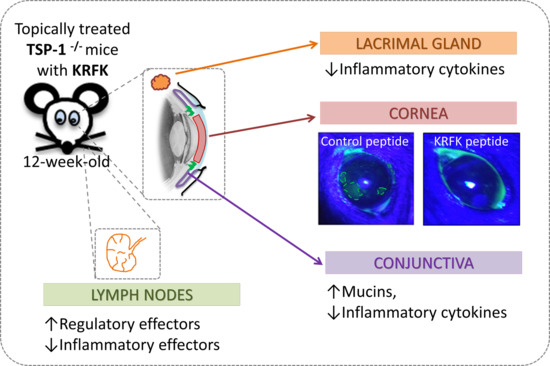
Topical Application of TGF-β-Activating Peptide, KRFK, Prevents Inflammatory Manifestations in the TSP-1-Deficient Mouse Model of Chronic Ocular Inflammation
Abstract
:1. Introduction
2. Results
2.1. The KRFK Peptide Activates Secreted TGF-β and Reduces the Expression of DC Maturation Markers in TSP-1-Deficient Bone Marrow-Derived Dendritic cells (BMDCs) In Vitro
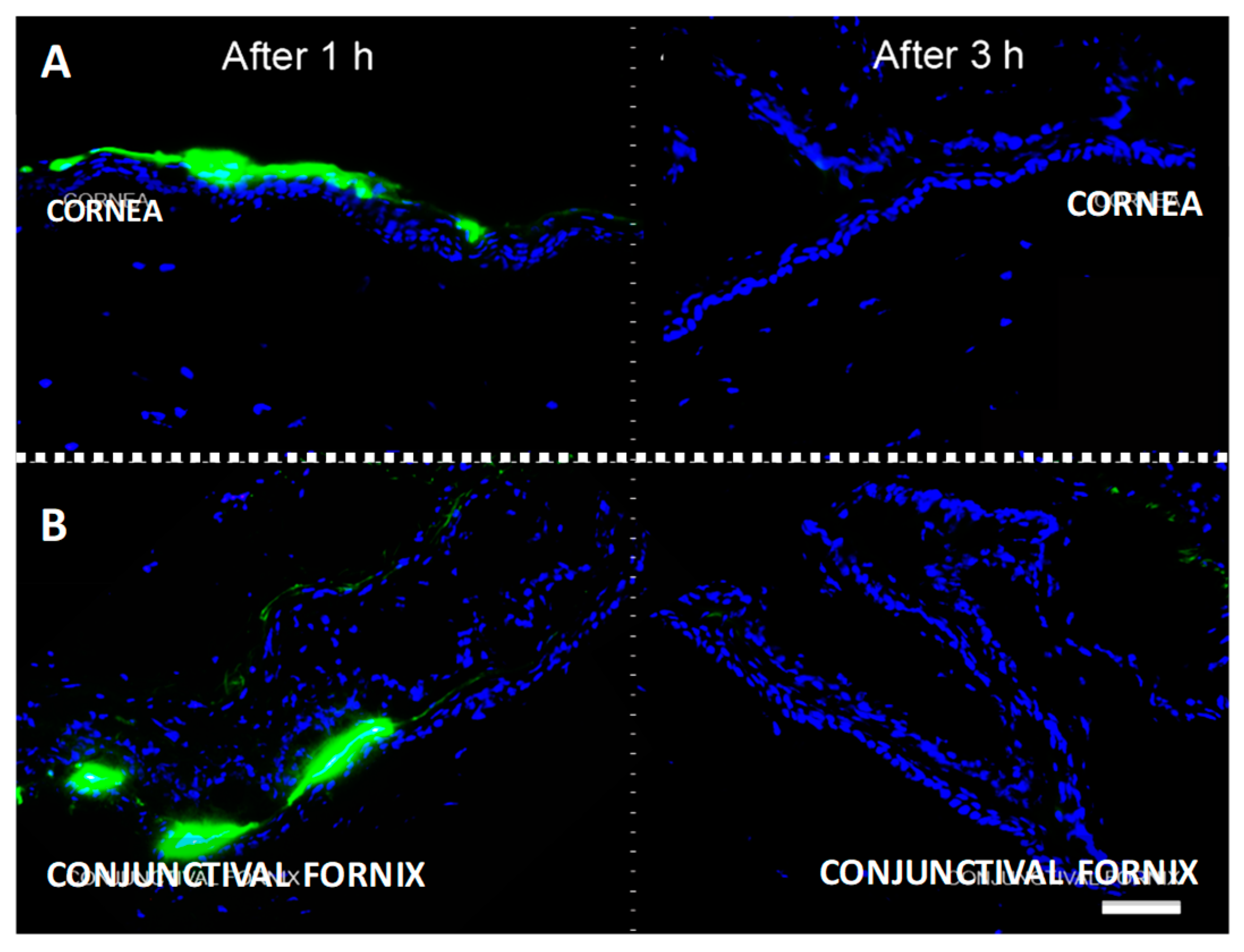
2.2. Topically Administered KRFK Peptide is Retained in Ocular Surface Tissues
2.3. Topically Administered KRFK Peptide to TSP-1-Deficient Mice Alters Peripheral Balance of CD4+ Inflammatory Effectors
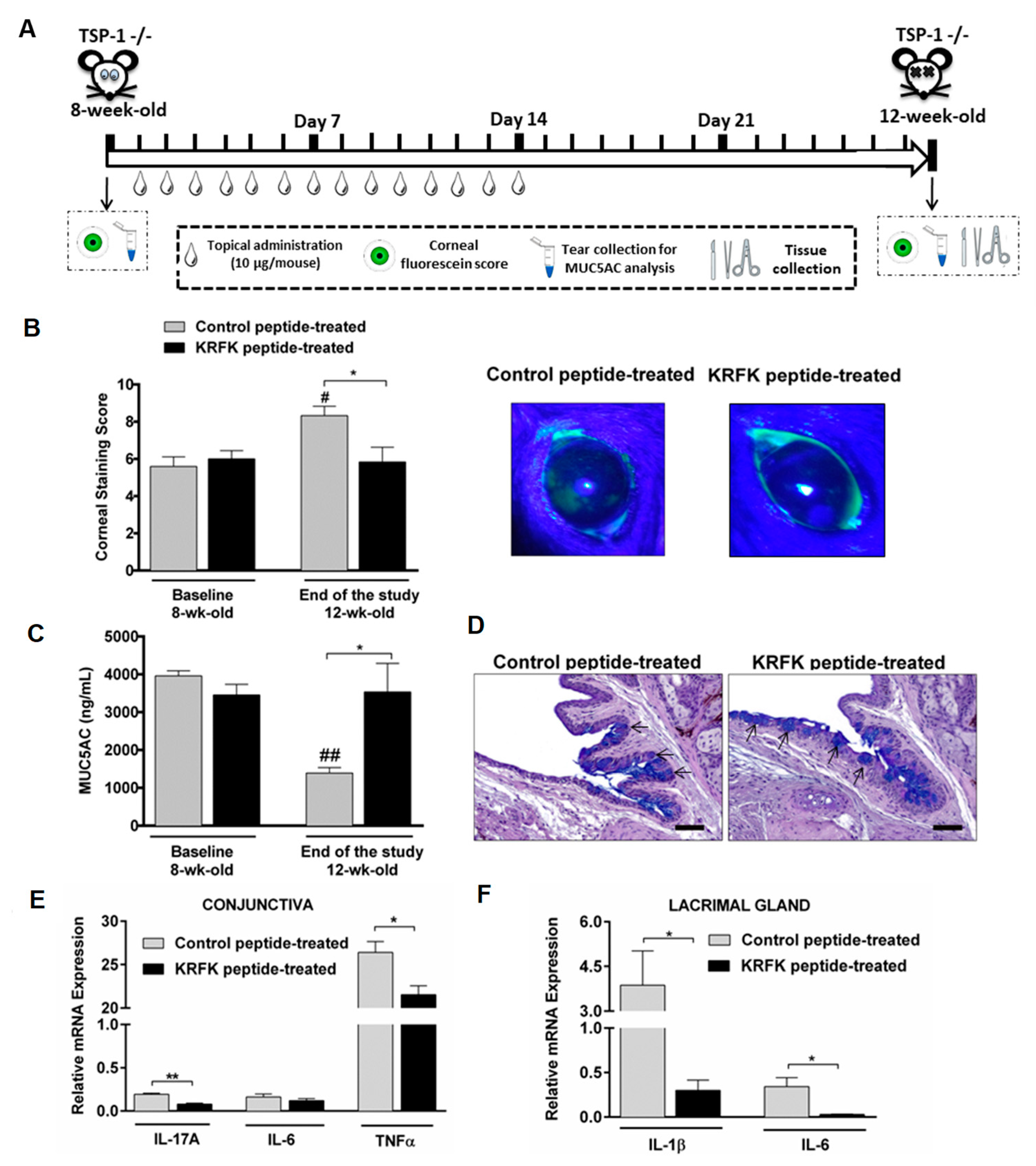
2.4. Topically Administered KRFK Peptide Prevents the Development of Chronic Ocular Inflammation Associated Signs in TSP-1-Deficient Mice
2.5. Topically Administered KRFK Peptide Does not Induce Fibrotic Changes in the Ocular Surface of TSP-1-Deficient Mice
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Antibodies and Reagents
4.3. Bone Marrow-Derived Dendritic Cell Cultures
4.4. TGF-β Bioassay
4.5. Topical FITC-Conjugated TSP-1-Derived Peptide KRFK Administration and In Vivo Localization
4.6. Treatment Set up and Disease Monitoring—Proof of Concept Study
4.7. Intracellular Cytokine Staining and Flow Cytometry
4.8. Tissue Processing and Histopathological Analysis
4.9. Immunofluorescence Staining
4.10. Real Time RT-PCR Analysis
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AB | alcian blue |
| SBE-SEAP | Smad-binding elements coupled to a secreted alkaline phosphatase reporter gene |
| SMA | smooth muscle actin |
References
- Grimbert, P.; Bouguermouh, S.; Baba, N.; Nakajima, T.; Allakhverdi, Z.; Braun, D.; Saito, H.; Rubio, M.; Delespesse, G.; Sarfati, M. Thrombospondin/CD47 interaction: A pathway to generate regulatory T cells from human CD4+ CD25− T cells in response to inflammation. J. Immunol. 2006, 177, 3534–3541. [Google Scholar] [CrossRef] [PubMed]
- Doyen, V.; Rubio, M.; Braun, D.; Nakajima, T.; Abe, J.; Saito, H.; Delespesse, G.; Sarfati, M. Thrombospondin 1 is an autocrine negative regulator of human dendritic cell activation. J. Exp. Med. 2003, 198, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Masli, S.; Turpie, B.; Streilein, J.W. Thrombospondin orchestrates the tolerance-promoting properties of TGFβ-treated antigen-presenting cells. Int. Immunol. 