Isolated Compounds from Turpinia formosana Nakai
Abstract
:1. Introduction
2. Results
2.1. Isolated Compounds from Turpinia formosana
2.2. Structural Elucidation of New Compound 1 from T. formosana
2.3. Cytotoxicity of Compounds Isolated from T. formosana toward HOb Cells
2.4. Effect of Isolated Compounds on ALP Activity in HOb Cells
2.5. Effect of Isolated Compounds on Mineralization in HOb Cells
2.6. Effect of Isolated Compounds on ER Expression in HOb Cells
2.7. Effect of Isolated Compounds on Genetic Markers of Bone Formation in HOb Cells
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. General Experimental Techniques
4.3. Plant Extract Preparation
4.4. Isolation and Purification of Active Compounds
4.5. Cell Culture
4.6. MTT Assay for Cell Viability Assay
4.7. ALP Activity Assay
4.8. Mineralization Assay
4.9. RNA Isolation and Reverse Transcription
4.10. Real-Time Quantitative PCR Analysis
4.11. Estrogen Receptor Expression Assay
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Harvey, N.; Dennison, E.; Cooper, C. Osteoporosis: Impact on health and economics. Nat. Rev. Rheumatol. 2010, 6, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Florencio-Silva, R.; Sasso, G.R.D.S.; Sasso-Cerri, E.; Sim, M.J.; Cerri, P.S. Biology of bone tissue: Structure, function, and factors that influence bone cells. Biomed. Res. Int. 2015, 2015, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Robling, A.G.; Castillo, A.B.; Turner, C.H. Biochemical and molecular regulation of bone remodelling. Annu. Rev. Biomed. Eng. 2006, 8, 455–498. [Google Scholar] [CrossRef] [PubMed]
- Hadjidakis, D.J.; Androulakis, I.I. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R. Efficacy, effectiveness and side effects of medications used to prevent fractures. J. Intern. Med. 2015, 277, 690–706. [Google Scholar] [CrossRef]
- Murad, M.H.; Drake, M.T.; Mullan, R.J.; Mauck, K.F.; Stuart, L.M.; Lane, M.A.; Abu Elnour, N.O.; Erwin, P.J.; Hazem, A.; Puhan, M.A.; et al. Comparative effectiveness of drug treatments to prevent fragility fractures: A systematic review and network meta-analysis. J. Clin. Endocrinol. Metab. 2012, 97, 1871–1880. [Google Scholar] [CrossRef]
- Lim, S.Y.; Bolster, M.B. Current approaches to osteoporosis treatment. Curr. Opin. Rheumatol. 2015, 27, 216–224. [Google Scholar] [CrossRef]
- Tabatabaei-Malazy, O.; Salari, P.; Khashayar, P.; Larijani, B. New horizons in treatment of osteoporosis. Daru. 2017, 25, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Watts, N.B.; Bilezikian, J.P.; Camacho, P.M.; Greenspan, S.L.; Harris, S.T.; Hodgson, S.F.; Kleerekoper, M.; Luckey, M.M.; McClung, M.R.; Pollack, R.P.; et al. American association of clinical endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr. pract. 2010, 16, 1–37. [Google Scholar] [CrossRef]
