Version]
- Alapi, K.; Erdős, M.; Kovács, G.; Maródi, L. Recurrent CXCR4 sequence variation in a girl with WHIM syndrome. Eur. J. Haematol. 2007, 78, 86–88. [Google Scholar] [CrossRef]
- Tarzi, M.D.; Jenner, M.; Hattotuwa, K.; Faruqi, A.Z.; Diaz, G.A.; Longhurst, H.J. Sporadic case of warts, hypogammaglobulinemia, immunodeficiency, and myelokathexis syndrome. J. Allergy Clin. Immunol. 2005, 116, 1101–1105. [Google Scholar] [CrossRef]
- Badolato, R.; Donadieu, J.; The WHIM Research Group. How I treat warts, hypogammaglobulinemia, infections, and myelokathexis syndrome. Blood 2017, 130, 2491–2498. [Google Scholar] [CrossRef] [Green Version]
- Bagri, A.; Gurney, T.; He, X.; Zou, Y.-R.; Littman, D.R.; Tessier-Lavigne, M.; Pleasure, S.J. The chemokine SDF1 regulates migration of dentate granule cells. Development 2002, 129, 4249–4260. [Google Scholar]
- Zou, Y.-R.; Kottmann, A.H.; Kuroda, M.; Taniuchi, I.; Littman, D.R. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature 1998, 393, 595–599. [Google Scholar] [CrossRef]
- Tachibana, K.; Hirota, S.; Iizasa, H.; Yoshida, H.; Kawabata, K.; Kataoka, Y.; Kitamura, Y.; Matsushima, K.; Yoshida, N.; Nishikawa, S.; et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature 1998, 393, 591–594. [Google Scholar] [CrossRef]
- Nagasawa, T.; Hirota, S.; Tachibana, K.; Takakura, N.; Nishikawa, S.; Kitamura, Y.; Yoshida, N.; Kikutani, H.; Kishimoto, T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 1996, 382, 635–638. [Google Scholar] [CrossRef]
- Badolato, R.; Dotta, L.; Tassone, L.; Amendola, G.; Porta, F.; Locatelli, F.; Notarangelo, L.D.; Bertrand, Y.; Bachelerie, F.; Donadieu, J. Tetralogy of Fallot is an Uncommon Manifestation of Warts, Hypogammaglobulinemia, Infections, and Myelokathexis Syndrome. J. Pediatr. 2012, 161, 763–765. [Google Scholar] [CrossRef] [Green Version]
- Balabanian, K.; Brotin, E.; Biajoux, V.; Bouchet-Delbos, L.; Lainey, E.; Fenneteau, O.; Bonnet, D.; Fiette, L.; Emilie, D.; Bachelerie, F. Proper desensitization of CXCR4 is required for lymphocyte development and peripheral compartmentalization in mice. Blood 2012, 119, 5722–5730. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Morrison, S.J. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature 2013, 495, 231–235. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, T.; Kohara, H.; Noda, M.; Nagasawa, T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity 2006, 25, 977–988. [Google Scholar] [CrossRef]
- Zhang, Y.; Dépond, M.; He, L.; Foudi, A.; Kwarteng, E.O.; Lauret, E.; Plo, I.; Desterke, C.; Dessen, P.; Fujii, N.; et al. CXCR4/CXCL12 axis counteracts hematopoietic stem cell exhaustion through selective protection against oxidative stress. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Nie, Y.; Han, Y.-C.; Zou, Y.-R. CXCR4 is required for the quiescence of primitive hematopoietic cells. J. Exp. Med. 2008, 205, 777–783. [Google Scholar] [CrossRef] [Green Version]
- Kawabata, K.; Ujikawa, M.; Egawa, T.; Kawamoto, H.; Tachibana, K.; Iizasa, H.; Katsura, Y.; Kishimoto, T.; Nagasawa, T. A cell-autonomous requirement for CXCR4 in long-term lymphoid and myeloid reconstitution. Proc. Natl. Acad. Sci. USA 1999, 96, 5663–5667. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Jones, D.; Borghesani, P.R.; Segal, R.A.; Nagasawa, T.; Kishimoto, T.; Bronson, R.T.; Springer, T.A. Impaired B.-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc. Natl. Acad. Sci. USA 1998, 95, 9448–9453. [Google Scholar] [CrossRef]
