P2X7 Receptor Signaling in Stress and Depression
Abstract
:1. Introduction
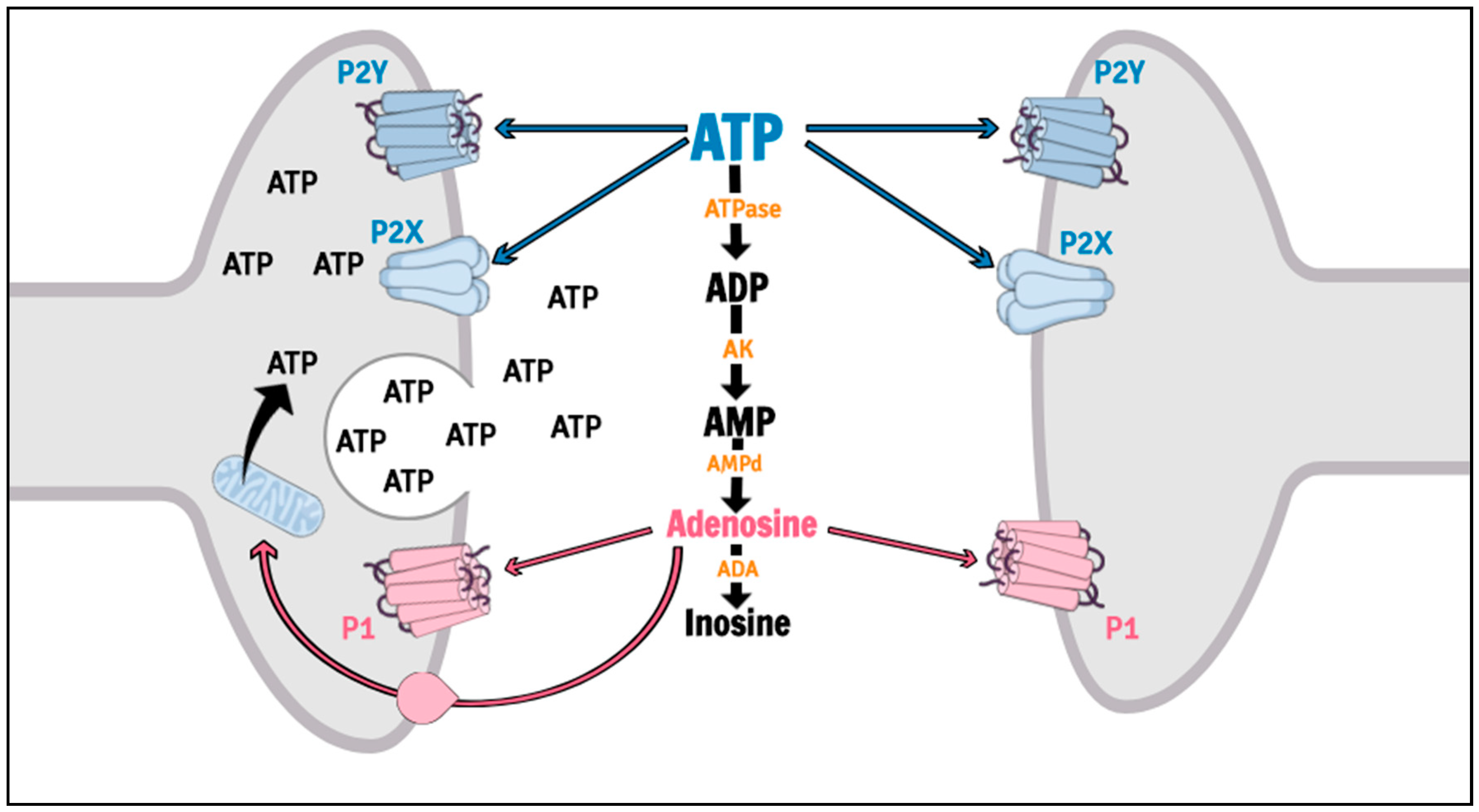
2. Overview of P2 Receptor-Mediated Signaling in the Brain
3. Targeting P2X7 Receptor in Stress and Depression
3.1. Human Studies
3.2. Pre-Clinical Studies
4. Mechanisms Regulated by P2X7 Receptor Signaling with Relevance to Stress and Depression
4.1. Neurochemical Mechanisms
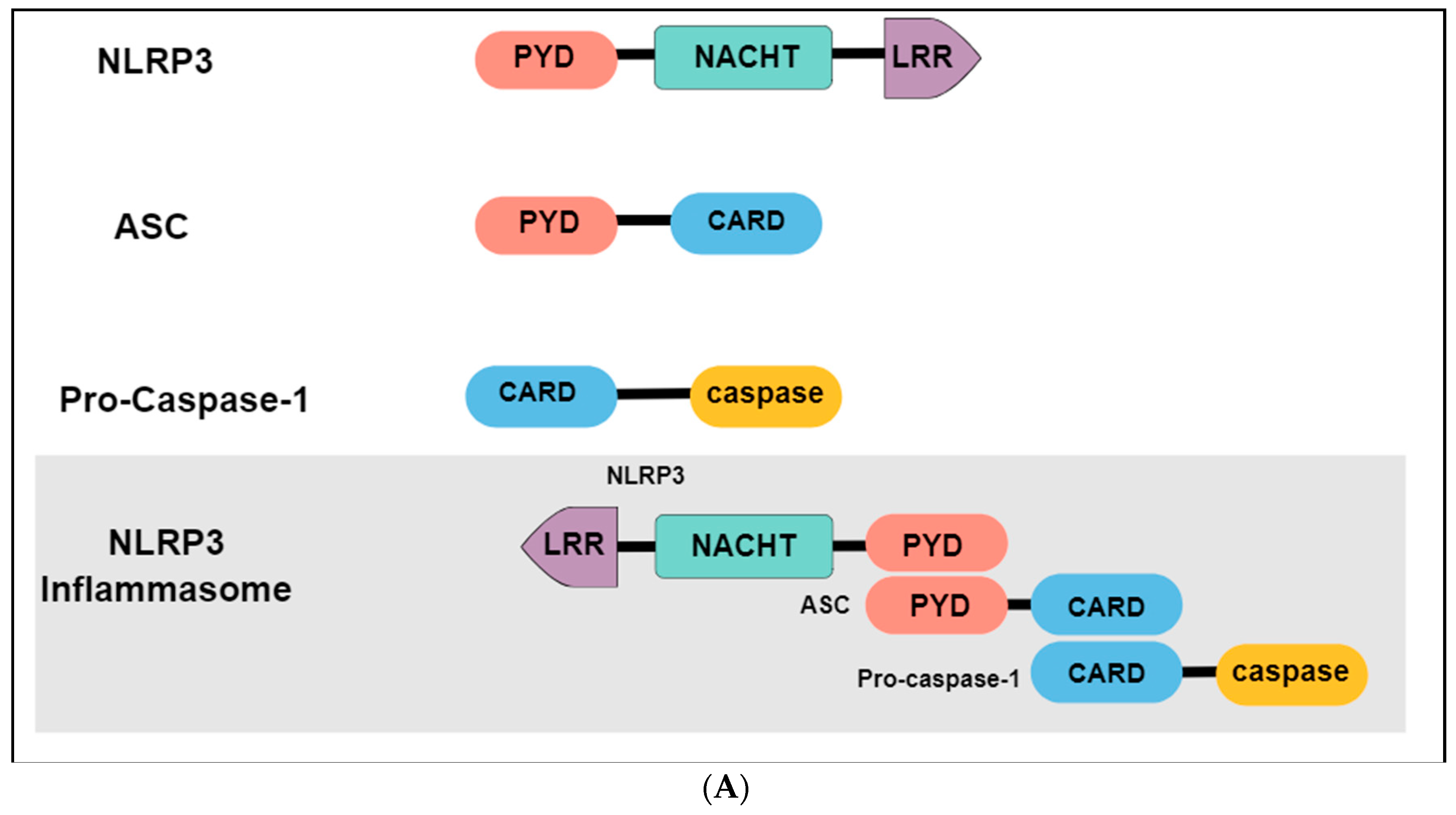
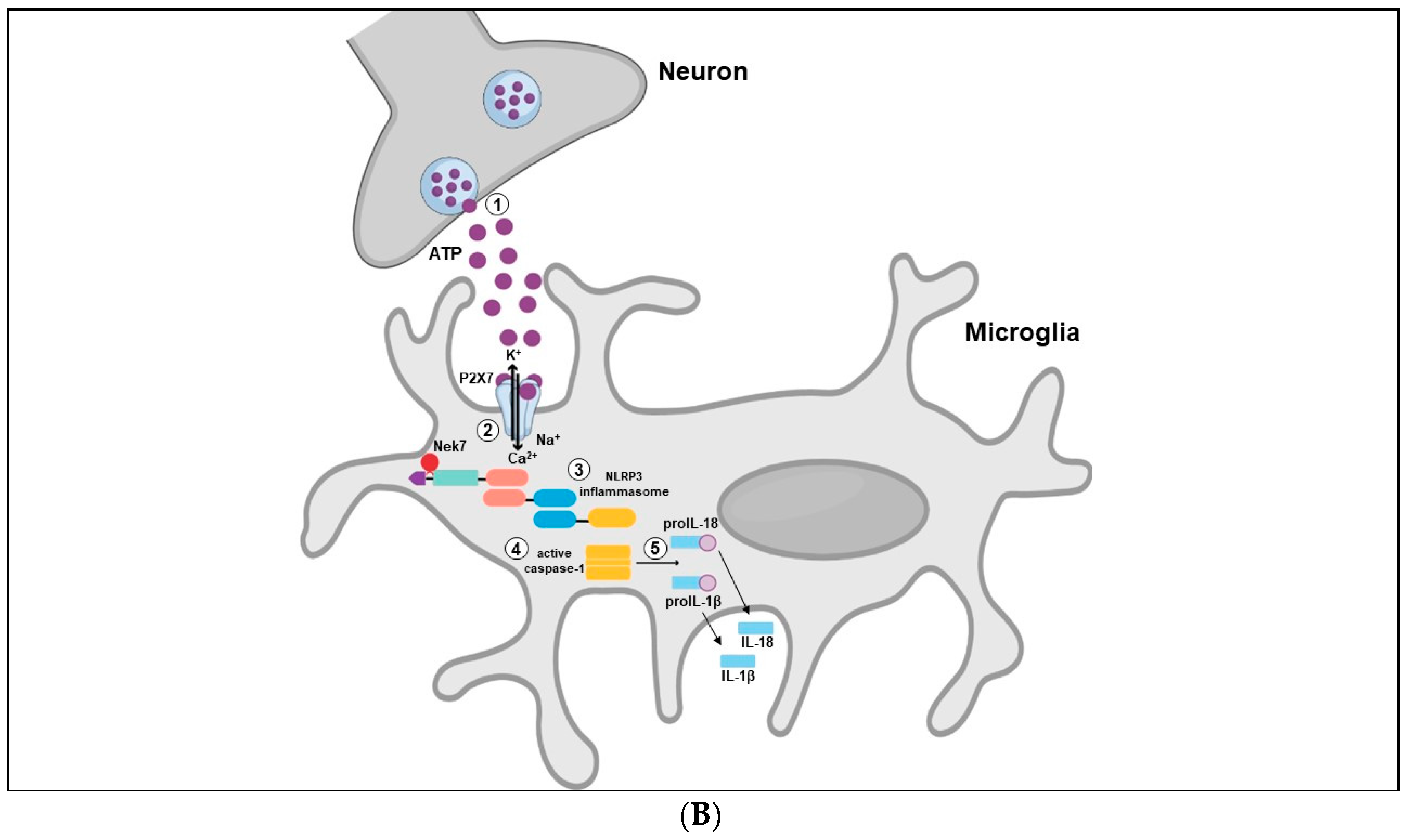
4.2. Neuroinflammatory Response and Inflammasome Activation
4.3. Neurogenesis and Neuroplasticity Process
5. Final Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kendler, K.S.; Gardner, C.O.; Prescott, C.A. Toward a comprehensive developmental model for major depression in men. Am. J. Psychiatry 2006, 163, 115–124. [Google Scholar] [CrossRef]
- Kendler, K.S.; Gardner, C.O.; Prescott, C.A. Toward a comprehensive developmental model for major depression in women. Am. J. Psychiatry 2002, 159, 1133–1145. [Google Scholar] [CrossRef] [PubMed]
- Post, R.M. Transduction of psychosocial stress into the neurobiology of recurrent affective disorder. Am. J. Psychiatry 1992, 149, 999–1010. [Google Scholar] [PubMed]
- Caan, W. The Global Crisis of Depression: The low of the 21st century? Perspect. Public Health 2015, 135, 62. [Google Scholar] [PubMed]
- Ferrari, A.J.; Charlson, F.J.; Norman, R.E.; Flaxman, A.D.; Patten, S.B.; Vos, T.; Whiteford, H.A. The Epidemiological Modelling of Major Depressive Disorder: Application for the Global Burden of Disease Study 2010. PLoS ONE 2013, 8, e69637. [Google Scholar] [CrossRef]
- World Health Organization. WHO the Global Burden OF Disease: 2004 Update; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Chang, C.K.; Hayes, R.D.; Perera, G.; Broadbent, M.T.M.; Fernandes, A.C.; Lee, W.E.; Hotopf, M.; Stewart, R. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLoS ONE 2011, 6, e19590. [Google Scholar] [CrossRef]
- Laursen, T.M.; Musliner, K.L.; Benros, M.E.; Vestergaard, M.; Munk-Olsen, T. Mortality and life expectancy in persons with severe unipolar depression. J. Affect. Disord. 2016, 193, 203–207. [Google Scholar] [CrossRef]
- Chisholm, D.; Sweeny, K.; Sheehan, P.; Rasmussen, B.; Smit, F.; Cuijpers, P.; Saxena, S. Scaling-up treatment of depression and anxiety: A global return on investment analysis. Lancet Psychiatry 2016, 3, 415–424. [Google Scholar] [CrossRef]
- Wang, P.S.; Simon, G.; Kessler, R.C. The economic burden of depression and the cost-effectiveness of treatment. Int. J. Methods Psychiatr. Res. 2003, 12, 22–33. [Google Scholar] [CrossRef]
- Greenberg, P.E.; Fournier, A.A.; Sisitsky, T.; Pike, C.T.; Kessler, R.C. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). J. Clin. Psychiatry 2015, 76, 155–162. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Philadelphia, PA, USA, 2013; ISBN 9780890425572. [Google Scholar]
- Blier, P. The pharmacology of putative early-onset antidepressant strategies. Eur. Neuropsychopharmacol. 2003, 13, 57–66. [Google Scholar] [CrossRef]
- Machado-Vieira, R.; Baumann, J.; Wheeler-Castillo, C.; Latov, D.; Henter, I.D.; Salvadore, G.; Zarate, C.A. The timing of antidepressant effects: A comparison of diverse pharmacological and somatic treatments. Pharmaceuticals 2010, 3, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Santarsieri, D.; Schwartz, T.L. Antidepressant efficacy and side-effect burden: A quick guide for clinicians. Drugs Context 2015, 4, 212290. [Google Scholar] [CrossRef] [PubMed]
- Schildkraut, J.J. The catecholamine hypothesis of affective disorders: A review of supporting evidence. Am. J. Psychiatry 1965, 122, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Dean, J.; Keshavan, M. The neurobiology of depression: An integrated view. Asian J. Psychiatr. 2017, 27, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Sluyter, R. The P2X7 receptor. In Advances in Experimental Medicine and Biology; Springer: Berlin, Germany, 2017; Volume 1051, pp. 17–53. [Google Scholar]
- Gölöncsér, F.; Baranyi, M.; Balázsfi, D.; Demeter, K.; Haller, J.; Freund, T.F.F.; Zelena, D.; Sperlágh, B. Regulation of Hippocampal 5-HT Release by P2X7 Receptors in Response to Optogenetic Stimulation of Median Raphe Terminals of Mice. Front. Mol. Neurosci. 2017, 10, 325. [Google Scholar] [CrossRef] [PubMed]
- Csölle, C.; Baranyi, M.; Zsilla, G.; Kittel, A.; Gölöncsér, F.; Illes, P.; Papp, E.; Vizi, E.S.; Sperlágh, B. Neurochemical Changes in the Mouse Hippocampus Underlying the Antidepressant Effect of Genetic Deletion of P2X7 Receptors. PLoS ONE 2013, 8, e66547. [Google Scholar] [CrossRef]
- Papp, L. P2X Receptor Activation Elicits Transporter-Mediated Noradrenaline Release from Rat Hippocampal Slices. J. Pharmacol. Exp. Ther. 2004, 310, 973–980. [Google Scholar] [CrossRef] [Green Version]
- Mayhew, J.; Graham, B.A.; Biber, K.; Nilsson, M.; Walker, F.R. Purinergic modulation of glutamate transmission: An expanding role in stress-linked neuropathology. Neurosci. Biobehav. Rev. 2018, 93, 26–37. [Google Scholar] [CrossRef]
- Iwata, M.; Ota, K.T.; Li, X.Y.; Sakaue, F.; Li, N.; Dutheil, S.; Banasr, M.; Duric, V.; Yamanashi, T.; Kaneko, K.; et al. Psychological Stress Activates the Inflammasome via Release of Adenosine Triphosphate and Stimulation of the Purinergic Type 2X7 Receptor. Biol. Psychiatry 2016, 80, 12–22. [Google Scholar] [CrossRef]
- Otrokocsi, L.; Kittel, Á.; Sperlágh, B. P2X7 receptors drive spine synapse plasticity in the learned helplessness model of depression. Int. J. Neuropsychopharmacol. 2017, 20, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Sperlagh, B.; Csolle, C.; Ando, R.D.; Goloncser, F.; Kittel, A.; Baranyi, M. The role of purinergic signaling in depressive disorders. Neuropsychopharmacol. Hung. 2012, 14, 231–238. [Google Scholar] [PubMed]
- Cheffer, A.; Castillo, A.R.G.; Corrêa-Velloso, J.; Gonçalves, M.C.B.; Naaldijk, Y.; Nascimento, I.C.; Burnstock, G.; Ulrich, H. Purinergic system in psychiatric diseases. Mol. Psychiatry 2018, 23, 94–106. [Google Scholar] [CrossRef]