- Rossouw, J.E.; Anderson, G.L.; Prentice, R.L.; LaCroix, A.Z.; Kooperberg, C.; Stefanick, M.L.; Jackson, R.D.; Beresford, S.A.; Howard, B.V.; Johnson, K.C.; et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: Principal results from the women’s health initiative randomized controlled trial. Jama 2002, 288, 321–333. [Google Scholar]
- Cosman, F.; de Beur, S.J.; LeBoff, M.S.; Lewiecki, E.M.; Tanner, B.; Randall, S.; Lindsay, R. Clinician’s guide to prevention and treatment of osteoporosis. Osteoporos Int. 2014, 25, 2359–2381. [Google Scholar] [CrossRef] [PubMed]
- Uusi-Rasi, K.; Sievänen, H.; Pasanen, M.; Beck, T.J.; Kannus, P. Influence of calcium intake and physical activity on proximal femur bone mass and structure among pre- and postmenopausal women. A 10-Year prospective study. Calcif. Tissue Int. 2008, 82, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.R.; Zhu, K.; Prince, R.L. Adverse events from calcium supplementation: Relationship to errors in myocardial infarction self-reporting in randomized controlled trials of calcium supplementation. J. Bone Miner. Res. 2012, 27, 719–722. [Google Scholar] [CrossRef] [PubMed]
- Golub, E.E.; Boesze-Battaglia, K. The role of alkaline phosphatase in mineralization. Curr. Opin. Orthop. 2007, 18, 444–448. [Google Scholar] [CrossRef]
- Yoshida, C.A.; Yamamoto, H.; Fujita, T.; Furuichi, T.; Ito, K.; Inoue, K.; Yamana, K.; Zanma, A.; Takada, K.; Ito, Y.; et al. Runx2 and Runx3 are essential for chondrocyte maturation, and Runx2 regulates limb growth through induction of Indian hedgehog. Genes Dev. 2004, 18, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Roles of Runx2 in skeletal development. Adv. Exp. Med. Biol. 2017, 962, 83–93. [Google Scholar]
- Viguet-Carrin, S.; Garnero, P.; Delmas, P.D. The role of collagen in bone strength. Osteoporos Int. 2006, 17, 319–336. [Google Scholar] [CrossRef]
- Nudelman, F.; Pieterse, K.; George, A.; Bomans, P.H.H.; Friedrich, H.; Brylka, L.J.; Hilbers, P.A.J.; de With, G.; Sommerdijk, N.A.J.M. The role of collagen in bone apatite formation in the presence of hydroxyapatite nucleation inhibitors. Nat. Mater. 2010, 9, 1004–1009. [Google Scholar] [CrossRef] [Green Version]
- Niyibizi, C.; Eyre, D.R. Structural characteristics of cross-linking sites in type V Collagen of bone. Eur. J. Biochem. 1994, 224, 943–950. [Google Scholar] [CrossRef]
- Standal, T.; Borset, M.; Sundan, A. Role of osteopontin in adhesion, migration, cell survival and bone remodeling. J. Clin. Exp. Oncol. 2004, 26, 179–184. [Google Scholar]