- Freitas, C.; Wittner, M.; Nguyen, J.; Rondeau, V.; Biajoux, V.; Aknin, M.-L.; Gaudin, F.; Beaussant-Cohen, S.; Bertrand, Y.; Bellanné-Chantelot, C.; et al. Lymphoid differentiation of hematopoietic stem cells requires efficient Cxcr4 desensitization. J. Exp. Med. 2017, 214, 2023–2040. [Google Scholar] [CrossRef]
- Gao, J.-L.; Yim, E.; Siwicki, M.; Yang, A.; Liu, Q.; Azani, A.; Owusu-Ansah, A.; McDermott, D.H.; Murphy, P.M. Cxcr4-haploinsufficient bone marrow transplantation corrects leukopenia in an unconditioned WHIM syndrome model. J. Clin. Investig. 2018, 128, 3312–3318. [Google Scholar] [CrossRef] [Green Version]
- Moens, L.; Frans, G.; Bosch, B.; Bossuyt, X.; Verbinnen, B.; Poppe, W.; Boeckx, N.; Slatter, M.; Brusselmans, C.; Diaz, G.; et al. Successful hematopoietic stem cell transplantation for myelofibrosis in an adult with warts-hypogammaglobulinemia-immunodeficiency-myelokathexis syndrome. J. Allergy Clin. Immunol. 2016, 138, 1485–1489.e2. [Google Scholar] [CrossRef] [Green Version]
- Saettini, F.; Notarangelo, L.D.; Biondi, A.; Bonanomi, S. Neutropenia, hypogammaglobulinemia, and pneumonia: A case of WHIM syndrome. Pediatr. Int. 2018, 60, 318–319. [Google Scholar] [CrossRef]
- Shin, D.W.; Park, S.N.; Kim, S.-M.; Im, K.; Kim, J.-A.; Hong, K.T.; Choi, J.Y.; Hong, C.R.; Park, K.D.; Shin, H.Y.; et al. WHIM Syndrome with a Novel CXCR4 Variant in a Korean Child. Ann. Lab. Med. 2017, 37, 446. [Google Scholar] [CrossRef]
- McDermott, D.H.; Liu, Q.; Velez, D.; Lopez, L.; Anaya-O’Brien, S.; Ulrick, J.; Kwatemaa, N.; Starling, J.; Fleisher, T.A.; Priel, D.A.L.; et al. A phase 1 clinical trial of long-term, low-dose treatment of WHIM syndrome with the CXCR4 antagonist plerixafor. Blood 2014, 123, 2308–2316. [Google Scholar] [CrossRef] [Green Version]
- Gulino, A.V. WHIM syndrome: A genetic disorder of leukocyte trafficking. Curr. Opin. Allergy Clin. Immunol. 2003, 3, 443–450. [Google Scholar] [CrossRef]
- Hatse, S.; Princen, K.; Bridger, G.; De Clercq, E.; Schols, D. Chemokine receptor inhibition by AMD3100 is strictly confined to CXCR4. FEBS Lett. 2002, 527, 255–262. [Google Scholar] [CrossRef] [Green Version]
- De Clercq, E. AMD3100/CXCR4 Inhibitor. Front. Immunol. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Li, X.; You, S.; Bhuyan, S.S.; Dong, L. Effectiveness of AMD3100 in treatment of leukemia and solid tumors: From original discovery to use in current clinical practice. Exp. Hematol. Oncol. 2015, 5. [Google Scholar] [CrossRef]
- Dale, D.C.; Bolyard, A.A.; Kelley, M.L.; Westrup, E.C.; Makaryan, V.; Aprikyan, A.; Wood, B.; Hsu, F.J. The CXCR4 antagonist plerixafor is a potential therapy for myelokathexis, WHIM syndrome. Blood 2011, 118, 4963–4966. [Google Scholar] [CrossRef] [Green Version]
- McDermott, D.H.; Liu, Q.; Ulrick, J.; Kwatemaa, N.; Anaya-O’Brien, S.; Penzak, S.R.; Filho, J.O.; Priel, D.A.L.; Kelly, C.; Garofalo, M.; et al. The CXCR4 antagonist plerixafor corrects panleukopenia in patients with WHIM syndrome. Blood 2011, 118, 4957–4962. [Google Scholar] [CrossRef] [Green Version]
