Immune Mechanisms Underlying Susceptibility to Endotoxin Shock in Aged Hosts: Implication in Age-Augmented Generalized Shwartzman Reaction
Abstract
:1. Introduction
2. Definition of Septic Shock
3. High Mortality and Morbidity of Septic Shock in the Elderly
4. Pivotal Role of Tumor Necrosis Factor-α (TNF) in Human Endotoxin Shock
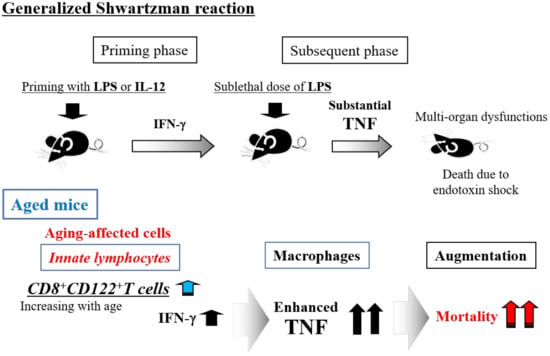
5. Generalized Shwartzman Reaction as an Experimental Endotoxin Shock Model
6. Generalized Shwartzman Reaction Induced by Interleukin (IL)-12 Priming and Sublethal LPS Challenge
7. Crucial Role of NKT Cells and Their Produced IFN-γ for the Murine Generalized Shwartzman Reaction
8. Involvement of Innate Lymphocytes in the Generalized Shwartzman Reaction
9. Age-Dependent Increase in Mortality Due to the Generalized Shwartzman Reaction in Mice
10. Immunosenescence and Thymus-Independent T Cells in Mice
11. Age-Dependent Increases in Murine CD8+CD122+ T Cells
12. Crucial Role of CD8+CD122+T Cells in Mortality Due to the Generalized Shwartzman Reaction in Aged Mice
13. Beneficial Roles of CD8+CD122+T Cells in Host Defense
14. In Vitro Shwartzman Reaction-Like Response in Human Peripheral Blood Mononuclear Cells (PBMCs)
15. Age-Dependent Augmentation of the In Vitro Shwartzman Reaction-Like Response in Human PBMCs
16. High Mortality and Morbidity of Septic Shock in Elderly Patients Showing Similar Plasma Endotoxin Levels as Adults (Elderly vs. Adults)
17. Management of Severe Sepsis/Septic Shock in Elderly Patients: Endotoxin Tolerance as a Potential Therapeutic Strategy in the Future
18. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Liu, V.; Escobar, G.J.; Greene, J.D.; Soule, J.; Whippy, A.; Angus, D.C.; Iwashyna, T.J. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 2014, 312, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Nasa, P.; Juneja, D.; Singh, O. Severe sepsis and septic shock in the elderly: An overview. World J. Crit. Care Med. 2012, 1, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Med. 2013, 39, 165–228. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.T.; Tsai, Y.J.; Tsai, P.R.; Yu, C.J.; Ko, W.J. Severe Sepsis and Septic Shock: Timing of Septic Shock Onset Matters. Shock 2016, 45, 518–524. [Google Scholar] [CrossRef] [PubMed]
- American College of Chest Physicians; Society of Critical Care Medicine Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit. Care Med. 1992, 20, 864–874. [Google Scholar]
- Bone, R.C.; Sibbald, W.J.; Sprung, C.L. The ACCP-SCCM consensus conference on sepsis and organ failure. Chest 1992, 101, 1481–1483. [Google Scholar] [CrossRef]
- Levy, M.M.; Fink, M.P.; Marshall, J.C.; Abraham, E.; Angus, D.; Cook, D.; Cohen, J.; Opal, S.M.; Vincent, J.L.; Ramsay, G. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003, 29, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef]
- Angus, D.C.; van der Poll, T. Severe sepsis and septic shock. N. Engl. J. Med. 2013, 369, 840–851. [Google Scholar] [CrossRef]
- Sherwin, R.; Winters, M.E.; Vilke, G.M.; Wardi, G. Does Early and Appropriate Antibiotic Administration Improve Mortality in Emergency Department Patients with Severe Sepsis or Septic Shock? J. Emerg. Med. 2017, 53, 588–595. [Google Scholar] [CrossRef]
- Banjas, N.; Hopf, H.B.; Hanisch, E.; Friedrichson, B.; Fichte, J.; Buia, A. ECMO-treatment in patients with acute lung failure, cardiogenic, and septic shock: Mortality and ECMO-learning curve over a 6-year period. J. Intensive Care 2018, 6, 84. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Ikeda, T.; Yokoyama, T.; Kihara, Y.; Konno, O.; Nakamura, Y.; Iwamoto, H.; Shimizu, T.; McGrath, M.M.; Chandraker, A. Reduction in circulating level of HMGB-1 following continuous renal replacement therapy in sepsis. Cytokine 2016, 83, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Yealy, D.M.; Kellum, J.A.; Huang, D.T.; Barnato, A.E.; Weissfeld, L.A.; Pike, F.; Terndrup, T.; Wang, H.E.; Hou, P.C.; LoVecchio, F.; et al. A randomized trial of protocol-based care for early septic shock. N. Engl. J. Med. 2014, 370, 1683–1693. [Google Scholar] [CrossRef] [PubMed]