2006, 18, 689–699. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mir, F.A.; Contreras-Ruiz, L.; Masli, S. Thrombospondin-1-dependent immune regulation by transforming growth factor-β2-exposed antigen-presenting cells. Immunology 2015, 146, 547–556. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Ruiz, L.; Masli, S. Immunomodulatory Cross-Talk between Conjunctival Goblet Cells and Dendritic Cells. PLoS ONE 2015, 10, e0120284. [Google Scholar] [CrossRef] [PubMed]
- Turpie, B.; Yoshimura, T.; Gulati, A.; Rios, J.D.; Dartt, D.A.; Masli, S. Sjogren’s syndrome-like ocular surface disease in thrombospondin-1 deficient mice. Am. J. Pathol. 2009, 175, 1136–1147. [Google Scholar] [CrossRef]
- Contreras-Ruiz, L.; Ryan, D.S.; Sia, R.K.; Bower, K.S.; Dartt, D.A.; Masli, S. Polymorphism in THBS1 gene is associated with post-refractive surgery chronic ocular surface inflammation. Ophthalmology 2014, 121, 1389–1397. [Google Scholar] [CrossRef] [PubMed]
- Soriano-Romani, L.; Contreras-Ruiz, L.; Garcia-Posadas, L.; Lopez-Garcia, A.; Masli, S.; Diebold, Y. Inflammatory Cytokine-Mediated Regulation of Thrombospondin-1 and CD36 in Conjunctival Cells. J. Ocul. Pharmacol. Ther. 2015, 31, 419–428. [Google Scholar] [CrossRef] [Green Version]
- Benito, M.J.; Calder, V.; Corrales, R.M.; Garcia-Vazquez, C.; Narayanan, S.; Herreras, J.M.; Stern, M.E.; Calonge, M.; Enriquez-de-Salamanca, A. Effect of TGF-β on ocular surface epithelial cells. Exp. Eye Res. 2013, 107, 88–100. [Google Scholar] [CrossRef]
- Munger, J.S.; Huang, X.; Kawakatsu, H.; Griffiths, M.J.; Dalton, S.L.; Wu, J.; Pittet, J.F.; Kaminski, N.; Garat, C.; Matthay, M.A.; et al. The integrin αvβ6 binds and activates latent TGF β1: A mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999, 96, 319–328. [Google Scholar] [CrossRef]
- Schultz-Cherry, S.; Lawler, J.; Murphy-Ullrich, J.E. The type 1 repeats of thrombospondin 1 activate latent transforming growth factor-β. J. Biol. Chem. 1994, 269, 26783–26788. [Google Scholar]
