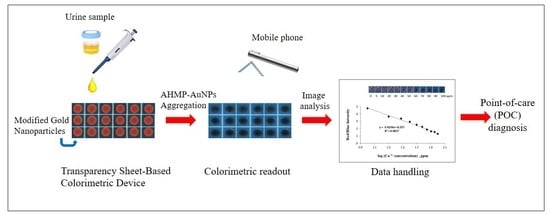
A Transparency Sheet-Based Colorimetric Device for Simple Determination of Calcium Ions Using Induced Aggregation of Modified Gold Nanoparticles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of AHMP-AuNPs
2.2. Colorimetric Assay of Ca2+
2.3. Optimization for the Determination of Ca2+ on Transparency Sheet-Based Devices
2.4. Selectivity for the Determination of Ca2+
2.5. Analytical Performances
2.6. Analysis of Artificial Urine Samples and Method Validation
3. Experimental
3.1. Materials and Instrumentation
3.2. Preparation of AHMP-Modified Gold Nanoparticles (AHMP-AuNPs)
3.3. Fabrication of Transparency Sheet-Based Devices
3.4. Transparency Sheet-Based Colorimetric Detection of Calcium
3.5. Analysis of Ca2+ in Artificial Urine Samples
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Joint FAO/WHO Expert Consultation on Human Vitamin and Mineral Requirements. In Proceedings of the Joint FAO/WHO Expert Consultation, Bangkok, Thailand, 21–30 September 1998. [Google Scholar]
- FAO/WHO Expert Group. Calcium requirements, Food and Agriculture Organization of the United Nations. In FAO Nutrition Meetings Report Series, No. 30; Food and Agriculture Organization of the United Nations: Rome, Italy, 1962. [Google Scholar]
- Carrie, E.B.; Richard, L.B.; Urquhart, A.C. 24-Hour Urinary Calcium in Primary Hyperparathyroidism. Clin. Med. Res. 2014, 11, 219–225. [Google Scholar]
- AACE/AAES Task Force on Primary Hyperparathyroidism. The American association of clinical endocrinologists position statement on the diagnosis and management of primary hyperparathyroidism. Endocr. Pract. 2005, 11, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Kratochvil, B.; Jeremy, P.G. Determination of calcium in urine by manual microtitration with EDTA. Talanta 1977, 24, 126–128. [Google Scholar] [CrossRef]
- Carubelli, R.; Smith, W.O.; Hammarsten, J.F. Determination of magnesium and calcium in urine. Clin. Chem. 1959, 5, 45–50. [Google Scholar]
- Parentoni, L.S.; Pozeti, R.C.S.; Figueiredo, J.F.; Faria, E.C.D. The determination of total calcium in urine: A comparison between the atomic absorption and the ortho-cresolphtalein complexone methods. J. Bras. Patol. Med. Lab. 2001, 37, 235–238. [Google Scholar] [CrossRef]
- Gomez-Nieto, B.; Gismera, M.J.; Sevilla, M.T.; Satrustegui, J.; Procopio, J.R. Micro-sampling method based on high-resolution continuum source graphite furnace atomic absorption spectrometry for calcium determination in blood and mitochondrial suspensions. Talanta 2017, 170, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Trudeau, D.L.; Freier, E.F. Determination of calcium in urine and serum by atomic absorption spectrophotometry (AAS). Clin. Chem. 1967, 13, 101–114. [Google Scholar] [PubMed]
- Chapoteau, E.; Czech, B.P.; Zazulak, W.; Kumar, A. New reagent for colorimetric assay of calcium in serum. Clin. Chem. 1993, 39, 1820–1824. [Google Scholar] [PubMed]
- Staden, V.J.F.; Taljaard, R.E. Determination of calcium in water, urine and pharmaceutical samples by sequential injection analysis. Anal. Chim. Acta 1996, 323, 75–85. [Google Scholar] [CrossRef]
- Radin, N.; Gramza, A.L. Differential spectrophotometric determination of calcium. Clin. Chem. 1964, 10, 704–720. [Google Scholar] [PubMed]
- Yua, J.; Zhang, X.; Lu, Q.; Wang, X.; Sun, D.; Wang, Y.; Yang, W. Determination of calcium and zinc in gluconates oral solution and blood samples by liquid cathode glow discharge-atomic emission spectrometry. Talanta 2017, 175, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Griffin, I.J.; Kriseman, Y.L.; Liang, L.K.; Abrams, S.A. Inductively coupled plasma mass spectrometric analysis of calcium isotopes in human serum: A low-sample-volume acid-equilibration method. Clin. Chem. 2003, 49, 2050–2055. [Google Scholar] [CrossRef] [PubMed]
- Fiedoruk-Pogrebniak, M.; Koncki, R. Multicommutated flow analysis system based on fluorescence microdetectors for simultaneous determination of phosphate and calcium ions in human serum. Talanta 2015, 144, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Ankireddy, S.R.; Kim, J. Highly Selective and Sensitive Detection of Calcium (II) Ions in Human Serum Using Novel Fluorescent Carbon Dots. Sens. Actuators B Chem. 2018, 255, 3425–3433. [Google Scholar] [CrossRef]
- Huanga, Y.; Zhang, Z.; Lva, J.; Chenga, H. Flow-injection analysis–fluorescence detection for the in vivo on-line determination of calcium in blood with microdialysis sampling. Anal. Chim. Acta 2000, 419, 175–184. [Google Scholar] [CrossRef]