- Robertson, P.; Means, T.K.; Luster, A.D.; Scadden, D.T. CXCR4 and CCR5 mediate homing of primitive bone marrow–derived hematopoietic cells to the postnatal thymus. Exp. Hematol. 2006, 34, 308–319. [Google Scholar] [CrossRef]
- Calderon, L.; Boehm, T. Three chemokine receptors cooperatively regulate homing of hematopoietic progenitors to the embryonic mouse thymus. Proc. Natl. Acad. Sci. USA 2011, 108, 7517–7522. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Lopez, C. Stromal cell-derived factor 1/CXCR4 signaling is critical for early human T.-cell development. Blood 2002, 99, 546–554. [Google Scholar] [CrossRef]
- Zaitseva, M.B.; Lee, S.; Rabin, R.L.; Tiffany, H.L.; Farber, J.M.; Peden, K.W.; Murphy, P.M.; Golding, H. CXCR4 and CCR5 on human thymocytes: Biological function and role in HIV-1 infection. J. Immunol. 1998, 161, 3103–3113. [Google Scholar]
- Plotkin, J.; Prockop, S.E.; Lepique, A.; Petrie, H.T. Critical role for CXCR4 signaling in progenitor localization and T cell differentiation in the postnatal thymus. J. Immunol. 2003, 171, 4521–4527. [Google Scholar] [CrossRef]
- Trampont, P.C.; Tosello-Trampont, A.-C.; Shen, Y.; Duley, A.K.; Sutherland, A.E.; Bender, T.P.; Littman, D.R.; Ravichandran, K.S. CXCR4 acts as a costimulator during thymic β-selection. Nat. Immunol. 2010, 11, 162–170. [Google Scholar] [CrossRef]
- Janas, M.L.; Varano, G.; Gudmundsson, K.; Noda, M.; Nagasawa, T.; Turner, M. Thymic development beyond β-selection requires phosphatidylinositol 3-kinase activation by CXCR4. J. Exp. Med. 2010, 207, 247–261. [Google Scholar] [CrossRef]
- Ara, T.; Itoi, M.; Kawabata, K.; Egawa, T.; Tokoyoda, K.; Sugiyama, T.; Fujii, N.; Amagai, T.; Nagasawa, T. A Role of CXC Chemokine Ligand 12/Stromal Cell-Derived Factor-1/Pre-B Cell Growth Stimulating Factor and Its Receptor CXCR4 in Fetal and Adult T Cell Development in Vivo. J. Immunol. 2003, 170, 4649–4655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lucas, B.; White, A.J.; Parnell, S.M.; Henley, P.M.; Jenkinson, W.E.; Anderson, G. Progressive Changes in CXCR4 Expression That Define Thymocyte Positive Selection Are Dispensable for Both Innate and Conventional αβT-cell Development. Sci. Rep. 2017, 7. [Google Scholar] [CrossRef]
- Swainson, L.; Kinet, S.; Manel, N.; Battini, J.-L.; Sitbon, M.; Taylor, N. Glucose transporter 1 expression identifies a population of cycling CD4+CD8+ human thymocytes with high CXCR4-induced chemotaxis. Proc. Natl. Acad. Sci. USA 2005, 102, 12867–12872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, G.; Nakata, Y.; Dan, Y.; Uzawa, A.; Nakagawa, K.; Saito, T.; Mita, K.; Shirasawa, T. Loss of SDF-1 receptor expression during positive selection in the thymus. Int. Immunol. 1998, 10, 1049–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poznansky, M.C.; Olszak, I.T.; Evans, R.H.; Wang, Z.; Foxall, R.B.; Olson, D.P.; Weibrecht, K.; Luster, A.D.; Scadden, D.T. Thymocyte emigration is mediated by active movement away from stroma-derived factors. J. Clin. Investig. 2002, 109, 1101–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vianello, F.; Kraft, P.; Mok, Y.T.; Hart, W.K.; White, N.; Poznansky, M.C. A CXCR4-dependent chemorepellent signal contributes to the emigration of mature single-positive CD4 cells from the fetal thymus. J. Immunol. 2005, 175, 5115–5125. [Google Scholar] [CrossRef] [PubMed]