- Denhardt, D.T.; Noda, M. Osteopontin expression and function: Role in bone remodeling. J. Cell. Biochem. 1998, 72, 92–102. [Google Scholar] [CrossRef]
- Vaananen, H.K.; Harkonen, P.L. Estrogen and bone metabolism. Maturitas 1996, 23, S65–S69. [Google Scholar] [CrossRef]
- Klein-Nulend, J.; van Oers, R.F.M.; Bakker, A.D.; Bacabac, R.G. Bone cell mechanosensitivity, estrogen deficiency, and osteoporosis. J. Biomech. 2015, 48, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Riggs, B.L. The mechanisms of estrogen regulation of bone resorption. J. Clin. Invest. 2000, 106, 1203–1204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horgen, F.; Edrada, R.; De Los Reyes, G.; Agcaoili, F.; Madulid, D.; Wongpanich, V.; Angerhofer, C.; Pezzuto, J.; Soejarto, D.; Farnsworth, N. Biological screening of rain forest plot trees from Palawan Island (Philippines). Phytomedicine 2001, 8, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Jantová, S.; Nagy, M.; Ruzeková, L.; Grancai, D. Antibacterial activity of plant extracts from the families Fabaceae, Oleaceae, Philadelphaceae, Rosaceae and Staphyleaceae. Phytother Res. 2000, 14, 601–603. [Google Scholar] [CrossRef]
- Matthew, S.; Kao, K.C.; Chang, Y.S.; Abreu, P. Ellagic acid glycosides from Turpinia ternata. Nat. Prod. Res. 2007, 21, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.C.; Chiou, C.T.; Hsiao, P.C.; Liaw, C.C.; Zhang, L.J.; Chang, C.L.; Chen, I.S.; Chen, W.C.; Lee, K.H.; Kuo, Y.H. Cytotoxic phenylpropanoids and a new triterpene, turformosinic acid, from Turpinia formosana Nakai. Molecules 2012, 17, 1837–1851. [Google Scholar] [CrossRef]
- Tang, C.; Zhang, H.; Wang, Y.; Xie, N.; Lv, W.; Yang, X.; Liu, D.; Li, Z.; Cheng, F. Method for manufacturing water extract of turpinia formosana for treating influenza. CN Patent CN101884651A, 17 Novomber 2010. [Google Scholar]
- Tang, C.; Wang, Y.; Zhang, H.; Xie, N.; Lv, W.; Yang, X.; Liu, D.; Li, Z.; Cheng, F. Method for manufacturing ethanol extract of turpinia formosana for preventing and treating influenza. CN Patent CN101879197A, 17 Novomber 2010. [Google Scholar]
- Ou, J.; Hsieh, W.; Lin, I.; Chang, Y.; Chen, I. The catalogue of medicinal plant resources in Taiwan; Department of Health, Executive Yuan: Taipei, Taiwan, 2003.
- Zhou, Y.; Chen, H.B.; Wang, B.; Liang, H.; Zhao, Y.Y.; Zhang, Q.Y. Sesquiterpenoid and phenolic glucoside gallates from Lagerstroemia balansae. Planta Med. 2011, 77, 1944–1956. [Google Scholar] [CrossRef]
- Kazaki, K. Casuarin, stachyurin and strictinin, new ellagitannins from casuarina stricta and stachyurus praecox. Chem. Pharm. Bull. 1982, 30, 766–769. [Google Scholar]
