Norepinephrine Inhibits Synovial Adipose Stem Cell Chondrogenesis via ?2a-Adrenoceptor-Mediated ERK1/2 Activation
Abstract
:1. Introduction
2. Results
2.1. Stem Cell-Specific Surface Markers and Chondrogenic Differentiation Potential of ASCs
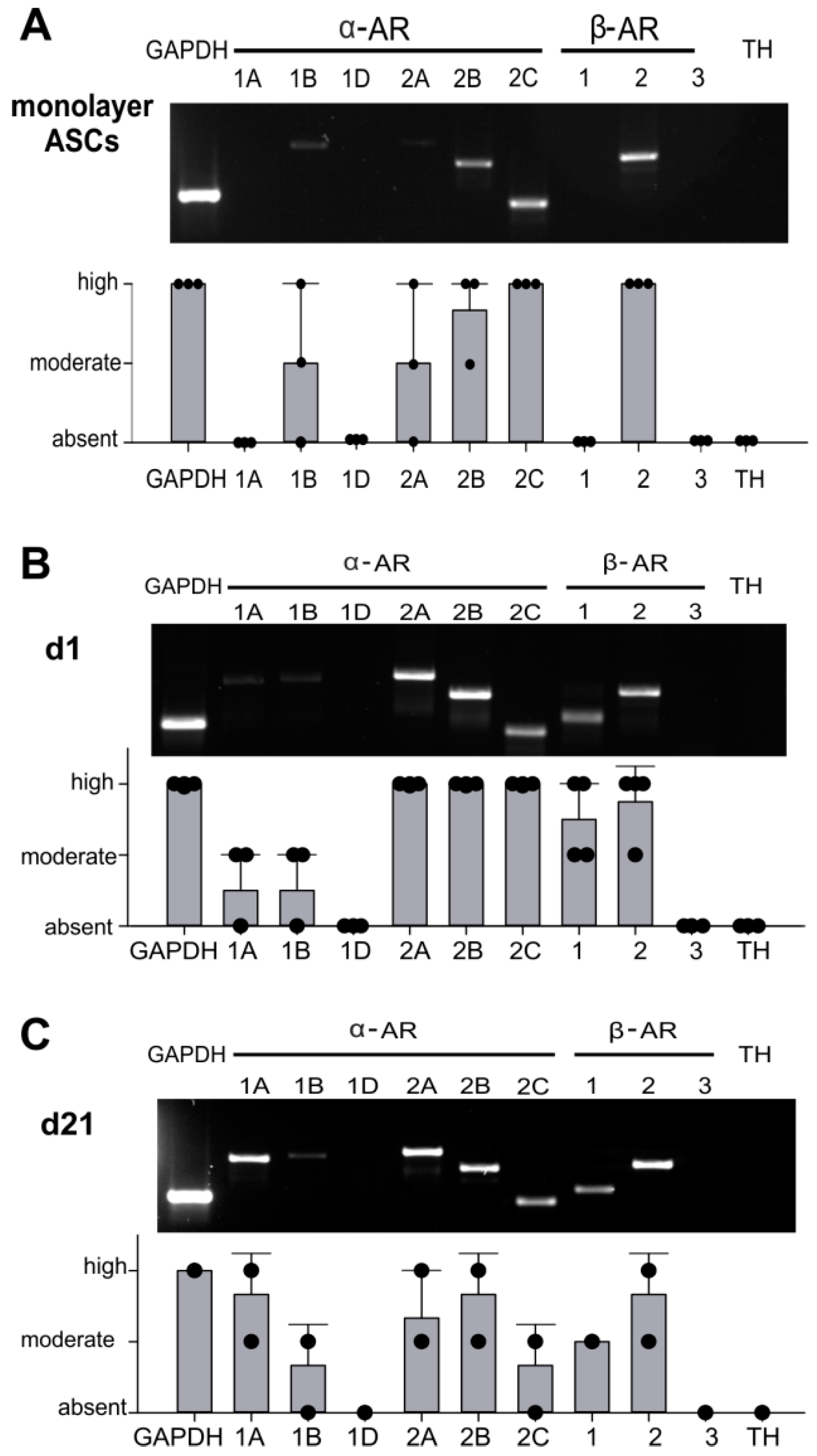
2.2. AR Expression Profile of ASCs in Monolayer and Pellet Cultures
2.3. NE-Mediated Intracellular Signaling
2.4. Effect of NE on ASC Proliferation
2.5. Effects of NE on sASC Chondrogenesis
2.6. Reversal of NE-Mediated Effects by Specific AR Antagonists
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Isolation and FACS Characterization of Human ASCs
4.3. In Vitro Proliferation of sASC
4.4. Determination of Cell Viability
4.5. Chondrogenic Differentiation of sASCs
4.6. Norepinephrine Quantification
4.7. Western Blot Analysis
4.8. RNA Isolation, Endpoint and Real-Time Quantitative PCR
4.9. Macroscopic and Histological Investigations
4.10. Immunohistochemistry
4.11. Biochemical Analysis of sGAGs and Type II Collagen Protein
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | Angiotensin converting enzyme |
| AEC | 3-Amino-9-ethylcarbazole |
| AP | Alkaline phosphatase |
| AR | Adrenoceptor |
| AT1 | Angiotensin II type 1 receptor |
| BMP-6 | Bone morphogenetic protein |
| BMSC | Bone marrow-derived MSCs |
| cAMP | Cyclic adenosine monophosphate |
| CCB | Calcium channel blockers |
| CD | Cluster of differentiation |
| cDNA | Complementary desoxy ribonucleic acid |
| COL10A1 | Collagen type X alpha 1 chain |
| COL1A1 | Collagen type I alpha 1 chain |
| COL2A1 | Collagen type II alpha 1 chain |
| DA | Dopamine |
| DMEM/F12 | Dulbecco’s modified eagle’s medium and Ham’s F-12 Medium |
| DMMB | 1,9-Dimethyl-methylene blue |
| DPBS | Dulbeccos phosphate-buffered saline |
| dsDNA | Double stranded DNA |
| E | Epinephrine |
| ECM | Extracellular matrix |
| ELISA | Enzyme-linked immunosorbent assay |
| ERK | Extracellular signal-regulated kinases |
| FBS | Fetal bovine serum |
| GAG | Glycosaminoglycan |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| HLA-DR | Human Leukocyte Antigen—DR isotype |
| HRP | Horseradish peroxidase |
| ITS | Insulin, human transferrin, and selenous acid |
| LDH | Lactate dehydrogenase |
| MMP13 | Matrix metallopeptidase 13 |
| mRNA | Messenger ribonucleic acid |
| MSC | Mesenchymal stem cells |
| NE | Norepinephrine |
| OA | Osteoarthritis |
| P/S | Penicillin streptomycin |
| PCR | Polymerase chain reaction |
| PFA | Paraformaldehyde |
| PKA | Proteinkinase A |
| PVDF | Polyvinylidene difluoride |
| RT-PCR | Reverse-transcriptase PCR |
| RUNX2 | Runt-related transcription factor 2 |
| sASC | Synovial adipose stem cell |
| SDS-Page | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| sGAG | Sulphated Glycosaminoglycans |
| SOX9 | SRY-box 9 |
| TBST | Tris buffered saline with Tween20 |
| TGF-β | Transforming growth factor β |
| TH | Tyrosine hydroxylase |
References