- Hernandezlopez, C.; Valencia, J.; Hidalgo, L.; Martinez, V.; Zapata, A.; Sacedon, R.; Varas, A.; Vicente, A. CXCL12/CXCR4 signaling promotes human thymic dendritic cell survival regulating the Bcl-2/Bax ratio. Immunol. Lett. 2008, 120, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Bleul, C.C.; Farzan, M.; Choe, H.; Parolin, C.; Clark-Lewis, I.; Sodroski, J.; Springer, T.A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 1996, 382, 829–833. [Google Scholar] [CrossRef]
- Feng, Y.; Broder, C.C.; Kennedy, P.E.; Berger, E.A. HIV-1 entry cofactor: Functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science 1996, 272, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, S.G.; Zack, J.A. CXCR4 expression during lymphopoiesis: Implications for human immunodeficiency virus type 1 infection of the thymus. J. Virol. 1997, 71, 6928–6934. [Google Scholar] [PubMed]
- Schmitt, N.; Chene, L.; Boutolleau, D.; Nugeyre, M.-T.; Guillemard, E.; Versmisse, P.; Jacquemot, C.; Barre-Sinoussi, F.; Israel, N. Positive Regulation of CXCR4 Expression and Signaling by Interleukin-7 in CD4+ Mature Thymocytes Correlates with Their Capacity To Favor Human Immunodeficiency X4 Virus Replication. J. Virol. 2003, 77, 5784–5793. [Google Scholar] [CrossRef] [Green Version]
- Salemi, M.; Burkhardt, B.R.; Gray, R.R.; Ghaffari, G.; Sleasman, J.W.; Goodenow, M.M. Phylodynamics of HIV-1 in Lymphoid and Non-Lymphoid Tissues Reveals a Central Role for the Thymus in Emergence of CXCR4-Using Quasispecies. PLoS ONE 2007, 2, e950. [Google Scholar] [CrossRef]
- Ma, Q.; Jones, D.; Springer, T.A. The Chemokine Receptor CXCR4 Is Required for the Retention of B Lineage and Granulocytic Precursors within the Bone Marrow Microenvironment. Immunity 1999, 10, 463–471. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Z.; Gao, J.-L.; Wan, W.; Ganesan, S.; McDermott, D.H.; Murphy, P.M. CXCR4 antagonist AMD3100 redistributes leukocytes from primary immune organs to secondary immune organs, lung, and blood in mice: Leukocyte signaling. Eur. J. Immunol. 2015, 45, 1855–1867. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Hobeika, E.; Jumaa, H.; Reth, M.; Maity, P.C. CXCR4 signaling and function require the expression of the IgD-class B-cell antigen receptor. Proc. Natl. Acad. Sci. USA 2017, 114, 5231–5236. [Google Scholar] [CrossRef] [Green Version]
- Roselli, G.; Martini, E.; Lougaris, V.; Badolato, R.; Viola, A.; Kallikourdis, M. CXCL12 Mediates Aberrant Costimulation of B Lymphocytes in Warts, Hypogammaglobulinemia, Infections, Myelokathexis Immunodeficiency. Front. Immunol. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Beck, T.C.; Gomes, A.C.; Cyster, J.G.; Pereira, J.P. CXCR4 and a cell-extrinsic mechanism control immature B lymphocyte egress from bone marrow. J. Exp. Med. 2014, 211, 2567–2581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nie, Y.; Waite, J.; Brewer, F.; Sunshine, M.-J.; Littman, D.R.; Zou, Y.-R. The Role of CXCR4 in Maintaining Peripheral B Cell Compartments and Humoral Immunity. J. Exp. Med. 2004, 200, 1145–1156. [Google Scholar] [CrossRef] [Green Version]