- Burnstock, G. Purinergic Signalling and Neurological Diseases: An Update. CNS Neurol. Disord. Drug Targets 2017, 16, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Sperlágh, B.; Illes, P. P2X7 receptor: An emerging target in central nervous system diseases. Trends Pharmacol. Sci. 2014, 35, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Verkhratsky, A. Receptors for Purines and Pyrimidines. In Purinergic Signalling and the Nervous System; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 978-3-642-28862-3. [Google Scholar]
- Knight, G.E. Purinergic Receptors. In Encyclopedia of Neuroscience; Elsevier: Amsterdam, The Netherlands, 2010; ISBN 9780080450469. [Google Scholar]
- Yegutkin, G.G. Enzymes involved in metabolism of extracellular nucleotides and nucleosides: Functional implications and measurement of activities. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 473–497. [Google Scholar] [CrossRef]
- Zimmermann, H.; Zebisch, M.; Sträter, N. Cellular function and molecular structure of ecto-nucleotidases. Purinergic Signal. 2012, 8, 437–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deussing, J.M.; Arzt, E. P2X7 Receptor: A Potential Therapeutic Target for Depression? Trends Mol. Med. 2018, 24, 736–747. [Google Scholar] [CrossRef]
- Surprenant, A.; Rassendren, F.; Kawashima, E.; North, R.A.; Buell, G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7). Science 1996, 272, 735–738. [Google Scholar] [CrossRef]
- Brown, G.C. Control of respiration and ATP synthesis in mammalian mitochondria and cells. Biochem. J. 2015, 284, 1–13. [Google Scholar] [CrossRef]
- White, T.D. Direct detection of depolarisation-induced release of ATP from a synaptosomal preparation. Nature 1977, 267, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Sawada, K.; Echigo, N.; Juge, N.; Miyaji, T.; Otsuka, M.; Omote, H.; Yamamoto, A.; Moriyama, Y. Identification of a vesicular nucleotide transporter. Proc. Natl. Acad. Sci. USA 2008, 105, 5683–5686. [Google Scholar] [CrossRef] [Green Version]
- Bodin, P.; Burnstock, G. Purinergic signalling: ATP release. Neurochem. Res. 2001, 26, 959–969. [Google Scholar] [CrossRef] [PubMed]
- Pankratov, Y.; Lalo, U.; Verkhratsky, A.; North, R.A. Vesicular release of ATP at central synapses. Pflugers Arch. Eur. J. Physiol. 2006, 452, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Imura, Y.; Morizawa, Y.; Komatsu, R.; Shibata, K.; Shinozaki, Y.; Kasai, H.; Moriishi, K.; Moriyama, Y.; Koizumi, S. Microglia release ATP by exocytosis. Glia 2013, 61, 1320–1330. [Google Scholar] [CrossRef]
- Makarenkova, H.P.; Shestopalov, V.I. The role of pannexin hemichannels in inflammation and regeneration. Front. Physiol. 2014, 5, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cotrina, M.L.; Lin, J.H.C.; Alves-Rodrigues, A.; Liu, S.; Li, J.; Azmi-Ghadimi, H.; Kang, J.; Naus, C.C.G.; Nedergaard, M. Connexins regulate calcium signaling by controlling ATP release. Proc. Natl. Acad. Sci. USA 1998, 95, 15735–15740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elliott, M.R.; Chekeni, F.B.; Trampont, P.C.; Lazarowski, E.R.; Kadl, A.; Walk, S.F.; Park, D.; Woodson, R.I.; Ostankovich, M.; Sharma, P.; et al. Nucleotides released by apoptotic cells act as a find-me signal to promote phagocytic clearance. Nature 2009, 461, 282–286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suadicani, S.O. P2X7 Receptors Mediate ATP Release and Amplification of Astrocytic Intercellular Ca2+ Signaling. J. Neurosci. 2006, 26, 1378–1385. [Google Scholar] [CrossRef] [PubMed]
- Brandao-Burch, A.; Key, M.L.; Patel, J.J.; Arnett, T.R.; Orriss, I.R. The P2X7 receptor is an important regulator of extracellular ATP levels. Front. Endocrinol. 2012, 3, 41. [Google Scholar] [CrossRef]
- Donnelly-Roberts, D.L.; Namovic, M.T.; Han, P.; Jarvis, M.F. Mammalian P2X7 receptor pharmacology: Comparison of recombinant mouse, rat and human P2X7 receptors. Br. J. Pharmacol. 2009, 157, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Illes, P.; Khan, T.M.; Rubini, P. Neuronal P2X7 Receptors Revisited: Do They Really Exist? J. Neurosci. 2017, 37, 7049–7062. [Google Scholar] [CrossRef] [PubMed]
- Miras-Portugal, M.T.; Sebastián-Serrano, Á.; de Diego García, L.; Díaz-Hernández, M. Neuronal P2X7 Receptor: Involvement in Neuronal Physiology and Pathology. J. Neurosci. 2017, 37, 7063–7072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sim, J.A. Reanalysis of P2X7 Receptor Expression in Rodent Brain. J. Neurosci. 2004, 24, 6307–6314. [Google Scholar] [CrossRef] [PubMed]
- Deuchars, S.A.; Atkinson, L.; Brooke, R.E.; Musa, H.; Milligan, C.J.; Batten, T.F.; Buckley, N.J.; Parson, S.H.; Deuchars, J. Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. J. Neurosci. 2001, 21, 7143–7152. [Google Scholar] [CrossRef] [PubMed]
- Sperlágh, B.; Köfalvi, A.; Deuchars, J.; Atkinson, L.; Milligan, C.J.; Buckley, N.J.; Vizi, E.S. Involvement of P2X7 receptors in the regulation of neurotransmitter release in the rat hippocampus. J. Neurochem. 2002, 81, 1196–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wirkner, K.; Köfalvi, A.; Fischer, W.; Günther, A.; Franke, H.; Gröger-Arndt, H.; Nörenberg, W.; Madarász, E.; Vizi, E.S.; Schneider, D.; et al. Supersensitivity of P2X7 receptors in cerebrocortical cell cultures after in vitro ischemia. J. Neurochem. 2005, 95, 1421–1437. [Google Scholar] [CrossRef] [PubMed]
- Yue, N.; Huang, H.; Zhu, X.; Han, Q.; Wang, Y.; Li, B.; Liu, Q.; Wu, G.; Zhang, Y.; Yu, J. Activation of P2X7 receptor and NLRP3 inflammasome assembly in hippocampal glial cells mediates chronic stress-induced depressive-like behaviors. J. Neuroinflamm. 2017, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Ugawa, S.; Ueda, T.; Ishida, Y.; Inoue, K.; Kyaw Nyunt, A.; Umemura, A.; Mase, M.; Yamada, K.; Shimada, S. Cellular localization of P2X7 receptor mRNA in the rat brain. Brain Res. 2008, 1194, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Metzger, M.W.; Walser, S.M.; Aprile-Garcia, F.; Dedic, N.; Chen, A.; Holsboer, F.; Arzt, E.; Wurst, W.; Deussing, J.M. Genetically dissecting P2rx7 expression within the central nervous system using conditional humanized mice. Purinergic Signal. 2017, 13, 153–170. [Google Scholar] [CrossRef]