- Contreras Ruiz, L.; Mir, F.A.; Turpie, B.; Masli, S. Thrombospondin-derived peptide attenuates Sjögren’s syndrome-associated ocular surface inflammation in mice. Clin. Exp. Immunol. 2017, 188, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Sweetwyne, M.T.; Murphy-Ullrich, J.E. Thrombospondin1 in tissue repair and fibrosis: TGF-β-dependent and independent mechanisms. Matrix Biol. J. Int. Soc. Matrix Biol. 2012, 31, 178–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, S.E.; Stellmach, V.; Murphy-Ullrich, J.E.; Ribeiro, S.M.; Lawler, J.; Hynes, R.O.; Boivin, G.P.; Bouck, N. Thrombospondin-1 is a major activator of TGF-β1 in vivo. Cell 1998, 93, 1159–1170. [Google Scholar] [CrossRef]
- Schultz-Cherry, S.; Chen, H.; Mosher, D.F.; Misenheimer, T.M.; Krutzsch, H.C.; Roberts, D.D.; Murphy-Ullrich, J.E. Regulation of transforming growth factor-β activation by discrete sequences of thrombospondin 1. J. Biol. Chem. 1995, 270, 7304–7310. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Ruiz, L.; Regenfuss, B.; Mir, F.A.; Kearns, J.; Masli, S. Conjunctival inflammation in thrombospondin-1 deficient mouse model of Sjogren’s syndrome. PLoS ONE 2013, 8, e75937. [Google Scholar] [CrossRef] [PubMed]
- Shatos, M.A.; Hodges, R.R.; Morinaga, M.; McNay, D.E.; Islam, R.; Bhattacharya, S.; Li, D.; Turpie, B.; Makarenkova, H.P.; Masli, S.; et al. Alteration in cellular turnover and progenitor cell population in lacrimal glands from thrombospondin 1−/− mice, a model of dry eye. Exp. Eye Res. 2016, 153, 27–41. [Google Scholar] [CrossRef]
- Tatematsu, Y.; Khan, Q.; Blanco, T.; Bair, J.A.; Hodges, R.R.; Masli, S.; Dartt, D.A. Thrombospondin-1 Is Necessary for the Development and Repair of Corneal Nerves. Int. J. Mol. Sci. 2018, 19, 3191. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Chin, I.; Gabriel, P.; Blaum, E.; Masli, S. Dysregulated Marginal Zone B Cell Compartment in a Mouse Model of Sjögren’s Syndrome with Ocular Inflammation. Int. J. Mol. Sci. 2018, 19, 3117. [Google Scholar] [CrossRef] [PubMed]
- Yamanaka, O.; Liu, C.-Y.; Kao, W.W.-Y. Fibrosis in the Anterior Segments of the Eye. Endocr. Metab. Immune Disord.-Drug Targets 2010, 10, 331–335. [Google Scholar] [CrossRef]
- Tandon, A.; Tovey, J.C.K.; Sharma, A.; Gupta, R.; Mohan, R.R. Role of transforming growth factor β in corneal function, biology and pathology. Curr. Mol. Med. 2010, 10, 565–578. [Google Scholar]