- Handisurya, A.; Schellenbacher, C.; Reininger, B.; Koszik, F.; Vyhnanek, P.; Heitger, A.; Kirnbauer, R.; Förster-Waldl, E. A quadrivalent HPV vaccine induces humoral and cellular immune responses in WHIM immunodeficiency syndrome. Vaccine 2010, 28, 4837–4841. [Google Scholar] [CrossRef] [Green Version]
- Mc Guire, P.J.; Cunningham-Rundles, C.; Ochs, H.; Diaz, G.A. Oligoclonality, impaired class switch and B-cell memory responses in WHIM syndrome. Clin. Immunol. 2010, 135, 412–421. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, Y.; Oh, Y.; Kato, T.; Zaha, K.; Morimoto, A. Transient Marked Increase of γδ T Cells in WHIM Syndrome After Successful HSCT. J. Clin. Immunol. 2018, 38, 553–555. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.C.; Ansel, K.M.; Low, C.; Lesley, R.; Tamamura, H.; Fujii, N.; Cyster, J.G. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat. Immunol. 2004, 5, 943–952. [Google Scholar] [CrossRef] [PubMed]
- Biajoux, V.; Natt, J.; Freitas, C.; Alouche, N.; Sacquin, A.; Hemon, P.; Gaudin, F.; Fazilleau, N.; Espéli, M.; Balabanian, K. Efficient Plasma Cell Differentiation and Trafficking Require Cxcr4 Desensitization. Cell. Rep. 2016, 17, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Kriván, G.; Erdős, M.; Kállay, K.; Benyó, G.; Tóth, Á.; Sinkó, J.; Goda, V.; Tóth, B.; Maródi, L. Successful umbilical cord blood stem cell transplantation in a child with WHIM syndrome. Eur. J. Haematol. 2010, 84, 274–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanki, T.; Lipsky, P.E. Cutting Edge: Stromal Cell-Derived Factor-1 Is a Costimulator for CD4+ T Cell Activation. J. Immunol. 2000, 164, 5010–5014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ticchioni, M.; Charvet, C.; Noraz, N.; Lamy, L.; Steinberg, M.; Bernard, A.; Deckert, M. Signaling through ZAP-70 is required for CXCL12-mediated T-cell transendothelial migration. Blood 2002, 99, 3111–3118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Humphreys, T.D.; Kremer, K.N.; Bramati, P.S.; Bradfield, L.; Edgar, C.E.; Hedin, K.E. CXCR4 Physically Associates with the T Cell Receptor to Signal in T Cells. Immunity 2006, 25, 213–224. [Google Scholar] [CrossRef]
- Smith, X.; Schneider, H.; Köhler, K.; Liu, H.; Lu, Y.; Rudd, C.E. The chemokine CXCL12 generates costimulatory signals in T cells to enhance phosphorylation and clustering of the adaptor protein SLP-76. Sci. Signal. 2013, 6, ra65. [Google Scholar] [CrossRef]
- Molon, B.; Gri, G.; Bettella, M.; Gómez-Moutón, C.; Lanzavecchia, A.; Martínez-A, C.; Mañes, S.; Viola, A. T cell costimulation by chemokine receptors. Nat. Immunol. 2005, 6, 465–471. [Google Scholar] [CrossRef]
- Contento, R.L.; Molon, B.; Boularan, C.; Pozzan, T.; Manes, S.; Marullo, S.; Viola, A. CXCR4-CCR5: A couple modulating T cell functions. Proc. Natl. Acad. Sci. USA 2008, 105, 10101–10106. [Google Scholar] [CrossRef] [Green Version]
- Sotsios, Y.; Whittaker, G.C.; Westwick, J.; Ward, S.G. The CXC chemokine stromal cell-derived factor activates a Gi-coupled phosphoinositide 3-kinase in T lymphocytes. J. Immunol. 1999, 163, 5954–5963. [Google Scholar]