- Masin, M.; Young, C.; Lim, K.; Barnes, S.J.; Xu, X.J.; Marschall, V.; Brutkowski, W.; Mooney, E.R.; Gorecki, D.C.; Murrell-Lagnado, R. Expression, assembly and function of novel C-terminal truncated variants of the mouse P2X7 receptor: Re-evaluation of P2X7 knockouts. Br. J. Pharmacol. 2012, 165, 978–993. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Nogueiro, J.; Marín-García, P.; Miras-Portugal, M.T. Characterization of a functional P2X7-like receptor in cerebellar granule neurons from P2X7 knockout mice. FEBS Lett. 2005, 579, 3783–3788. [Google Scholar] [CrossRef] [PubMed]
- Rubini, P.; Pagel, G.; Mehri, S.; Marquardt, P.; Riedel, T.; Illes, P. Functional P2X7 receptors at cultured hippocampal astrocytes but not neurons. Naunyn Schmiedebergs Arch. Pharmacol. 2014, 387, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.T.; Deussing, J.; Tang, Y.; Illes, P. Astrocytic rather than neuronal P2X7 receptors modulate the function of the tri-synaptic network in the rodent hippocampus. Brain Res. Bull. 2018. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.M.; Nedergaard, M. Emerging challenges of assigning P2X7 receptor function and immunoreactivity in neurons. Trends Neurosci. 2006, 29, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Carrasquero, L.M.G.; Delicado, E.G.; Bustillo, D.; Gutiérrez-Martín, Y.; Artalejo, A.R.; Miras-Portugal, M.T. P2X7 and P2Y13 purinergic receptors mediate intracellular calcium responses to BzATP in rat cerebellar astrocytes. J. Neurochem. 2009, 110, 879–889. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Hernandez, M.; del Puerto, A.; Díaz-Hernandez, J.I.; Diez-Zaera, M.; Lucas, J.J.; Garrido, J.J.; Miras-Portugal, M.T. Inhibition of the ATP-gated P2X7 receptor promotes axonal growth and branching in cultured hippocampal neurons. J. Cell Sci. 2008, 121, 3717–3728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hervás, C.; Pérez-Sen, R.; Miras-Portugal, M.T. Coexpression of functional P2X and P2Y nucleotide receptors in single cerebellar granule cells. J. Neurosci. Res. 2003, 73, 384–399. [Google Scholar] [CrossRef]
- Ortega, F.; Pérez-Sen, R.; Delicado, E.G.; Miras-Portugal, M.T. P2X7 Nucleotide Receptor is Coupled to GSK-3 Inhibition and Neuroprotection in Cerebellar Granule Neurons. Neurotox. Res. 2009, 15, 193–204. [Google Scholar] [CrossRef] [Green Version]
- Jimenez-Mateos, E.M.; Smith, J.; Nicke, A.; Engel, T. Regulation of P2X7 receptor expression and function in the brain. Brain Res. Bull. 2019. [Google Scholar] [CrossRef]
- Burnstock, G.; Knight, G.E. The potential of P2X7 receptors as a therapeutic target, including inflammation and tumour progression. Purinergic Signal. 2018, 14, 1–18. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Biber, K. The microglial ATP-gated ion channel P2X7 as a CNS drug target. Glia 2016, 64, 1772–1787. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A. Recent advances in CNS P2X7 physiology and pharmacology: Focus on neuropsychiatric disorders. Front. Pharmacol. 2018, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Shink, E.; Morissette, J.; Sherrington, R.; Barden, N. A genome-wide scan points to a susceptibility locus for bipolar disorder on chromosome 12. Mol. Psychiatry 2005, 10, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Luo, Z.; Gu, C.; Hall, L.S.; McIntosh, A.M.; Zeng, Y.; Porteous, D.J.; Hayward, C.; Li, M.; Yao, Y.G.; et al. Common variants on 6q16.2, 12q24.31 and 16p13.3 are associated with major depressive disorder. Neuropsychopharmacology 2018, 43, 2146–2153. [Google Scholar] [CrossRef]
- Lucae, S.; Salyakina, D.; Barden, N.; Harvey, M.; Gagné, B.; Labbé, M.; Binder, E.B.; Uhr, M.; Paez-Pereda, M.; Sillaber, I.; et al. P2RX7, a gene coding for a purinergic ligand-gated ion channel, is associated with major depressive disorder. Hum. Mol. Genet. 2006, 15, 2438–2445. [Google Scholar] [CrossRef]
- Hejjas, K.; Szekely, A.; Domotor, E.; Halmai, Z.; Balogh, G.; Schilling, B.; Sarosi, A.; Faludi, G.; Sasvari-Szekely, M.; Nemoda, Z. Association between depression and the Gln460Arg polymorphism of P2RX7 gene: A dimensional approach. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2009, 150B, 295–299. [Google Scholar] [CrossRef]
- Halmai, Z.; Dome, P.; Vereczkei, A.; Abdul-Rahman, O.; Szekely, A.; Gonda, X.; Faludi, G.; Sasvari-Szekely, M.; Nemoda, Z. Associations between depression severity and purinergic receptor P2RX7 gene polymorphisms. J. Affect. Disord. 2013, 150, 104–109. [Google Scholar] [CrossRef]
- Soronen, P.; Mantere, O.; Melartin, T.; Suominen, K.; Vuorilehto, M.; Rytsälä, H.; Arvilommi, P.; Holma, I.; Holma, M.; Jylhä, P.; et al. P2RX7 gene is associated consistently with mood disorders and predicts clinical outcome in three clinical cohorts. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2011, 156B, 435–447. [Google Scholar] [CrossRef]
- Feng, W.P.; Zhang, B.; Li, W.; Liu, J. Lack of association of P2RX7 gene rs2230912 polymorphism with mood disorders: A meta-analysis. PLoS ONE 2014, 9, e88575. [Google Scholar] [CrossRef]
- Czamara, D.; Müller-Myhsok, B.; Lucae, S. The P2RX7 polymorphism rs2230912 is associated with depression: A meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 82, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Metzger, M.W.; Walser, S.M.; Dedic, N.; Aprile-Garcia, F.; Jakubcakova, V.; Adamczyk, M.; Webb, K.J.; Uhr, M.; Refojo, D.; Schmidt, M.V.; et al. Heterozygosity for the mood disorder-associated variant Gln460Arg alters P2X7 receptor function and sleep quality. J. Neurosci. 2017, 37, 11688–11700. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xiang, Z.H.; Jiang, C.L.; Liu, W.Z.; Shang, Z.L. Effects of antidepressants on P2X7 receptors. Psychiatry Res. 2016, 242, 281–287. [Google Scholar] [CrossRef] [PubMed]
- Dao-Ung, P.; Skarratt, K.K.; Fuller, S.J.; Stokes, L. Paroxetine suppresses recombinant human P2X7 responses. Purinergic Signal. 2015, 11, 481–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, W.-J.; Zhang, T.; Jiang, C.-L.; Wang, W. Clemastine Alleviates Depressive-Like Behavior Through Reversing the Imbalance of Microglia-Related Pro-inflammatory State in Mouse Hippocampus. Front. Cell. Neurosci. 2018, 12, 412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, S.; Wang, Y.; Chen, K.; Long, Z.; Zou, J. Ketamine Alleviates Depressive-Like Behaviors via Down-Regulating Inflammatory Cytokines Induced by Chronic Restraint Stress in Mice. Biol. Pharm. Bull 2017, 40, 1260–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ribeiro, D.E.; Stanquini, L.A.; Biojone, C.; Casarotto, P.C.; Elfving, B.; Müller, H.K.; Wegener, G.; Joca, S.R.L. P2X7 receptors are involved in stress-related behaviours and antidepressant effect. Eur. Neuropsychopharmacol. 2019, 29, S213–S214. [Google Scholar] [CrossRef]