- Maheshwari, A.; Kelly, D.R.; Nicola, T.; Ambalavanan, N.; Jain, S.K.; Murphy-Ullrich, J.; Athar, M.; Shimamura, M.; Bhandari, V.; Aprahamian, C.; et al. TGF-β2 suppresses macrophage cytokine production and mucosal inflammatory responses in the developing intestine. Gastroenterology 2011, 140, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.W.; Balzar, S.; Seedorf, G.J.; Westcott, J.Y.; Trudeau, J.B.; Silkoff, P.; Wenzel, S.E. Transforming growth factor-β2 induces bronchial epithelial mucin expression in asthma. Am. J. Pathol. 2004, 165, 1097–1106. [Google Scholar] [CrossRef]
- McDermott, A.M.; Perez, V.; Huang, A.J.W.; Pflugfelder, S.C.; Stern, M.E.; Baudouin, C.; Beuerman, R.W.; Burns, A.R.; Calder, V.L.; Calonge, M.; et al. Pathways of corneal and ocular surface inflammation: A perspective from the cullen symposium. Ocul. Surf. 2005, 3, S131–S138. [Google Scholar] [CrossRef]
- Stevenson, W.; Chauhan, S.K.; Dana, R. Dry eye disease: An immune-mediated ocular surface disorder. Arch. Ophthalmol. 2012, 130, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Ibraheem, D.; Elaissari, A.; Fessi, H. Administration strategies for proteins and peptides. Int. J. Pharm. 2014, 477, 578–589. [Google Scholar] [CrossRef] [PubMed]
- Yee, K.O.; Streit, M.; Hawighorst, T.; Detmar, M.; Lawler, J. Expression of the type-1 repeats of thrombospondin-1 inhibits tumor growth through activation of transforming growth factor-β. Am. J. Pathol. 2004, 165, 541–552. [Google Scholar] [CrossRef]
- Nor, J.E.; Dipietro, L.; Murphy-Ullrich, J.E.; Hynes, R.O.; Lawler, J.; Polverini, P.J. Activation of Latent TGF-β1 by Thrombospondin-1 is a Major Component of Wound Repair. Oral Biosci. Med. 2005, 2, 153–161. [Google Scholar]
- Soriano-Romaní, L.; Álvarez-Trabado, J.; López-García, A.; Molina-Martínez, I.; Herrero-Vanrell, R.; Diebold, Y. Improved in vitro corneal delivery of a thrombospondin-1-derived peptide using a liposomal formulation. Exp. Eye Res. 2018, 167, 118–121. [Google Scholar] [CrossRef]
- Urtti, A. Challenges and obstacles of ocular pharmacokinetics and drug delivery. Adv. Drug Deliv. Rev. 2006, 58, 1131–1135. [Google Scholar] [CrossRef]
- Jonckheere, N.; Van Der Sluis, M.; Velghe, A.; Buisine, M.-P.; Sutmuller, M.; Ducourouble, M.-P.; Pigny, P.; Büller, H.A.; Aubert, J.-P.; Einerhand, A.W.C.; et al. Transcriptional activation of the murine Muc5ac mucin gene in epithelial cancer cells by TGF-β/Smad4 signalling pathway is potentiated by Sp1. Biochem. J. 2004, 377, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Contreras-Ruiz, L.; Ghosh-Mitra, A.; Shatos, M.A.; Dartt, D.A.; Masli, S. Modulation of conjunctival goblet cell function by inflammatory cytokines. Mediat. Inflamm. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.W.; Byun, Y.J.; Choi, W.; Jeong, E.; Kim, J.S.; Noh, H.; Kim, E.S.; Song, Y.J.; Park, S.K.; Lee, H.K. Neutralization of Ocular Surface TNF-α Reduces Ocular Surface and Lacrimal Gland Inflammation Induced by In Vivo Dry Eye. Investig. Opthalmol. Vis. Sci. 2013, 54, 7557–7566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lam, H.; Bleiden, L.; de Paiva, C.S.; Farley, W.; Stern, M.E.; Pflugfelder, S.C. Tear cytokine profiles in dysfunctional tear syndrome. Am. J. Ophthalmol. 2009, 147, 198–205.e1. [Google Scholar] [CrossRef] [PubMed]
- Massingale, M.L.; Li, X.; Vallabhajosyula, M.; Chen, D.; Wei, Y.; Asbell, P.A. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea 2009, 28, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Boehm, N.; Riechardt, A.I.; Wiegand, M.; Pfeiffer, N.; Grus, F.H. Proinflammatory cytokine profiling of tears from dry eye patients by means of antibody microarrays. Investig. Ophthalmol. Vis. Sci. 2011, 52, 7725–7730. [Google Scholar] [CrossRef] [PubMed]
- Na, K.S.; Mok, J.W.; Kim, J.Y.; Rho, C.R.; Joo, C.K. Correlations between tear cytokines, chemokines, and soluble receptors and clinical severity of dry eye disease. Investig. Ophthalmol. Vis. Sci. 2012, 53, 5443–5450. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, P.J.; Pflugfelder, S.C.; Stern, M.E.; Hardten, D.R.; Conway, T.; Villanueva, L.; Hollander, D.A. Study design and baseline findings from the progression of ocular findings (PROOF) natural history study of dry eye. BMC Ophthalmol. 2017, 17, 265. [Google Scholar] [CrossRef]
- Kimura, K.; Teranishi, S.; Nishida, T. Interleukin-1β-induced disruption of barrier function in cultured human corneal epithelial cells. Investig. Ophthalmol. Vis. Sci. 2009, 50, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Gallup, M.; Chen, Y.-T.; McNamara, N.A. Molecular Mechanism of Proinflammatory Cytokine-Mediated Squamous Metaplasia in Human Corneal Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 2010, 51, 2466–2475. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.-T.; Nikulina, K.; Lazarev, S.; Bahrami, A.F.; Noble, L.B.; Gallup, M.; McNamara, N.A. Interleukin-1 as a Phenotypic Immunomodulator in Keratinizing Squamous Metaplasia of the Ocular Surface in Sjögren’s Syndrome. Am. J. Pathol. 2010, 177, 1333–1343. [Google Scholar] [CrossRef]