- Ottoson, N.C.; Pribila, J.T.; Chan, A.S.; Shimizu, Y. Cutting edge: T cell migration regulated by CXCR4 chemokine receptor signaling to ZAP-70 tyrosine kinase. J. Immunol. 2001, 167, 1857–1861. [Google Scholar] [CrossRef]
- Okabe, S. Stromal cell-derived factor-1/CXCL12-induced chemotaxis of T cells involves activation of the RasGAP-associated docking protein p62Dok-1. Blood 2005, 105, 474–480. [Google Scholar] [CrossRef]
- Kallikourdis, M.; Trovato, A.E.; Anselmi, F.; Sarukhan, A.; Roselli, G.; Tassone, L.; Badolato, R.; Viola, A. The CXCR4 mutations in WHIM syndrome impair the stability of the T-cell immunologic synapse. Blood 2013, 122, 666–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balabanian, K.; Levoye, A.; Klemm, L.; Lagane, B.; Hermine, O.; Harriague, J.; Baleux, F.; Arenzana-Seisdedos, F.; Bachelerie, F. Leukocyte analysis from WHIM syndrome patients reveals a pivotal role for GRK3 in CXCR4 signaling. J. Clin. Investig. 2008. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Pan, C.; Lopez, L.; Gao, J.; Velez, D.; Anaya-O’Brien, S.; Ulrick, J.; Littel, P.; Corns, J.S.; Ellenburg, D.T.; et al. WHIM Syndrome Caused by Waldenström’s Macroglobulinemia-Associated Mutation CXCR4 L329fs. J. Clin. Immunol. 2016, 36, 397–405. [Google Scholar] [CrossRef]
- Chaix, J.; Nish, S.A.; Lin, W.-H.W.; Rothman, N.J.; Ding, L.; Wherry, E.J.; Reiner, S.L. Cutting Edge: CXCR4 Is Critical for CD8+ Memory T Cell Homeostatic Self-Renewal but Not Rechallenge Self-Renewal. J. Immunol. 2014, 193, 1013–1016. [Google Scholar] [CrossRef] [Green Version]
- Beider, K. Involvement of CXCR4 and IL-2 in the homing and retention of human NK and NK T cells to the bone marrow and spleen of NOD/SCID mice. Blood 2003, 102, 1951–1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kean, L.S.; Sen, S.; Onabajo, O.; Singh, K.; Robertson, J.; Stempora, L.; Bonifacino, A.C.; Metzger, M.E.; Promislow, D.E.L.; Mattapallil, J.J.; et al. Significant mobilization of both conventional and regulatory T cells with AMD3100. Blood 2011, 118, 6580–6590. [Google Scholar] [CrossRef] [Green Version]
- Zou, L.; Barnett, B.; Safah, H.; Larussa, V.F.; Evdemon-Hogan, M.; Mottram, P.; Wei, S.; David, O.; Curiel, T.J.; Zou, W. Bone marrow is a reservoir for CD4+CD25+ regulatory T cells that traffic through CXCL12/CXCR4 signals. Cancer Res. 2004, 64, 8451–8455. [Google Scholar] [CrossRef]
- Noda, M.; Omatsu, Y.; Sugiyama, T.; Oishi, S.; Fujii, N.; Nagasawa, T. CXCL12-CXCR4 chemokine signaling is essential for NK-cell development in adult mice. Blood 2011, 117, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Mayol, K.; Biajoux, V.; Marvel, J.; Balabanian, K.; Walzer, T. Sequential desensitization of CXCR4 and S1P5 controls natural killer cell trafficking. Blood 2011, 118, 4863–4871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaeger, B.N.; Donadieu, J.; Cognet, C.; Bernat, C.; Ordoñez-Rueda, D.; Barlogis, V.; Mahlaoui, N.; Fenis, A.; Narni-Mancinelli, E.; Beaupain, B.; et al. Neutrophil depletion impairs natural killer cell maturation, function, and homeostasis. J. Exp. Med. 2012, 209, 565–580. [Google Scholar] [CrossRef] [Green Version]
- Pastrana, D.V.; Perettia, A.; Welch, N.L.; Borgogna, C.; Olivero, C.; Badolato, R.; Notarangelo, L.; Gariglio, M.; FitzGerald, P.C.; McIntosh, C.E.; et al. Metagenomic discovery of 83 new HPV types in patients with immunodeficiency. mSphere 2018, 3, e00645-18. [Google Scholar] [CrossRef] [PubMed]