- Pereira, D.; Peleteiro, B.; Araujo, J.; Branco, J.; Santos, R.A.; Ramos, E. The effect of osteoarthritis definition on prevalence and incidence estimates: A systematic review. Osteoarthr. Cartil. 2011, 19, 1270–1285. [Google Scholar] [CrossRef] [PubMed]
- Hoy, D.G.; Smith, E.; Cross, M.; Sanchez-Riera, L.; Blyth, F.M.; Buchbinder, R.; Woolf, A.D.; Driscoll, T.; Brooks, P.; March, L.M. Reflecting on the global burden of musculoskeletal conditions: Lessons learnt from the global burden of disease 2010 study and the next steps forward. Ann. Rheum. Dis. 2015, 74, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Litwic, A.; Edwards, M.H.; Dennison, E.M.; Cooper, C. Epidemiology and burden of osteoarthritis. Br. Med Bull. 2013, 105, 185–199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Reviews. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGonagle, D.; Tan, A.L.; Carey, J.; Benjamin, M. The anatomical basis for a novel classification of osteoarthritis and allied disorders. J. Anat. 2010, 216, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Kalamegam, G.; Musumeci, G.; Batt, M.E. Chondrocyte and mesenchymal stem cell-based therapies for cartilage repair in osteoarthritis and related orthopaedic conditions. Maturitas 2014, 78, 188–198. [Google Scholar] [CrossRef] [PubMed]
- De Bari, C.; Dell’Accio, F.; Tylzanowski, P.; Luyten, F.P. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001, 44, 1928–1942. [Google Scholar] [CrossRef]
- McGonagle, D.; Baboolal, T.G.; Jones, E. Native joint-resident mesenchymal stem cells for cartilage repair in osteoarthritis. Nat. Reviews. Rheumatol. 2017, 13, 719–730. [Google Scholar] [CrossRef]
- Murphy, J.M.; Dixon, K.; Beck, S.; Fabian, D.; Feldman, A.; Barry, F. Reduced chondrogenic and adipogenic activity of mesenchymal stem cells from patients with advanced osteoarthritis. Arthritis Rheum. 2002, 46, 704–713. [Google Scholar] [CrossRef]
- Sekiya, I.; Muneta, T.; Horie, M.; Koga, H. Arthroscopic transplantation of synovial stem cells improves clinical outcomes in knees with cartilage defects. Clin. Orthop. Relat. Res. 2015, 473, 2316–2326. [Google Scholar] [CrossRef] [PubMed]
- Grässel, S.G. The role of peripheral nerve fibers and their neurotransmitters in cartilage and bone physiology and pathophysiology. Arthritis Res. 2014, 16, 485. [Google Scholar] [CrossRef] [Green Version]
- Jones, K.B.; Mollano, A.V.; Morcuende, J.A.; Cooper, R.R.; Saltzman, C.L. Bone and brain: A review of neural, hormonal, and musculoskeletal connections. Iowa Orthop. J. 2004, 24, 123–132. [Google Scholar] [PubMed]
- Maestroni, G.J. Neurohormones and catecholamines as functional components of the bone marrow microenvironment. Ann. New York Acad. Sci. 2000, 917, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Bjurholm, A.; Kreicbergs, A.; Terenius, L.; Goldstein, M.; Schultzberg, M. Neuropeptide y-, tyrosine hydroxylase- and vasoactive intestinal polypeptide-immunoreactive nerves in bone and surrounding tissues. J. Auton. Nerv. Syst. 1988, 25, 119–125. [Google Scholar] [CrossRef]