- Madaan, A.; Verma, R.; Singh, A.T.; Jain, S.K.; Jaggi, M. A stepwise procedure for isolation of murine bone marrow and generation of dendritic cells. J. Biol. Methods 2014, 1, e1. [Google Scholar] [CrossRef]
- Tesseur, I.; Zou, K.; Berber, E.; Zhang, H.; Wyss-Coray, T. Highly sensitive and specific bioassay for measuring bioactive TGF-β. BMC Cell Biol. 2006, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Lemp, M.A. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995, 21, 221–232. [Google Scholar] [PubMed]



| Gen | Sequence for forward Primer | Sequence for Reverse Primer |
|---|---|---|
| MHC II 1 | 5’-AGG GCA TTT CGT GTA CCA GTT-3’ | 5’-GTA CTC CTC CCG GTT GTA GAT-3’ |
| CD80 | 5’-GAA TTA CCT GGC ATC AAT ACG-3’ | 5’-CTT AAT GGT GTG GTT GCG AGT C-3’ |
| IL-6 2 | 5’-AGT CAA TTC CAG AAA CCG CTA TGA-3’ | 5’-TAG GGA AGG CCG TGG TTG T-3’ |
| IL-1β 2 | 5’-TCT GAA GCA GCT ATG GCA ACT GTT-3’ | 5’-CAT CTT TTG GGG TCC GTC AAC T-3’ |
| TNFα 3 | 5’-GGC CTC CCT CTC ATC AGT TCT ATG-3’ | 5’-GTT TGC TAC GAC GTG GGC TAC A-3’ |
| IL-17A 2 | 5’-AGT GAA GGC AGC AGC GAT CAT-3’ | 5’-CGC CAA GGG AGT TAA AG-3’ |
| GAPDH 4 | 5’-GAACGTGAAGGTCGGAGTCAAC-3’ | 5’-CGTGAAGATGGTGATGGGATTTC-3’ |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soriano-Romaní, L.; Contreras-Ruiz, L.; López-García, A.; Diebold, Y.; Masli, S. Topical Application of TGF-β-Activating Peptide, KRFK, Prevents Inflammatory Manifestations in the TSP-1-Deficient Mouse Model of Chronic Ocular Inflammation. Int. J. Mol. Sci. 2019, 20, 9. https://doi.org/10.3390/ijms20010009
Soriano-Romaní L, Contreras-Ruiz L, López-García A, Diebold Y, Masli S. Topical Application of TGF-β-Activating Peptide, KRFK, Prevents Inflammatory Manifestations in the TSP-1-Deficient Mouse Model of Chronic Ocular Inflammation. International Journal of Molecular Sciences. 2019; 20(1):9. https://doi.org/10.3390/ijms20010009
Chicago/Turabian StyleSoriano-Romaní, Laura, Laura Contreras-Ruiz, Antonio López-García, Yolanda Diebold, and Sharmila Masli. 2019. "Topical Application of TGF-β-Activating Peptide, KRFK, Prevents Inflammatory Manifestations in the TSP-1-Deficient Mouse Model of Chronic Ocular Inflammation" International Journal of Molecular Sciences 20, no. 1: 9. https://doi.org/10.3390/ijms20010009