- Peake, S.L.; Delaney, A.; Bailey, M.; Bellomo, R.; Cameron, P.A.; Cooper, D.J.; Higgins, A.M.; Holdgate, A.; Howe, B.D.; Webb, S.A.; et al. Goal-directed resuscitation for patients with early septic shock. N. Engl. J. Med. 2014, 371, 1496–1506. [Google Scholar] [CrossRef] [PubMed]
- Mouncey, P.R.; Osborn, T.M.; Power, G.S.; Harrison, D.A.; Sadique, M.Z.; Grieve, R.D.; Jahan, R.; Harvey, S.E.; Bell, D.; Bion, J.F.; et al. Trial of early, goal-directed resuscitation for septic shock. N. Engl. J. Med. 2015, 372, 1301–1311. [Google Scholar] [CrossRef]
- Abe, R.; Oda, S.; Sadahiro, T.; Nakamura, M.; Hirayama, Y.; Tateishi, Y.; Shinozaki, K.; Hirasawa, H. Gram-negative bacteremia induces greater magnitude of inflammatory response than Gram-positive bacteremia. Crit. Care 2010, 14, R27. [Google Scholar] [CrossRef]
- Sligl, W.I.; Dragan, T.; Smith, S.W. Nosocomial Gram-negative bacteremia in intensive care: Epidemiology, antimicrobial susceptibilities, and outcomes. Int. J. Infect. Dis. 2015, 37, 129–134. [Google Scholar] [CrossRef]
- Saito, N.; Sugiyama, K.; Ohnuma, T.; Kanemura, T.; Nasu, M.; Yoshidomi, Y.; Tsujimoto, Y.; Adachi, H.; Koami, H.; Tochiki, A.; et al. Efficacy of polymyxin B-immobilized fiber hemoperfusion for patients with septic shock caused by Gram-negative bacillus infection. PLoS ONE 2017, 12, e0173633. [Google Scholar] [CrossRef]
- Valenti, W.M.; Trudell, R.G.; Bentley, D.W. Factors predisposing to oropharyngeal colonization with gram-negative bacilli in the aged. N. Engl. J. Med. 1978, 298, 1108–1111. [Google Scholar] [CrossRef]
- Martin, G.S.; Mannino, D.M.; Moss, M. The effect of age on the development and outcome of adult sepsis. Crit. Care Med. 2006, 34, 15–21. [Google Scholar] [CrossRef]
- Motegi, A.; Kinoshita, M.; Sato, K.; Shinomiya, N.; Ono, S.; Nonoyama, S.; Hiraide, H.; Seki, S. An In Vitro Shwartzman reaction-like response is augmented age-dependently in human peripheral blood mononuclear cells. J. Leukoc. Biol. 2006, 79, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Opal, S.M.; Scannon, P.J.; Vincent, J.L.; White, M.; Carroll, S.F.; Palardy, J.E.; Parejo, N.A.; Pribble, J.P.; Lemke, J.H. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J. Infect. Dis. 1999, 180, 1584–1589. [Google Scholar] [CrossRef] [PubMed]
- Munford, R.S. Endotoxemia-menace, marker, or mistake? J. Leukoc. Biol. 2016, 100, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.J.; Derzko, A.; Foster, D.; Seely, A.J.; Brunet, F.; Romaschin, A.D.; Marshall, J.C. Daily variation in endotoxin levels is associated with increased organ failure in critically ill patients. Shock 2007, 28, 524–529. [Google Scholar] [CrossRef]
- Laichalk, L.L.; Bucknell, K.A.; Huffnagle, G.B.; Wilkowski, J.M.; Moore, T.A.; Romanelli, R.J.; Standiford, T.J. Intrapulmonary delivery of tumor necrosis factor agonist peptide augments host defense in murine gram-negative bacterial pneumonia. Infect. Immun. 1998, 66, 2822–2826. [Google Scholar] [PubMed]
- Havell, E.A. Evidence that tumor necrosis factor has an important role in antibacterial resistance. J. Immunol. 1989, 143, 2894–2899. [Google Scholar]
- Cauwels, A.; Brouckaert, P. Survival of TNF toxicity: Dependence on caspases and NO. Arch. Biochem. Biophys. 2007, 462, 132–139. [Google Scholar] [CrossRef]
- Brenner, D.; Blaser, H.; Mak, T.W. Regulation of tumour necrosis factor signalling: Live or let die. Nat. Rev. Immunol. 2015, 15, 362–374. [Google Scholar] [CrossRef]
- Oberholzer, A.; Souza, S.M.; Tschoeke, S.K.; Oberholzer, C.; Abouhamze, A.; Pribble, J.P.; Moldawer, L.L. Plasma cytokine measurements augment prognostic scores as indicators of outcome in patients with severe sepsis. Shock 2005, 23, 488–493. [Google Scholar]