- Cui, C.B.; Tezuka, Y.; Kikuchi, T.; Nakano, H.; Tamaoki, T.; Park, J.H. Constituents of a Fern, Davallia mariesii MOORE. IV. Isolation and structures of a novel norcarotane sesquiterpene glycoside, a chromone glucuronide, and two epicatechin glycosides. Chem. Pharm. Bull. 1992, 40, 2035–2040. [Google Scholar] [CrossRef]
- Li, X.C.; Elsohly, H.N.; Hufford, C.D.; Clark, A.M. NMR assignments of ellagic acid derivatives. Magn. Reson. Chem. 1999, 37, 856–859. [Google Scholar] [CrossRef]
- Benesi, A.J.; Falzone, C.J.; Banerjee, S.; Farber, G.K. NMR assignments for the aldopentoses. Carbohydr. Res. 1994, 258, 27–33. [Google Scholar] [CrossRef]
- Murshed, M. Mechanism of bone mineralization. Cold Spring Harb Perspect Med. 2018, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.P.; Sheu, S.Y.; Sun, J.S.; Chen, M.H.; Liu, M.H. Icariin isolated from Epimedium pubescens regulates osteoblasts anabolism through BMP-2, SMAD4, and Cbfa1 expression. Phytomedicine 2010, 17, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; He, X.; Yang, Y.; Li, M.; Hao, D.; Jia, Z. The genus Epimedium: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2011, 134, 519–541. [Google Scholar] [CrossRef] [PubMed]
- Jolly, J.J.; Chin, K.-Y.; Alias, E.; Chua, K.H.; Soelaiman, I.N. Protective effects of selected botanical agents on bone. Int. J. Environ. Res. Public Health 2018, 15, 963. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.L.; Li, J.H.; Yang, Y.F.; Zhu, J.Y. Floral development of gymnospermium microrrhynchum (berberidaceae) and its systematic significance in the nandinoideae. Flora 2017, 228, 10–16. [Google Scholar] [CrossRef]
- Lambert, M.N.T.; Thybo, C.B.; Lykkeboe, S.; Rasmussen, L.M.; Frette, X.; Christensen, L.P.; Jeppesen, P.B. Combined bioavailable isoflavones and probiotics improve bone status and estrogen metabolism in postmenopausal osteopenic women: A randomized controlled trial. Am. J. Clin. Nutr. 2017, 106, 909–920. [Google Scholar] [CrossRef]
- Ricci, E.; Cipriani, S.; Chiaffarino, F.; Malvezzi, M.; Parazzini, F. Soy isoflavones and bone mineral density in perimenopausal and postmenopausal Western women: A systematic review and meta-analysis of randomized controlled trials. J. Womens Health 2010, 19, 1609–1617. [Google Scholar] [CrossRef]