- Kongsui, R.; Beynon, S.B.; Johnson, S.J.; Mayhew, J.; Kuter, P.; Nilsson, M.; Walker, F.R. Chronic stress induces prolonged suppression of the P2X7 receptor within multiple regions of the hippocampus: A cumulative threshold spectra analysis. Brain. Behav. Immun. 2014, 42, 69–80. [Google Scholar] [CrossRef]
- Basso, A.M.; Bratcher, N.A.; Harris, R.R.; Jarvis, M.F.; Decker, M.W.; Rueter, L.E. Behavioral profile of P2X7 receptor knockout mice in animal models of depression and anxiety: Relevance for neuropsychiatric disorders. Behav. Brain Res. 2009, 198, 83–90. [Google Scholar] [CrossRef]
- Csölle, C.; Andó, R.D.; Kittel, Á.; Gölöncsér, F.; Baranyi, M.; Soproni, K.; Zelena, D.; Haller, J.; Németh, T.; Mócsai, A.; et al. The absence of P2X7 receptors (P2rx7) on non-haematopoietic cells leads to selective alteration in mood-related behaviour with dysregulated gene expression and stress reactivity in mice. Int. J. Neuropsychopharmacol. 2013, 16, 213–233. [Google Scholar] [CrossRef] [Green Version]
- Boucher, A.A.; Arnold, J.C.; Hunt, G.E.; Spiro, A.; Spencer, J.; Brown, C.; McGregor, I.S.; Bennett, M.R.; Kassiou, M. Resilience and reduced c-Fos expression in P2X7 receptor knockout mice exposed to repeated forced swim test. Neuroscience 2011, 189, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.S.; Casarotto, P.C.; Hiroaki-Sato, V.A.; Sartim, A.G.; Guimarães, F.S.; Joca, S.R. Antidepressant- and anticompulsive-like effects of purinergic receptor blockade: Involvement of nitric oxide. Eur. Neuropsychopharmacol. 2013, 23, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Farooq, R.K.; Tanti, A.; Ainouche, S.; Roger, S.; Belzung, C.; Camus, V. A P2X7 receptor antagonist reverses behavioural alterations, microglial activation and neuroendocrine dysregulation in an unpredictable chronic mild stress (UCMS) model of depression in mice. Psychoneuroendocrinology 2018, 97, 120–130. [Google Scholar] [CrossRef] [PubMed]
- Overstreet, D.H.; Friedman, E.; Mathé, A.A.; Yadid, G. The Flinders Sensitive Line rat: A selectively bred putative animal model of depression. Neurosci. Biobehav. Rev. 2005, 29, 739–759. [Google Scholar] [CrossRef] [PubMed]
- Overstreet, D.H.; Wegener, G. The Flinders Sensitive Line Rat Model of Depression—25 Years and Still Producing. Pharmacol. Rev. 2013, 65, 143–155. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, J.M.; Hueston, C.M.; Deak, M.M.; Deak, T. The impact of the P2X7 receptor antagonist A-804598 on neuroimmune and behavioral consequences of stress. Behav. Pharmacol. 2014, 25, 582–598. [Google Scholar] [PubMed]
- Fujino, K.; Yoshitake, T.; Inoue, O.; Ibii, N.; Kehr, J.; Ishida, J.; Nohta, H.; Yamaguchi, M. Increased serotonin release in mice frontal cortex and hippocampus induced by acute physiological stressors. Neurosci. Lett. 2002, 320, 91–95. [Google Scholar] [CrossRef]
- De Torre-Minguela, C.; del Castillo, P.M.; Pelegrín, P. The NLRP3 and pyrin inflammasomes: Implications in the pathophysiology of autoinflammatory diseases. Front. Immunol. 2017, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Von Kügelgen, I.; Koch, H.; Starke, K. P2-receptor-mediated inhibition of serotonin release in the rat brain cortex. Neuropharmacology 1997, 36, 1221–1227. [Google Scholar] [CrossRef]
- Adell, A.; Garcia-Marquez, C.; Armario, A.; Gelpi, E. Chronic Stress Increases Serotonin and Noradrenaline in Rat Brain and Sensitizes Their Responses to a Further Acute Stress. J. Neurochem. 1988, 50, 1678–1681. [Google Scholar] [CrossRef] [PubMed]
- Diniz, C.; Rodrigues, M.; Casarotto, P.C.; Pereira, V.S.; Crestani, C.C.; Joca, S.R.L. Monoamine involvement in the antidepressant-like effect induced by P2 blockade. Brain Res. 2017, 1676, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, G.R.J.; Baimoukhametova, D.V.; Hewitt, S.A.; Rajapaksha, W.R.A.K.J.S.; Fisher, T.E.; Bains, J.S. Norepinephrine triggers release of glial ATP to increase postsynaptic efficacy. Nat. Neurosci. 2005, 8, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, L.; Batten, T.F.C.; Moores, T.S.; Varoqui, H.; Erickson, J.D.; Deuchars, J. Differential co-localisation of the P2X7 receptor subunit with vesicular glutamate transporters VGLUT1 and VGLUT2 in rat CNS. Neuroscience 2004, 123, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Papp, L.; Vizi, E.S.; Sperlágh, B. Lack of ATP-evoked GABA and glutamate release in the hippocampus of P2X7 receptor-/- mice. Neuroreport 2004, 15, 2387–2391. [Google Scholar] [CrossRef] [PubMed]
- Koványi, B.; Csölle, C.; Calovi, S.; Hanuska, A.; Kató, E.; Köles, L.; Bhattacharya, A.; Haller, J.; Sperlágh, B. The role of P2X7 receptors in a rodent PCP-induced schizophrenia model. Sci. Rep. 2016, 6, 36680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angulo, M.C. Glutamate Released from Glial Cells Synchronizes Neuronal Activity in the Hippocampus. J. Neurosci. 2004, 24, 6920–6927. [Google Scholar] [CrossRef] [Green Version]
- Kukley, M. Ecto-Nucleotidases and Nucleoside Transporters Mediate Activation of Adenosine Receptors on Hippocampal Mossy Fibers by P2X7 Receptor Agonist 2′-3′-O-(4-Benzoylbenzoyl)-ATP. J. Neurosci. 2004, 24, 7128–7139. [Google Scholar] [CrossRef] [PubMed]
- Popoli, P.; Frank, C.; Tebano, M.T.; Potenza, R.L.; Pintor, A.; Domenici, M.R. Modulation of glutamate release and excitotoxicity by adenosine A2A receptors. Neurology 2003, 61, S69–S71. [Google Scholar] [CrossRef]
- Inoue, K.; Nakazawa, K.; Fujimori, K.; Watano, T.; Takanaka, A. Extracellular adenosine 5′-triphosphate-evoked glutamate release in cultured hippocampal neurons. Neurosci. Lett. 1992, 134, 215–218. [Google Scholar] [CrossRef]
- Popoli, P.; Betto, P.; Reggio, R.; Ricciarello, G. Adenosine A2A receptor stimulation enhances striatal extracellular glutamate levels in rats. Eur. J. Pharmacol. 1995, 287, 215–217. [Google Scholar] [CrossRef]