- KhdeejaJabbar, A.; Abd, F.F. Determination of calcium by new sequential injection unit using a chemical dye. Int. J. Chemtech Res. 2016, 9, 121–131. [Google Scholar]
- Haj-Hussein, A.T.; Christian, G.D. Multicomponent flow injection analysis using spectrophotometric detection with reagent spectral overlap: Application to determination of calcium and magnesium in blood serum using Eriochrome Black T. Microchemica 1986, 34, 67–75. [Google Scholar] [CrossRef]
- Bowers, G.N., Jr.; Brassard, C.; Sena, S.F. Measurement of ionized calcium in serum with ion-selective electrodes: A mature technology that can meet the daily service needs. Clin. Chem. 1986, 32, 1437–1447. [Google Scholar] [PubMed]
- Maj-Zurawska, M.; Lewenstam, A. Selectivity coefficients of ion-selective magnesium electrodes used for simultaneous determination of magnesium and calcium ions. Talanta 2011, 87, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Sahli, R.; Raouafia, N.; Maisonhautec, E.; Boujlela, K.; Schöllhorn, B. Thiophene-based electrochemically active probes for selective calcium detection. Electrochim. Acta 2012, 63, 228–231. [Google Scholar] [CrossRef]
- Abramova, N.; Moral-Vico, J.; Soley, J.; Ocana, C.; Bratov, A. Solid contact ion sensor with conducting polymer layer copolymerized with the ion-selective membrane for determination of calcium in blood serum. Anal. Chim. Acta 2016, 943, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Gemene, K.L.; Bakker, E. Measurement of total calcium by flash chronopotentiometry at polymer membrane ion-selective electrodes. Anal. Chim. Acta 2009, 648, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Elghanian, R.; Storhoff, J.J.; Mucic, R.C.; Letsinger, R.L.; Mirkin, C.A. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science 1997, 277, 1078–1081. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, T.; Yamaguchi, I. Optical absorption study of the surface plasmon resonance in gold nanoparticles immobilized onto a gold substrate by self-assembly technique. J. Phys. Chem. B 2003, 107, 10321–10324. [Google Scholar] [CrossRef]
- Hongkun, L.; Jiajia, G.; Hong, P.; Lurui, L.; Minwei, Z.; Fengrui, G.; Chunyan, S.; Qian, Z. Visual detection of organophosphorus pesticides represented by mathamidophos using Au nanoparticles as colorimetric probe. Talanta 2011, 87, 93–99. [Google Scholar]
- Aldewachi, H.S.; Woodroofe, N.; Turega, S.; Gardiner, P.H.E. Optimization of gold nanoparticle-based real-time colorimetric assay of dipeptidyl peptidase IV activity. Talanta 2017, 169, 13–19. [Google Scholar] [CrossRef]
- Shankar, S.; John, A.S. 4-Amino-6-hydroxy-2-mercaptopyrimidine capped gold nanoparticles as fluorophore for the ultrasensitive and selective determination of l-cysteine. Sens. Actuators B Chem. 2015, 221, 1202–1208. [Google Scholar] [CrossRef]
- Shankar, S.; John, A.S. Sensitive and highly selective determination of vitamin B1 in the presence of other vitamin B complexes using functionalized gold nanoparticles as fluorophore. RSC Adv. 2015, 5, 49920–49925. [Google Scholar] [CrossRef]
- Gong, W.; Bai, L.; Cui, C.; Zhou, Y.; Zhao, X. Rapid visual detection of calcium ions using glutathione functionalized gold nanoparticles. In Proceedings of the Third International Conference on Measuring Technology and Mechatronics Automation, Shangshai, China, 6–7 January 2011. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Pradhan, N. Gold nanoparticles as efficient sensors in colorimetric detection oftoxic metal ions: A review. Sens. Actuators B Chem. 2017, 238, 888–902. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duenchay, P.; Chailapakul, O.; Siangproh, W. A Transparency Sheet-Based Colorimetric Device for Simple Determination of Calcium Ions Using Induced Aggregation of Modified Gold Nanoparticles. Int. J. Mol. Sci. 2019, 20, 2954. https://doi.org/10.3390/ijms20122954
Duenchay P, Chailapakul O, Siangproh W. A Transparency Sheet-Based Colorimetric Device for Simple Determination of Calcium Ions Using Induced Aggregation of Modified Gold Nanoparticles. International Journal of Molecular Sciences. 2019; 20(12):2954. https://doi.org/10.3390/ijms20122954
Chicago/Turabian StyleDuenchay, Paweenar, Orawon Chailapakul, and Weena Siangproh. 2019. "A Transparency Sheet-Based Colorimetric Device for Simple Determination of Calcium Ions Using Induced Aggregation of Modified Gold Nanoparticles" International Journal of Molecular Sciences 20, no. 12: 2954. https://doi.org/10.3390/ijms20122954