- Taku, K.; Melby, M.K.; Kurzer, M.S.; Mizuno, S.; Watanabe, S.; Ishimi, Y. Effects of soy isoflavone supplements on bone turnover markers in menopausal women: Systematic review and meta-analysis of randomized controlled trials. Bone 2010, 47, 413–423. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.R. Proinflammatory cytokines and osteoporosis. Curr. Osteoporos. Rep. 2009, 7, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.L.; Klein, A.; Chin, K.Y.; Mo, H.; Tsai, P.; Yang, R.S.; Chyu, M.C.; Ima-Nirwana, S. Tocotrienols for bone health: A translational approach. Ann. N. Y. Acad. Sci. 2017, 1401, 150–165. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaidi, M.M.; Al-Bayaty, F.H.; Al Batran, R.; Ibrahim, O.E.; Daher, A.M. Ellagic acid increases osteocalcin and alkaline phosphatase after tooth extraction in nicotinic-treated rats. Curr. Pharm. Des. 2016, 22, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Guo, A.J.; Choi, R.C.; Zheng, K.Y.; Chen, V.P.; Dong, T.T.; Wang, Z.T.; Vollmer, G.; Lau, D.T.; Tsim, K.W. Kaempferol as a flavonoid induces osteoblastic differentiation via estrogen receptor signaling. Chin. Med. 2012, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Huang, Y.L.; Liao, J.F.; Chiou, W.F. Ugonin K-stimulated osteogenesis involves estrogen receptor-dependent activation of non-classical Src signaling pathway and classical pathway. Eur. J. Pharmacol. 2012, 676, 26–33. [Google Scholar] [CrossRef]
- Baek, K.H.; Oh, K.W.; Lee, W.Y.; Lee, S.S.; Kim, M.K.; Kwon, H.S.; Rhee, E.J.; Han, J.H.; Song, K.H.; Cha, B.Y.; et al. Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif. Tissue Int. 2010, 87, 226–235. [Google Scholar] [CrossRef]
- Ostman, B.; Michaelsson, K.; Helmersson, J.; Byberg, L.; Gedeborg, R.; Melhus, H.; Basu, S. Oxidative stress and bone mineral density in elderly men: Antioxidant activity of alpha-tocopherol. Free Radic. Biol. Med. 2009, 47, 668–673. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Feng, Z.; Wang, X.; Zeng, M.; Liu, J.; Han, S.; Xu, J.; Chen, L.; Cao, K.; Long, J.; et al. SIRT3/SOD2 maintains osteoblast differentiation and bone formation by regulating mitochondrial stress. Cell Death Differ. 2018, 25, 229–240. [Google Scholar] [CrossRef]
- Abdollahi, M.; Larijani, B.; Rahimi, R.; Salari, P. Role of oxidative stress in osteoporosis. Therapy 2005, 2, 787–796. [Google Scholar] [CrossRef]
- Lean, J.M.; Jagger, C.J.; Kirstein, B.; Fuller, K.; Chambers, T.J. Hydrogen peroxide is essential for estrogen-deficiency bone loss and osteoclast formation. Endocrinology 2005, 146, 728–735. [Google Scholar] [CrossRef] [PubMed]