- León, D.; Sánchez-Nogueiro, J.; Marín-García, P.; Miras-Portugal, M.T. Glutamate release and synapsin-I phosphorylation induced by P2X7 receptors activation in cerebellar granule neurons. Neurochem. Int. 2008, 52, 1148–1159. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.H.; Choi, I.S.; Jang, I.S. P2X7 receptors enhance glutamate release in hippocampal hilar neurons. Neuroreport 2010, 21, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Marcoli, M.; Cervetto, C.; Paluzzi, P.; Guarnieri, S.; Alloisio, S.; Thellung, S.; Nobile, M.; Maura, G. P2X7 pre-synaptic receptors in adult rat cerebrocortical nerve terminals: A role in ATP-induced glutamate release. J. Neurochem. 2008, 105, 2330–2342. [Google Scholar] [CrossRef] [PubMed]
- Barros-Barbosa, A.R.; Oliveira, Â.; Lobo, M.G.; Cordeiro, J.M.; Correia-de-Sá, P. Under stressful conditions activation of the ionotropic P2X7 receptor differentially regulates GABA and glutamate release from nerve terminals of the rat cerebral cortex. Neurochem. Int. 2018, 112, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Anderson, C.M.; Keung, E.C.; Chen, Y.; Chen, Y.; Swanson, R.A. P2X7 receptor-mediated release of excitatory amino acids from astrocytes. J. Neurosci. 2003, 23, 1320–1328. [Google Scholar] [CrossRef] [PubMed]
- Khakpay, R.; Polster, D.; Köles, L.; Skorinkin, A.; Szabo, B.; Wirkner, K.; Illes, P. Potentiation of the glutamatergic synaptic input to rat locus coeruleus neurons by P2X7 receptors. Purinergic Signal. 2010, 6, 349–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barros-Barbosa, A.R.; Lobo, M.G.; Ferreirinha, F.; Correia-de-Sá, P.; Cordeiro, J.M. P2X7 receptor activation downmodulates Na+-dependent high-affinity GABA and glutamate transport into rat brain cortex synaptosomes. Neuroscience 2015, 306, 74–90. [Google Scholar] [CrossRef] [PubMed]
- Barros-Barbosa, A.R.; Fonseca, A.L.; Guerra-Gomes, S.; Ferreirinha, F.; Santos, A.; Rangel, R.; Lobo, M.G.; Correia-De-Sá, P.; Cordeiro, J.M. Up-regulation of P2X7 receptor-mediated inhibition of GABA uptake by nerve terminals of the human epileptic neocortex. Epilepsia 2016, 57, 99–110. [Google Scholar] [CrossRef]
- Lo, J.C.; Huang, W.C.; Chou, Y.C.; Tseng, C.H.; Lee, W.L.; Sun, S.H. Activation of P2X7 receptors decreases glutamate uptake and glutamine synthetase activity in RBA-2 astrocytes via distinct mechanisms. J. Neurochem. 2008, 105, 151–164. [Google Scholar] [CrossRef]
- Liu, Y.P.; Yang, C.S.; Chen, M.C.; Sun, S.H.; Tzeng, S.F. Ca2+-dependent reduction of glutamate aspartate transporter GLAST expression in astrocytes by P2X7 receptor-mediated phosphoinositide 3-kinase signaling. J. Neurochem. 2010, 113, 213–227. [Google Scholar] [CrossRef]
- Popoli, M.; Yan, Z.; McEwen, B.S.; Sanacora, G. The stressed synapse: The impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 2012, 13, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Sanacora, G.; Zarate, C.A.; Krystal, J.H.; Manji, H.K. Targeting the glutamatergic system to develop novel, improved therapeutics for mood disorders. Nat. Rev. Drug Discov. 2008, 7, 426–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Musazzi, L.; Milanese, M.; Farisello, P.; Zappettini, S.; Tardito, D.; Barbiero, V.S.; Bonifacino, T.; Mallei, A.; Baldelli, P.; Racagni, G.; et al. Acute Stress Increases Depolarization-Evoked Glutamate Release in the Rat Prefrontal/Frontal Cortex: The Dampening Action of Antidepressants. PLoS ONE 2010, 5, e8566. [Google Scholar] [CrossRef]
- Rada, P.; Moreno, S.A.; Tucci, S.; Gonzalez, L.E.; Harrison, T.; Chau, D.T.; Hoebel, B.G.; Hernandez, L. Glutamate release in the nucleus accumbens is involved in behavioral depression during the Porsolt swim test. Neuroscience 2003, 119, 557–565. [Google Scholar] [CrossRef]
- Hashimoto, K.; Sawa, A.; Iyo, M. Increased Levels of Glutamate in Brains from Patients with Mood Disorders. Biol. Psychiatry 2007, 62, 1310–1316. [Google Scholar] [CrossRef] [PubMed]
- Levine, J.; Panchalingam, K.; Rapoport, A.; Gershon, S.; McClure, R.J.; Pettegrew, J.W. Increased cerebrospinal fluid glutamine levels in depressed patients. Biol. Psychiatry 2000, 47, 586–593. [Google Scholar] [CrossRef]
- Paul, I.A.; Skolnick, P. Glutamate and depression—Clinical and preclinical studies. Glutamate Disord. Cogn. Motiv. 2003, 1003, 250–272. [Google Scholar]
- Sanacora, G.; Treccani, G.; Popoli, M. Towards a glutamate hypothesis of depression An emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology 2012, 62, 63–77. [Google Scholar] [CrossRef]
- Bobula, B.; Hess, G. Antidepressant treatments-induced modifications of glutamatergic transmission in rat frontal cortex. Pharmacol. Rep. 2008, 60, 865–871. [Google Scholar]
- Tokarski, K.; Bobula, B.; Wabno, J.; Hess, G. Repeated administration of imipramine attenuates glutamatergic transmission in rat frontal cortex. Neuroscience 2008, 153, 789–795. [Google Scholar] [CrossRef]
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 2000, 47, 351–354. [Google Scholar] [CrossRef]
- Targum, S.D.; Daly, E.; Fedgchin, M.; Cooper, K.; Singh, J.B. Comparability of blinded remote and site-based assessments of response to adjunctive esketamine or placebo nasal spray in patients with treatment resistant depression. J. Psychiatr. Res. 2019, 111, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Kittner, H.; Franke, H.; Fischer, W.; Schultheis, N.; Krugel, U.; Illes, P. Stimulation of P2Y1 receptors causes anxiolytic-like effects in the rat elevated plus-maze: Implications for the involvement of P2Y1 receptor-mediated nitric oxide production. Neuropsychopharmacology 2003, 28, 435–444. [Google Scholar] [CrossRef]
- Joca, S.R.L.; Sartim, A.G.; Roncalho, A.L.; Diniz, C.F.A.; Wegener, G. Nitric oxide signalling and antidepressant action revisited. Cell Tissue Res. 2019. [Google Scholar] [CrossRef]
- Luscher, B.; Shen, Q.; Sahir, N. The GABAergic deficit hypothesis of major depressive disorder. Mol. Psychiatry 2011, 16, 383–406. [Google Scholar] [CrossRef] [PubMed]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef]
- Martinon, F.; Burns, K.; Tschopp, J. The Inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Broz, P.; Dixit, V.M. Inflammasomes: Mechanism of assembly, regulation and signalling. Nat. Rev. Immunol. 2016, 16, 407–420. [Google Scholar] [CrossRef]
- Schroder, K.; Tschopp, J. The Inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [Green Version]