- Miller, L.E.; Justen, H.P.; Scholmerich, J.; Straub, R.H. The loss of sympathetic nerve fibers in the synovial tissue of patients with rheumatoid arthritis is accompanied by increased norepinephrine release from synovial macrophages. Faseb J. 2000, 14, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Pongratz, G.; Straub, R.H. Role of peripheral nerve fibres in acute and chronic inflammation in arthritis. Nat. Reviews. Rheumatol. 2013, 9, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Jenei-Lanzl, Z.; Grässel, S.; Pongratz, G.; Kees, F.; Miosge, N.; Angele, P.; Straub, R.H. Norepinephrine inhibition of mesenchymal stem cell and chondrogenic progenitor cell chondrogenesis and acceleration of chondrogenic hypertrophy. Arthritis Rheumatol 2014, 66, 2472–2481. [Google Scholar] [CrossRef]
- Pongratz, G.; Straub, R.H. The sympathetic nervous response in inflammation. Arthritis Res. Ther. 2014, 16, 504. [Google Scholar] [CrossRef]
- Molinoff, P.B. Alpha- and beta-adrenergic receptor subtypes properties, distribution and regulation. Drugs 1984, 28, 1–15. [Google Scholar] [CrossRef]
- Venkatakrishnan, A.J.; Deupi, X.; Lebon, G.; Tate, C.G.; Schertler, G.F.; Babu, M.M. Molecular signatures of g-protein-coupled receptors. Nature 2013, 494, 185. [Google Scholar] [CrossRef] [PubMed]
- Blesen, T.v.; Hawes, B.E.; Luttrell, D.K.; Krueger, K.M.; Touhara, K.; Porfflri, E.; Sakaue, M.; Luttrell, L.M.; Lefkowitz, R.J. Receptor-tyrosine-kinase- and gβγ-mediated map kinase activation by a common signalling pathway. Nature 1995, 376, 781. [Google Scholar] [CrossRef] [PubMed]
- Bogoyevitch, M.A.; Andersson, M.B.; Gillespie-Brown, J.; Clerk, A.; Glennon, P.E.; Fuller, S.J.; Sugden, P.H. Adrenergic receptor stimulation of the mitogen-activated protein kinase cascade and cardiac hypertrophy. Biochem. J. 1996, 314, 115–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alblas, J.; van Corven, E.J.; Hordijk, P.L.; Milligan, G.; Moolenaar, W.H. Gi-mediated activation of the p21ras-mitogen-activated protein kinase pathway by alpha 2-adrenergic receptors expressed in fibroblasts. J. Biol. Chem. 1993, 268, 22235–22238. [Google Scholar] [PubMed]
- Jiao, K.; Zeng, G.; Niu, L.N.; Yang, H.X.; Ren, G.T.; Xu, X.Y.; Li, F.F.; Tay, F.R.; Wang, M.Q. Activation of alpha2a-adrenergic signal transduction in chondrocytes promotes degenerative remodelling of temporomandibular joint. Sci. Rep. 2016, 6, 30085. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, J.; Schafer, N.; Bauer, R.; Jenei-Lanzl, Z.; Springorum, R.H.; Grassel, S. Norepinephrine modulates osteoarthritic chondrocyte metabolism and inflammatory responses. Osteoarthr. Cartil. 2016, 24, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.; Lai, L.P.; Peralta, F.; Xu, Y.; Sugamori, K. Beta2-adrenergic receptors inhibit the expression of collagen type ii in growth plate chondrocytes by stimulating the ap-1 factor jun-b. Am. J. Physiology. Endocrinol. Metab. 2011, 300, E633–E639. [Google Scholar] [CrossRef]