- Huh, Y.J.; Kim, J.M.; Kim, H.; Song, H.; So, H.; Lee, S.Y.; Kwon, S.B.; Kim, H.J.; Kim, H.H.; Lee, S.H.; et al. Regulation of osteoclast differentiation by the redox-dependent modulation of nuclear import of transcription factors. Cell Death Differ. 2006, 13, 1138–1146. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, K.; Neutzsky-Wulff, A.V.; Bonewald, L.F.; Karsdal, M.A. Local communication on and within bone controls bone remodeling. Bone 2009, 44, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Wilson, C. Oxidative stress and osteoporosis. Nat. Rev. Endocrinol. 2013, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Orimo, H. The mechanism of mineralization and the role of alkaline phosphatase in health and disease. J. Nippon. Med. Sch. 2010, 77, 4–12. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.H.; Lin, Y.L.; Hsu, W.L.; Hsieh, S.K.; Tzen, J.T.C. Significant elevation of antiviral activity of strictinin from Pu’er tea after thermal degradation to ellagic acid and gallic acid. J. Food Drug Anal. 2015, 23, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, S.-K.; Xu, J.-R.; Lin, N.-H.; Li, Y.-C.; Chen, G.-H.; Kuo, P.-C.; Chen, W.-Y.; Tzen, J.T.C. Antibacterial and laxative activities of strictinin isolated from Pu’er tea (Camellia sinensis). J. Food Drug Anal. 2016, 24, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.-J.; Wu, J.-B.; Ho, H.-Y.; Lin, W.-C. Antiosteoporotic activity of Davallia formosana. J. Ethnopharmacol. 2012, 139, 558–565. [Google Scholar] [CrossRef]
- Phan, T.C.; Xu, J.; Zheng, M.H. Interaction between osteoblast and osteoclast: Impact in bone disease. Histol Histopathol. 2004, 19, 1325–1344. [Google Scholar]
- Yang, F.; Yuan, P.-W.; Hao, Y.-Q.; Lu, Z.-M. Emodin enhances osteogenesis and inhibits adipogenesis. BMC Complement. Altern. Med. 2014, 14, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Gratacòs, E.; Checa, N.; Pérez-Navarro, E.; Alberch, J. Brain-derived neurotrophic factor (BDNF) mediates bone morphogenetic protein-2 (BMP-2) effects on cultured striatal neurones. J. Neurochem. 2001, 79, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Zamurovic, N.; Cappellen, D.; Rohner, D.; Susa, M. Coordinated activation of notch, Wnt, and transforming growth factor-beta signaling pathways in bone morphogenic protein 2-induced osteogenesis. Notch target gene Hey1 inhibits mineralization and Runx2 transcriptional activity. J. Biol. Chem. 2004, 279, 37704–37715. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Y.W.; Lin, R.D.; Hung, H.C.; Lee, M.H. Stimulation of osteogenic activity in human osteoblast cells by edible Uraria crinita. J. Agric. Food Chem. 2014, 62, 5581–5588. [Google Scholar] [CrossRef] [PubMed]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar] [PubMed]
- Hsu, Y.L.; Chang, J.K.; Tsai, C.H.; Chien, T.T.; Kuo, P.L. Myricetin induces human osteoblast differentiation through bone morphogenetic protein-2/p38 mitogen-activated protein kinase pathway. Biochem. Pharmacol. 2007, 73, 504–514. [Google Scholar] [CrossRef]