- Fernandes-Alnemri, T.; Wu, J.; Yu, J.W.; Datta, P.; Miller, B.; Jankowski, W.; Rosenberg, S.; Zhang, J.; Alnemri, E.S. The pyroptosome: A supramolecular assembly of ASC dimers mediating inflammatory cell death via caspase-1 activation. Cell Death Differ. 2007, 14, 1590–1604. [Google Scholar] [CrossRef]
- Maes, M.; Bosmans, E.; Suy, E.; Minner, B.; Raus, J. A further exploration of the relationships between immune parameters and the HPA-axis activity in depressed patients. Psychol. Med. 1991, 21, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Weizman, R.; Laor, N.; Podliszewski, E.; Notti, I.; Djaldetti, M.; Bessler, H. Cytokine production in major depressed patients before and after clomipramine treatment. Biol. Psychiatry 1994, 35, 42–47. [Google Scholar] [CrossRef]
- Young, J.J.; Bruno, D.; Pomara, N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J. Affect. Disord. 2014, 169, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Mao, R.; Zhang, C.; Chen, J.; Zhao, G.; Zhou, R.; Wang, F.; Xu, J.; Yang, T.; Su, Y.; Huang, J.; et al. Different levels of pro- and anti-inflammatory cytokines in patients with unipolar and bipolar depression. J. Affect. Disord. 2018, 237, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Dahl, J.; Ormstad, H.; Aass, H.C.D.; Malt, U.F.; Bendz, L.T.; Sandvik, L.; Brundin, L.; Andreassen, O.A. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology 2014, 45, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Gao, Z.; Zhang, H.; Fang, Z.; Wu, C.; Xu, H.; Huang, Q.J. Changes in proinflammatory cytokines and white matter in chronically stressed rats. Neuropsychiatr. Dis. Treat. 2015, 11, 597–607. [Google Scholar] [PubMed] [Green Version]
- Stepanichev, M.Y.; Peregud, D.I.; Manolova, A.O.; Lazareva, N.A.; Onufriev, M.V.; Gulyaeva, N.V. Chronic Mild Stress Increases the Expression of Genes Encoding Proinflammatory Cytokines in the Rat Brain. Biol. Bull. 2018, 45, 186–191. [Google Scholar] [CrossRef]
- Lu, Y.; Ho, C.S.; Liu, X.; Chua, A.N.; Wang, W.; McIntyre, R.S.; Ho, R.C. Chronic administration of fluoxetine and pro-inflammatory cytokine change in a rat model of depression. PLoS ONE 2017, 12, e0186700. [Google Scholar] [CrossRef] [PubMed]
- Cisneros-Mejorado, A.; Pérez-Samartín, A.; Gottlieb, M.; Matute, C. ATP Signaling in Brain: Release, Excitotoxicity and Potential Therapeutic Targets. Cell. Mol. Neurobiol. 2014, 35, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Alcocer-Gómez, E.; de Miguel, M.; Casas-Barquero, N.; Núñez-Vasco, J.; Sánchez-Alcazar, J.A.; Fernández-Rodríguez, A.; Cordero, M.D. NLRP3 inflammasome is activated in mononuclear blood cells from patients with major depressive disorder. Brain Behav. Immun. 2014, 36, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Kenis, G.; Maes, M. Effects of antidepressants on the production of cytokines. Int. J. Neuropsychopharmacol. 2002, 5, 401–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannestad, J.; Dellagioia, N.; Bloch, M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: A meta-analysis. Neuropsychopharmacology 2011, 36, 2452–2459. [Google Scholar] [CrossRef] [PubMed]
- Więdłocha, M.; Marcinowicz, P.; Krupa, R.; Janoska-Jaździk, M.; Janus, M.; Dębowska, W.; Mosiołek, A.; Waszkiewicz, N.; Szulc, A. Effect of antidepressant treatment on peripheral inflammation markers—A meta-analysis. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 80, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, L.; Liu, Y.Z.; Shen, X.L.; Wu, T.Y.; Zhang, T.; Wang, W.; Wang, Y.X.; Jiang, C.L. NLRP3 inflammasome mediates chronic mild stress-induced depression in mice via neuroinflammation. Int. J. Neuropsychopharmacol. 2015, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Alcocer-Gómez, E.; Ulecia-Morón, C.; Marín-Aguilar, F.; Rybkina, T.; Casas-Barquero, N.; Ruiz-Cabello, J.; Ryffel, B.; Apetoh, L.; Ghiringhelli, F.; Bullón, P.; et al. Stress-Induced Depressive Behaviors Require a Functional NLRP3 Inflammasome. Mol. Neurobiol. 2016, 53, 4874–4882. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zeng, M.Y.; Yang, D.; Motro, B.; Núñez, G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 2016, 530, 354–357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pétrilli, V.; Papin, S.; Dostert, C.; Mayor, A.; Martinon, F.; Tschopp, J. Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 2007, 14, 1583–1589. [Google Scholar] [CrossRef]
- Gustin, A.; Kirchmeyer, M.; Koncina, E.; Felten, P.; Losciuto, S.; Heurtaux, T.; Tardivel, A.; Heuschling, P.; Dostert, C. NLRP3 inflammasome is expressed and functional in mouse brain microglia but not in astrocytes. PLoS ONE 2015, 10, e0130624. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Jones, D.N.C. Emerging role of the P2X7-NLRP3-IL1β pathway in mood disorders. Psychoneuroendocrinology 2018, 98, 95–100. [Google Scholar] [CrossRef]
- Ma, M.; Ren, Q.; Zhang, J.C.; Hashimoto, K. Effects of Brilliant Blue G on Serum Tumor Necrosis Factor-alpha Levels and Depression-like Behavior in Mice after Lipopolysaccharide Administration. Clin. Psychopharmacol. Neurosci. 2014, 12, 31–36. [Google Scholar] [CrossRef]
- Li, J.M.; Liu, L.L.; Su, W.J.; Wang, B.; Zhang, T.; Zhang, Y.; Jiang, C.L. Ketamine may exert antidepressant effects via suppressing NLRP3 inflammasome to upregulate AMPA receptors. Neuropharmacology 2019, 146, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Cao, F.S.; Feng, J.; Chen, H.W.; Wan, J.R.; Lu, Q.; Wang, J. NLRP3 inflammasome activation contributes to long-term behavioral alterations in mice injected with lipopolysaccharide. Neuroscience 2017, 343, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, L.; Peng, Y.L.; Liu, Y.Z.; Wu, T.Y.; Shen, X.L.; Zhou, J.R.; Sun, D.Y.; Huang, A.J.; Wang, X.; et al. Involvement of inflammasome activation in lipopolysaccharide-induced mice depressive-like behaviors. CNS Neurosci. Ther. 2014, 20, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Gage, F.H. Mammalian neural stem cells. Science 2000, 287, 1433–1438. [Google Scholar] [CrossRef] [PubMed]
- Bonaguidi, M.A.; Wheeler, M.A.; Shapiro, J.S.; Stadel, R.P.; Sun, G.J.; Ming, G.L.; Song, H. In vivo clonal analysis reveals self-renewing and multipotent adult neural stem cell characteristics. Cell 2011, 145, 1142–1155. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.A.; Alvarez-Buylla, A. The Adult Ventricular-Subventricular Zone (V-SVZ) and Olfactory Bulb (OB) Neurogenesis. Cold Spring Harb. Perspect. Biol. 2016, 8, a018820. [Google Scholar] [CrossRef] [PubMed]