- Meuris, F.; Gaudin, F.; Aknin, M.-L.; Hémon, P.; Berrebi, D.; Bachelerie, F. Symptomatic Improvement in Human Papillomavirus-Induced Epithelial Neoplasia by Specific Targeting of the CXCR4 Chemokine Receptor. J. Investig. Dermatol. 2016, 136, 473–480. [Google Scholar] [CrossRef]
- Komdeur, F.L.; Prins, T.M.; van de Wall, S.; Plat, A.; Wisman, G.B.A.; Hollema, H.; Daemen, T.; Church, D.N.; de Bruyn, M.; Nijman, H.W. CD103+ tumor-infiltrating lymphocytes are tumor-reactive intraepithelial CD8+ T cells associated with prognostic benefit and therapy response in cervical cancer. Oncoimmunology 2017, 6, e1338230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.J.; Jin, H.-T.; Hur, S.-Y.; Yang, H.G.; Seo, Y.B.; Hong, S.R.; Lee, C.-W.; Kim, S.; Woo, J.-W.; Park, K.S.; et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef]
- Diniz, M.O.; Sales, N.S.; Silva, J.R.; Ferreira, L.C.S. Protection against HPV-16-Associated Tumors Requires the Activation of CD8+ Effector Memory T Cells and the Control of Myeloid-Derived Suppressor Cells. Mol. Cancer Ther. 2016, 15, 1920–1930. [Google Scholar] [CrossRef]
- Tassone, L.; Moratto, D.; Vermi, W.; De Francesco, M.; Notarangelo, L.D.; Porta, F.; Lougaris, V.; Facchetti, F.; Plebani, A.; Badolato, R. Defect of plasmacytoid dendritic cells in warts, hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome patients. Blood 2010, 116, 4870–4873. [Google Scholar] [CrossRef] [Green Version]
- Dale, D.; Bolyard, A.A.; Dick, E.; Kelley, M.L.; Makaryan, V.; Johnson, R.; Gan, L.; Parasuraman, S. X4P-001: A Novel Molecularly-Targeted Oral Therapy for Whim Syndrome. Blood 2017, 130, 995. [Google Scholar]
- Vater, A.; Sahlmann, J.; Kröger, N.; Zöllner, S.; Lioznov, M.; Maasch, C.; Buchner, K.; Vossmeyer, D.; Schwoebel, F.; Purschke, W.G.; et al. Hematopoietic Stem and Progenitor Cell Mobilization in Mice and Humans by a First-in-Class Mirror-Image Oligonucleotide Inhibitor of CXCL12. Clin. Pharmacol. Ther. 2013, 94, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Hachet-Haas, M.; Balabanian, K.; Rohmer, F.; Pons, F.; Franchet, C.; Lecat, S.; Chow, K.Y.C.; Dagher, R.; Gizzi, P.; Didier, B.; et al. Small Neutralizing Molecules to Inhibit Actions of the Chemokine CXCL12. J. Biol. Chem. 2008, 283, 23189–23199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Wit, R.H.; Heukers, R.; Brink, H.J.; Arsova, A.; Maussang, D.; Cutolo, P.; Strubbe, B.; Vischer, H.F.; Bachelerie, F.; Smit, M.J. CXCR4-Specific Nanobodies as Potential Therapeutics for WHIM syndrome. J. Pharmacol. Exp. Ther. 2017, 363, 35–44. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majumdar, S.; Murphy, P.M. Adaptive Immunodeficiency in WHIM Syndrome. Int. J. Mol. Sci. 2019, 20, 3. https://doi.org/10.3390/ijms20010003
Majumdar S, Murphy PM. Adaptive Immunodeficiency in WHIM Syndrome. International Journal of Molecular Sciences. 2019; 20(1):3. https://doi.org/10.3390/ijms20010003
Chicago/Turabian StyleMajumdar, Shamik, and Philip M. Murphy. 2019. "Adaptive Immunodeficiency in WHIM Syndrome" International Journal of Molecular Sciences 20, no. 1: 3. https://doi.org/10.3390/ijms20010003