- Lafont, J.E. Lack of oxygen in articular cartilage: Consequences for chondrocyte biology. Int. J. Exp. Pathol. 2010, 91, 99–106. [Google Scholar] [CrossRef]
- Pattappa, G.; Johnstone, B.; Zellner, J.; Docheva, D.; Angele, P. The importance of physioxia in mesenchymal stem cell chondrogenesis and the mechanisms controlling its response. Int. J. Mol. Sci. 2019, 20, 484. [Google Scholar] [CrossRef]
- Mandl, L.A. Osteoarthritis year in review 2018: Clinical. Osteoarthr. Cartil. 2019, 27, 359–364. [Google Scholar] [CrossRef]
- Huang, Y.Z.; Xie, H.Q.; Silini, A.; Parolini, O.; Zhang, Y.; Deng, L.; Huang, Y.C. Mesenchymal stem/progenitor cells derived from articular cartilage, synovial membrane and synovial fluid for cartilage regeneration: Current status and future perspectives. Stem Cell Rev. 2017, 13, 575–586. [Google Scholar] [CrossRef] [PubMed]
- Grogan, S.P.; Miyaki, S.; Asahara, H.; D’Lima, D.D.; Lotz, M.K. Mesenchymal progenitor cell markers in human articular cartilage: Normal distribution and changes in osteoarthritis. Arthritis Res. 2009, 11, R85. [Google Scholar] [CrossRef] [PubMed]
- Capellino, S.; Cosentino, M.; Wolff, C.; Schmidt, M.; Grifka, J.; Straub, R.H. Catecholamine-producing cells in the synovial tissue during arthritis: Modulation of sympathetic neurotransmitters as new therapeutic target. Ann. Rheum. Dis. 2010, 69, 1853–1860. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The international society for cellular therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Kotova, P.D.; Sysoeva, V.Y.; Rogachevskaja, O.A.; Bystrova, M.F.; Kolesnikova, A.S.; Tyurin-Kuzmin, P.A.; Fadeeva, J.I.; Tkachuk, V.A.; Kolesnikov, S.S. Functional expression of adrenoreceptors in mesenchymal stromal cells derived from the human adipose tissue. Biochim. Et Biophys. Acta 2014, 1843, 1899–1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tyurin-Kuzmin, P.A.; Fadeeva, J.I.; Kanareikina, M.A.; Kalinina, N.I.; Sysoeva, V.Y.; Dyikanov, D.T.; Stambolsky, D.V.; Tkachuk, V.A. Activation of beta-adrenergic receptors is required for elevated alpha1a-adrenoreceptors expression and signaling in mesenchymal stromal cells. Sci. Rep. 2016, 6, 32835. [Google Scholar] [CrossRef] [PubMed]
- Speichert, S.; Molotkov, N.; El Bagdadi, K.; Meurer, A.; Zaucke, F.; Jenei-Lanzl, Z. Role of norepinephrine in il-1beta-induced chondrocyte dedifferentiation under physioxia. Int. J. Mol. Sci. 2019, 20, 1212. [Google Scholar] [CrossRef] [PubMed]
- Sheng, G. The developmental basis of mesenchymal stem/stromal cells (mscs). Bmc Dev. Biol. 2015, 15, 44. [Google Scholar] [CrossRef]
- Jenei-Lanzl, Z.; Meurer, A.; Zaucke, F. Interleukin-1beta signaling in osteoarthritis - chondrocytes in focus. Cell. Signal. 2019, 53, 212–223. [Google Scholar] [CrossRef]
- Anderson, D.E.; Markway, B.D.; Weekes, K.J.; McCarthy, H.E.; Johnstone, B. Physioxia promotes the articular chondrocyte-like phenotype in human chondroprogenitor-derived self-organized tissue. Tissue Engineering. Part. A 2018, 24, 264–274. [Google Scholar] [CrossRef]