- Feliciano, D.M.; Bordey, A.; Bonfanti, L. Noncanonical Sites of Adult Neurogenesis in the Mammalian Brain. Cold Spring Harb. Perspect. Biol. 2015, 7, a018846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieberwirth, C.; Pan, Y.; Liu, Y.; Zhang, Z.; Wang, Z. Hippocampal adult neurogenesis: Its regulation and potential role in spatial learning and memory. Brain Res. 2016, 1644, 127–140. [Google Scholar] [CrossRef]
- Knoth, R.; Singec, I.; Ditter, M.; Pantazis, G.; Capetian, P.; Meyer, R.P.; Horvat, V.; Volk, B.; Kempermann, G. Murine features of neurogenesis in the human hippocampus across the lifespan from 0 to 100 years. PLoS ONE 2010, 5, e8809. [Google Scholar] [CrossRef]
- Eriksson, P.S.; Perfilieva, E.; Bjork-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef]
- Toni, N.; Schinder, A.F. Maturation and Functional Integration of New Granule Cells into the Adult Hippocampus. Cold Spring Harb. Perspect. Biol. 2016, 8, a018903. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.J.; Sailor, K.A.; Mahmood, Q.A.; Chavali, N.; Christian, K.M.; Song, H.; Ming, G.L. Seamless reconstruction of intact adult-born neurons by serial end-block imaging reveals complex axonal guidance and development in the adult hippocampus. J. Neurosci. 2013, 33, 11400–11411. [Google Scholar] [CrossRef] [PubMed]
- Toni, N.; Teng, E.M.; Bushong, E.A.; Aimone, J.B.; Zhao, C.; Consiglio, A.; van Praag, H.; Martone, M.E.; Ellisman, M.H.; Gage, F.H. Synapse formation on neurons born in the adult hippocampus. Nat. Neurosci. 2007, 10, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Bergami, M.; Masserdotti, G.; Temprana, S.G.; Motori, E.; Eriksson, T.M.; Gobel, J.; Yang, S.M.; Conzelmann, K.K.; Schinder, A.F.; Gotz, M.; et al. A critical period for experience-dependent remodeling of adult-born neuron connectivity. Neuron 2015, 85, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Opendak, M.; Gould, E. Adult neurogenesis: A substrate for experience-dependent change. Trends Cogn. Sci. 2015, 19, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, B.L.; van Praag, H.; Gage, F.H. Adult brain neurogenesis and psychiatry: A novel theory of depression. Mol. Psychiatry 2000, 5, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Demic, S.; Cheng, S. The reduction of adult neurogenesis in depression impairs the retrieval of new as well as remote episodic memory. PLoS ONE 2018, 13, e0198406. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, T.J.; Rhee, D.; Martin, L.; Smith, J.A.; Sonti, A.N.; Padmanaban, V.; Cameron, H.A. New neurons restore structural and behavioral abnormalities in a rat model of PTSD. Hippocampus 2019. [Google Scholar] [CrossRef] [PubMed]
- Santarelli, L.; Saxe, M.; Gross, C.; Surget, A.; Battaglia, F.; Dulawa, S.; Weisstaub, N.; Lee, J.; Duman, R.; Arancio, O.; et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003, 301, 805–809. [Google Scholar] [CrossRef]
- Airan, R.D.; Meltzer, L.A.; Roy, M.; Gong, Y.; Chen, H.; Deisseroth, K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science 2007, 317, 819–823. [Google Scholar] [CrossRef]
- Holick, K.A.; Lee, D.C.; Hen, R.; Dulawa, S.C. Behavioral effects of chronic fluoxetine in BALB/cJ mice do not require adult hippocampal neurogenesis or the serotonin 1A receptor. Neuropsychopharmacology 2008, 33, 406–417. [Google Scholar] [CrossRef] [PubMed]
- Sahay, A.; Hen, R. Adult hippocampal neurogenesis in depression. Nat. Neurosci. 2007, 10, 1110–1115. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, V.A.; Fernandes, K.; Jha, S. Regulation of adult hippocampal neurogenesis: Relevance to depression. Expert Rev. Neurother. 2007, 7, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Pastuzyn, E.D.; Day, C.E.; Kearns, R.B.; Kyrke-Smith, M.; Taibi, A.V.; McCormick, J.; Yoder, N.; Belnap, D.M.; Erlendsson, S.; Morado, D.R.; et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 2018, 555, 377–381. [Google Scholar]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.; Frisen, J. Adult neurogenesis in humans- common and unique traits in mammals. PLoS Biol. 2015, 13, e1002045. [Google Scholar] [CrossRef] [PubMed]
- Frisen, J. Neurogenesis and Gliogenesis in Nervous System Plasticity and Repair. Annu. Rev. Cell Dev. Biol. 2016, 32, 127–141. [Google Scholar] [CrossRef]
- Spalding, K.L.; Bergmann, O.; Alkass, K.; Bernard, S.; Salehpour, M.; Huttner, H.B.; Bostrom, E.; Westerlund, I.; Vial, C.; Buchholz, B.A.; et al. Dynamics of hippocampal neurogenesis in adult humans. Cell 2013, 153, 1219–1227. [Google Scholar] [CrossRef]
- Liu, W.; Ge, T.T.; Leng, Y.S.; Pan, Z.X.; Fan, J.E.; Yang, W.; Cui, R.J. The Role of Neural Plasticity in Depression: From Hippocampus to Prefrontal Cortex. Neural Plast. 2017, 2017, 6871089. [Google Scholar] [CrossRef]
- Qiao, H.; Li, M.X.; Xu, C.; Chen, H.B.; An, S.C.; Ma, X.M. Dendritic Spines in Depression: What We Learned from Animal Models. Neural Plast. 2016, 2016, 8056370. [Google Scholar] [CrossRef]
- Jia, N.; Yang, K.; Sun, Q.; Cai, Q.; Li, H.; Cheng, D.; Fan, X.; Zhu, Z. Prenatal stress causes dendritic atrophy of pyramidal neurons in hippocampal CA3 region by glutamate in offspring rats. Dev. Neurobiol. 2010, 70, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Magarinos, A.M.; McEwen, B.S.; Flugge, G.; Fuchs, E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J. Neurosci. 1996, 16, 3534–3540. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Xiao, B.; Wen, L. Loss of Glial Cells of the Hippocampus in a Rat Model of Post-traumatic Stress Disorder. Neurochem. Res. 2015, 40, 942–951. [Google Scholar] [CrossRef] [PubMed]
- Noorafshan, A.; Abdollahifar, M.A.; Karbalay-Doust, S. Stress changes the spatial arrangement of neurons and glial cells of medial prefrontal cortex and sertraline and curcumin prevent it. Psychiatry Investig. 2015, 12, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Castren, E.; Antila, H. Neuronal plasticity and neurotrophic factors in drug responses. Mol. Psychiatry 2017, 22, 1085–1095. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.J.; Lane, H.Y.; Lin, C.H. New Treatment Strategies of Depression: Based on Mechanisms Related to Neuroplasticity. Neural Plast. 2017, 2017, 4605971. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.E.; Glaser, T.; Oliveira-Giacomelli, A.; Ulrich, H. Purinergic receptors in neurogenic processes. Brain Res. Bull. 2018. [Google Scholar] [CrossRef]