- Wang, Y.H.; Liu, Y.; Maye, P.; Rowe, D.W. Examination of mineralized nodule formation in living osteoblastic cultures using fluorescent dyes. Biotechnol. progr. 2006, 22, 1697–1701. [Google Scholar] [CrossRef]
- Bhadang, K.A.; Holding, C.A.; Thissen, H.; McLean, K.M.; Forsythe, J.S.; Haynes, D.R. Biological responses of human osteoblasts and osteoclasts to flame-sprayed coatings of hydroxyapatite and fluorapatite blends. Acta biomater. 2010, 6, 1575–1583. [Google Scholar] [CrossRef]
- Chou, C.W.; Chiang, T.I.; Chang, I.C.; Huang, C.H.; Cheng, Y.W. Expression levels of estrogen receptor alpha mRNA in peripheral blood cells are an independent biomarker for postmenopausal osteoporosis. BBA Clin. 2016, 5, 124–129. [Google Scholar] [CrossRef]
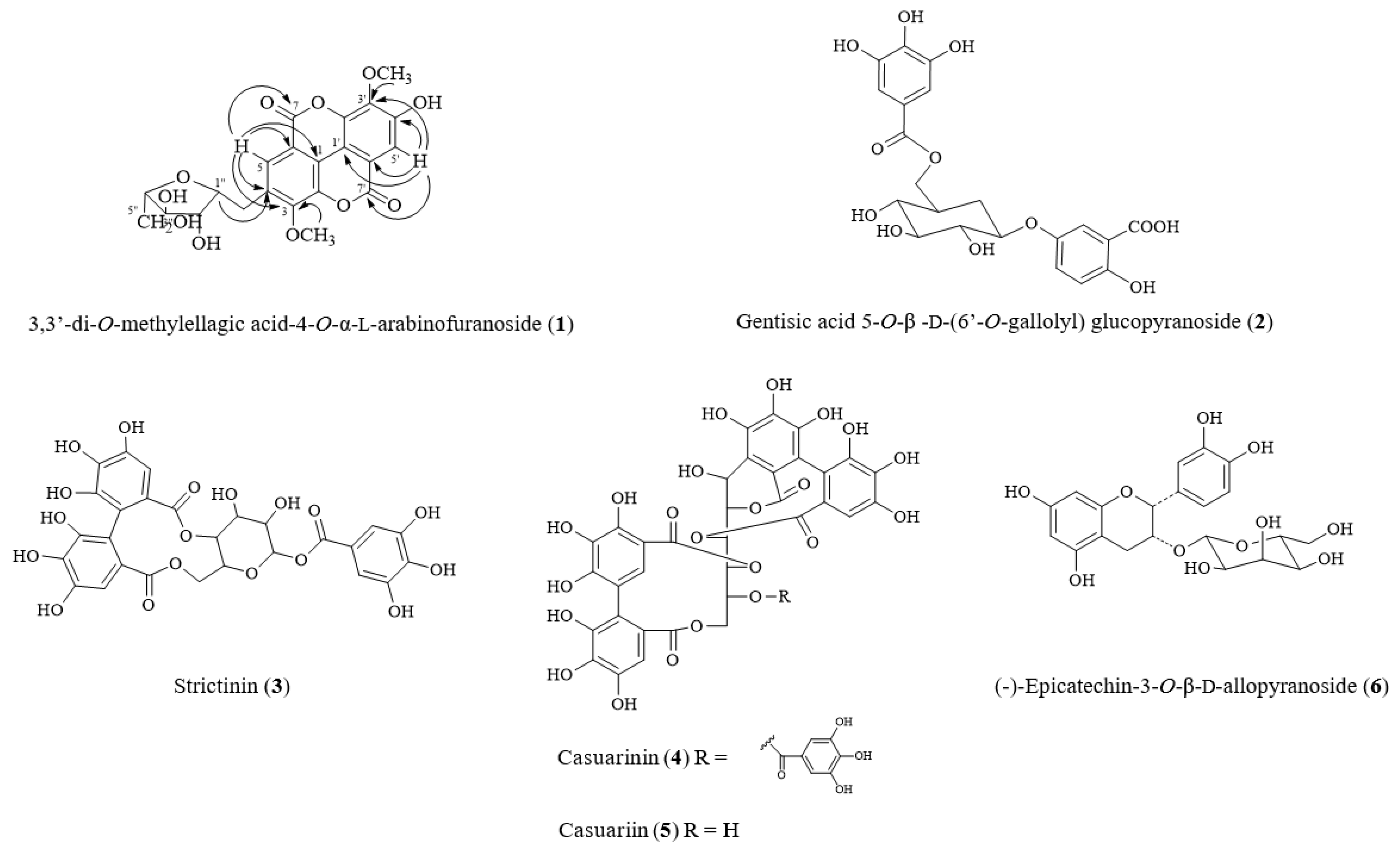





| Position | δC | δH (multi, J in Hz) | HMBC (H→C) |
|---|---|---|---|
| methylellagic acid | |||
| 1 | 113.8 | ||
| 2 | 141.4 | ||
| 3 | 141.9 | ||
| 4 | 150.7 | ||
| 5 | 111.6 | 7.70 (1H, s) | C-1, C-3, C-4, C-6, C-7 |
| 6 | 111.6 | ||
| 7 | 158.4 | ||
| 1′ | 110.8 | ||
| 2′ | 140.8 | ||
| 3′ | 140.2 | ||
| 4′ | 152.9 | ||
| 5′ | 111.7 | 7.45 (1H, s) | C-1′, C-3′, C-4′, C-6′, C-7′ |
| 6′ | 112.5 | ||
| 7′ | 158.2 | ||
| 3-OCH3 | 61.4 | 4.03 (3H, s) | |
| 3′-OCH3 | 61.0 | 4.07 (3H, s) | |
| arabinose | |||
| 1″ | 107.5 | 5.63 (1H, brs) | C-4 |
| 2″ | 82.1 | 4.23 (1H, d, J = 3.9 Hz) | |
| 3″ | 76.6 | 3.87 (1H, m) | |
| 4″ | 86.1 | 3.97 (1H, m) | |
| 5″ | 60.9 | 3.50 (1H, m) 3.62 (1H, m) |
| Sequence (5′→ 3′) | Probe Number | ||
|---|---|---|---|
| Runx-2 | Forward Reverse | CAGTGACACCATGTCAGCAA GCTCACGTCGCTCATTTTG | 41 |
| OPN | Forward Reverse | GGGCTTGGTTGTCAGCAG TGCAATTCTCATGGTAGTGAGTTT | 63 |
| BMP-2 | Forward Reverse | CGGACTGCGGTCTCCTAA GGAAGCAGCAACGCTAGAAG | 49 |
| BSP | Forward Reverse | GATTTCCAGTTCAGGGCAGT TCTCCTTCATTTGAAGTCTCCTCT | 63 |
| Col-1 | Forward Reverse | AGGTCCCCCTGGAAAGAA AATCCTCGAGCACCCTGA | 60 |
| BDNF | Forward Reverse | AGAATCGGAACCACGATTTG TCTCACCTGGTGGAACTCG | 70 |
| GAPDH | Forward Reverse | AGCCACATCGCTCAGACAC GCCCAATACGACCAAATCC | 60 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imtiyaz, Z.; Wang, Y.-F.; Lin, Y.-T.; Liu, H.-K.; Lee, M.-H.
Isolated Compounds from Turpinia formosana Nakai
Imtiyaz Z, Wang Y-F, Lin Y-T, Liu H-K, Lee M-H.
Isolated Compounds from Turpinia formosana Nakai
Imtiyaz, Zuha, Yi-Fang Wang, Yi-Tzu Lin, Hui-Kang Liu, and Mei-Hsien Lee.
2019. "Isolated Compounds from Turpinia formosana Nakai
Imtiyaz, Z., Wang, Y.-F., Lin, Y.-T., Liu, H.-K., & Lee, M.-H.
(2019). Isolated Compounds from Turpinia formosana Nakai