- Jenei-Lanzl, Z.; Zwingenberg, J.; Lowin, T.; Anders, S.; Straub, R.H. Proinflammatory receptor switch from galphas to galphai signaling by beta-arrestin-mediated pde4 recruitment in mixed ra synovial cells. Brain Behav. Immun. 2015, 50, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Jenei-Lanzl, Z.; Straub, R.H.; Dienstknecht, T.; Huber, M.; Hager, M.; Grassel, S.; Kujat, R.; Angele, M.K.; Nerlich, M.; Angele, P. Estradiol inhibits chondrogenic differentiation of mesenchymal stem cells via nonclassic signaling. Arthritis Rheum. 2010, 62, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Hennig, T.; Lorenz, H.; Thiel, A.; Goetzke, K.; Dickhut, A.; Geiger, F.; Richter, W. Reduced chondrogenic potential of adipose tissue derived stromal cells correlates with an altered tgfbeta receptor and bmp profile and is overcome by bmp-6. J. Cell. Physiol. 2007, 211, 682–691. [Google Scholar] [CrossRef] [PubMed]
- Kees, M.G.; Pongratz, G.; Kees, F.; Scholmerich, J.; Straub, R.H. Via beta-adrenoceptors, stimulation of extrasplenic sympathetic nerve fibers inhibits lipopolysaccharide-induced tnf secretion in perfused rat spleen. J. Neuroimmunol. 2003, 145, 77–85. [Google Scholar] [CrossRef] [PubMed]





| Characteristics | Number/Mean/(%)/(Range) |
|---|---|
| total (number) | 32 |
| female/male (number, %) | 12/20 (37.5%/62.5%) |
| age (years—mean ± stdd., (range)) | 65.92 ± 9.84 (46–88) |
| C-reactive protein (mg/L) | 1.48 ± 1.11 |
| medication | |
| non-steroidal antiinflammatory drugs | 31 (97%) |
| steroids (other than prednisolone) | n.a. |
| opioid analgesics | 2 (6.25%) |
| biologicals | n.a. |
| antihypertensive drugs | 19 (59.3%) |
| non-selective beta blockers (β1 and β2) | 0 (0%) |
| selective beta blockers (β1) | 8 (25%) |
| other (AT1, ACE, CCB) | 11 (34.4%) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Bagdadi, K.; Zaucke, F.; Meurer, A.; Straub, R.H.; Jenei-Lanzl, Z. Norepinephrine Inhibits Synovial Adipose Stem Cell Chondrogenesis via ?2a-Adrenoceptor-Mediated ERK1/2 Activation. Int. J. Mol. Sci. 2019, 20, 3127. https://doi.org/10.3390/ijms20133127
El Bagdadi K, Zaucke F, Meurer A, Straub RH, Jenei-Lanzl Z. Norepinephrine Inhibits Synovial Adipose Stem Cell Chondrogenesis via ?2a-Adrenoceptor-Mediated ERK1/2 Activation. International Journal of Molecular Sciences. 2019; 20(13):3127. https://doi.org/10.3390/ijms20133127
Chicago/Turabian StyleEl Bagdadi, Karima, Frank Zaucke, Andrea Meurer, Rainer H. Straub, and Zsuzsa Jenei-Lanzl. 2019. "Norepinephrine Inhibits Synovial Adipose Stem Cell Chondrogenesis via ?2a-Adrenoceptor-Mediated ERK1/2 Activation" International Journal of Molecular Sciences 20, no. 13: 3127. https://doi.org/10.3390/ijms20133127
APA StyleEl Bagdadi, K., Zaucke, F., Meurer, A., Straub, R. H., & Jenei-Lanzl, Z. (2019). Norepinephrine Inhibits Synovial Adipose Stem Cell Chondrogenesis via ?2a-Adrenoceptor-Mediated ERK1/2 Activation. International Journal of Molecular Sciences, 20(13), 3127. https://doi.org/10.3390/ijms20133127