- Tsao, H.K.; Chiu, P.H.; Sun, S.H. PKC-dependent ERK phosphorylation is essential for P2X7 receptor-mediated neuronal differentiation of neural progenitor cells. Cell Death Dis. 2013, 4, e751. [Google Scholar] [CrossRef]
- Lledo, P.M.; Alonso, M.; Grubb, M.S. Adult neurogenesis and functional plasticity in neuronal circuits. Nat. Rev. Neurosci. 2006, 7, 179–193. [Google Scholar] [CrossRef]
- Mu, Y.; Lee, S.W.; Gage, F.H. Signaling in adult neurogenesis. Curr. Opin. Neurobiol. 2010, 20, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Delarasse, C.; Gonnord, P.; Galante, M.; Auger, R.; Daniel, H.; Motta, I.; Kanellopoulos, J.M. Neural progenitor cell death is induced by extracellular ATP via ligation of P2X7 receptor. J. Neurochem. 2009, 109, 846–857. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, H.; Illes, P. P2X receptors in maintenance and differentiation of neural progenitor cells. Neural Regen. Res. 2014, 9, 2040–2041. [Google Scholar] [CrossRef] [PubMed]
- Messemer, N.; Kunert, C.; Grohmann, M.; Sobottka, H.; Nieber, K.; Zimmermann, H.; Franke, H.; Nörenberg, W.; Straub, I.; Schaefer, M.; et al. P2X7 receptors at adult neural progenitor cells of the mouse subventricular zone. Neuropharmacology 2013, 73, 122–137. [Google Scholar] [CrossRef] [PubMed]
- Levy, M.J.F.; Boulle, F.; Steinbusch, H.W.; van den Hove, D.L.A.; Kenis, G.; Lanfumey, L. Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology 2018, 235, 2195–2220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rantamäki, T. TrkB neurotrophin receptor at the core of antidepressant effects, but how? Cell Tissue Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Diniz, C.R.A.F.; Casarotto, P.C.; Resstel, L.; Joca, S.R.L. Beyond good and evil: A putative continuum-sorting hypothesis for the functional role of proBDNF/BDNF-propeptide/mBDNF in antidepressant treatment. Neurosci. Biobehav. Rev. 2018, 90, 70–83. [Google Scholar] [CrossRef] [Green Version]


| Stress/Treatment | P2X7R | Cell Type/Brain Structure | Analysis Technique | References |
|---|---|---|---|---|
| paroxetine | ↓ BzATP-evoked inward currents | cloned rat P2X7R expressed in HEK 293 cells | Whole-cell patch-clamp | [78] |
| fluoxetine or desipramine | no change | |||
| paroxetine | ↓ ATP-induced dye uptake | recombinant human P2X7R expressed in HEK-293 cells | dye uptake assay | [79] |
| fluoxetine or clomipramine | ↑ ATP-induced dye uptake | |||
| inescapable foot shocks | no change | ventral hippocampus of stressed rats | WB | [82] |
| imipramine | ↓ expression | |||
| CUMS | ↑ expression | hippocampus of stressed mice | WB | [80] |
| clemastine | ↓ expression | |||
| chronic restraint stress | ↑ expression | hippocampus of stressed mice | WB | [81] |
| ketamine | ↓ expression | |||
| CUS | no change | hippocampus of stressed rats | IHC | [53] |
| Acute or chronic restraint stress | ↓ expression | hippocampus of stressed rats | IHC | [83] |
| P2X7R Modulation | Specie | Model | Effect | Mechanism Involved | References |
|---|---|---|---|---|---|
| Genetic deletion | mice | FST, TST | Antidepressant-like | IL-1β release absence. | [82] |
| Genetic deletion | mice | FST | Antidepressant-like effect after 3 sessions of FST, but not 1 | Decreased activation of hippocampus dentate gyrus and basolateral amygdala. | [84] |
| Genetic deletion/pharmacological blockade (BBG) | mice | FST, TST | Antidepressant-like | Increased levels of NA in amygdala; and attenuated stress-induced ACTH and corticosterone responses. | [83] |
| Genetic deletion/pharmacological blockade (BBG) | mice | TST and SPT after LPS challenge | Antidepressant-like | Absence of P2RX7-mediated glutamate release, elevated basal BDNF production, enhanced neurogenesis and increased 5-HT bioavailability in the hippocampus. | [19] |
| Pharmacological blockade (iso-PPADS) | mice | FST | Antidepressant-like | Decreased NOS1 activation and NO synthesis in prefrontal cortex. | [85] |
| Pharmacological blockade (PPADS) | mice | FST | Antidepressant-like | 5-HT and NA availability. | [92] |
| Pharmacological blockade (A-804598) | FRL/FSL rats | FST | Antidepressant-like | Activation of BDNF signaling pathway in ventral hippocampus. | [80] |
| Pharmacological blockade (A-804598) | rats | CUS/restraint stress | Antidepressant-like | Inhibition of inflammasome activation in the hippocampus. | [22] |
| Pharmacological blockade (A-804598) | rats | Foot shocks | No effect | Partially attenuated the increase in IL-1β and CD14 mRNA in the paraventricular nucleus induced by stress. | [89] |
| Pharmacological blockade (BBG) | mice | TST and SPT after LPS challenge | Antidepressant-like | Decreased serum levels of TNF-α in serum after LPS treatment. | [93] |
| Pharmacological blockade (combined hippocampal microinjection of BBG and A-438079 | rats | CUS | Antidepressant-like | CUS increased ATP and NLRP3 inflammasomal activation in the hippocampus but the effect of P2RX7 blockade was not investigated. | [52] |
| Pharmacological blockade (BBG) | mice | CUMS | Antidepressant-like | Regulation of HPA axis and decrease microglial activation in cortex, hippocampus and basal nuclei. | [86] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, D.E.; Roncalho, A.L.; Glaser, T.; Ulrich, H.; Wegener, G.; Joca, S. P2X7 Receptor Signaling in Stress and Depression. Int. J. Mol. Sci. 2019, 20, 2778. https://doi.org/10.3390/ijms20112778
Ribeiro DE, Roncalho AL, Glaser T, Ulrich H, Wegener G, Joca S. P2X7 Receptor Signaling in Stress and Depression. International Journal of Molecular Sciences. 2019; 20(11):2778. https://doi.org/10.3390/ijms20112778
Chicago/Turabian StyleRibeiro, Deidiane Elisa, Aline Lulho Roncalho, Talita Glaser, Henning Ulrich, Gregers Wegener, and Sâmia Joca. 2019. "P2X7 Receptor Signaling in Stress and Depression" International Journal of Molecular Sciences 20, no. 11: 2778. https://doi.org/10.3390/ijms20112778