- Rice, T.W.; Wheeler, A.P.; Morris, P.E.; Paz, H.L.; Russell, J.A.; Edens, T.R.; Bernard, G.R. Safety and efficacy of affinity-purified, anti-tumor necrosis factor-alpha, ovine fab for injection (CytoFab) in severe sepsis. Crit. Care Med. 2006, 34, 2271–2281. [Google Scholar] [CrossRef]
- Michie, H.R.; Manogue, K.R.; Spriggs, D.R.; Revhaug, A.; O’Dwyer, S.; Dinarello, C.A.; Cerami, A.; Wolff, S.M.; Wilmore, D.W. Detection of circulating tumor necrosis factor after endotoxin administration. N. Engl. J. Med. 1988, 318, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Hesse, D.G.; Tracey, K.J.; Fong, Y.; Manogue, K.R.; Palladino, M.A., Jr.; Cerami, A.; Shires, G.T.; Lowry, S.F. Cytokine appearance in human endotoxemia and primate bacteremia. Surg. Gynecol. Obstet. 1988, 166, 147–153. [Google Scholar] [PubMed]
- Van Deventer, S.J.; Buller, H.R.; ten Cate, J.W.; Aarden, L.A.; Hack, C.E.; Sturk, A. Experimental endotoxemia in humans: Analysis of cytokine release and coagulation, fibrinolytic, and complement pathways. Blood 1990, 76, 2520–2526. [Google Scholar] [PubMed]
- Van der Poll, T.; Buller, H.R.; ten Cate, H.; Wortel, C.H.; Bauer, K.A.; van Deventer, S.J.; Hack, C.E.; Sauerwein, H.P.; Rosenberg, R.D.; ten Cate, J.W. Activation of coagulation after administration of tumor necrosis factor to normal subjects. N. Engl. J. Med. 1990, 322, 1622–1627. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Miyazaki, H.; Nakashima, H.; Nakashima, M.; Nishikawa, M.; Ishikiriyama, T.; Kato, S.; Iwaya, K.; Hiroi, S.; Shinomiya, N.; et al. In Vivo Lipopolysaccharide Tolerance Recruits CD11b+ Macrophages to the Liver with Enhanced Bactericidal Activity and Low Tumor Necrosis Factor-Releasing Capability, Resulting in Drastic Resistance to Lethal Septicemia. J. Innate Immun. 2017, 9, 493–510. [Google Scholar] [CrossRef] [PubMed]
- Chahin, A.B.; Opal, J.M.; Opal, S.M. Whatever happened to the Shwartzman phenomenon? Innate Immun. 2018, 24, 466–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shwartzman, G. Studies on bacillus typhosus toxic substances: I. Phenomenon of local skin reactivity to B. typhosus culture filtrate. J. Exp. Med. 1928, 48, 247–268. [Google Scholar] [CrossRef] [PubMed]
- Thomas, L.; Good, R.A. Studies on the generalized Shwartzman reaction: I. General observations concerning the phenomenon. J. Exp. Med. 1952, 96, 605–624. [Google Scholar] [CrossRef]
- Sato, K.; Kinoshita, M.; Motegi, A.; Habu, Y.; Takayama, E.; Nonoyama, S.; Hiraide, H.; Seki, S. Critical role of the liver CD8+ CD122+ T cells in the generalized Shwartzman reaction of mice. Eur. J. Immunol. 2005, 35, 593–602. [Google Scholar] [CrossRef]
- Biswas, S.K.; Lopez-Collazo, E. Endotoxin tolerance: New mechanisms, molecules and clinical significance. Trends Immunol. 2009, 30, 475–487. [Google Scholar] [CrossRef]
- Ozmen, L.; Pericin, M.; Hakimi, J.; Chizzonite, R.A.; Wysocka, M.; Trinchieri, G.; Gately, M.; Garotta, G. Interleukin 12, interferon gamma, and tumor necrosis factor alpha are the key cytokines of the generalized Shwartzman reaction. J. Exp. Med. 1994, 180, 907–915. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. Interleukin-12: A proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu. Rev. Immunol. 1995, 13, 251–276. [Google Scholar] [CrossRef] [PubMed]
- Tait Wojno, E.D.; Hunter, C.A.; Stumhofer, J.S. The Immunobiology of the Interleukin-12 Family: Room for Discovery. Immunity 2019, 50, 851–870. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, K.; Takeda, K.; Hashimoto, W.; Satoh, M.; Okuyama, R.; Yanai, N.; Obinata, M.; Kumagai, K.; Takada, H.; Hiraide, H.; et al. Involvement of NK1+ T cells and their IFN-gamma production in the generalized Shwartzman reaction. J. Immunol. 1998, 160, 3522–3527. [Google Scholar] [PubMed]
- Kinoshita, M.; Seki, S.; Ono, S.; Shinomiya, N.; Hiraide, H. Paradoxical effect of IL-18 therapy on the severe and mild Escherichia coli infections in burn-injured mice. Ann. Surg. 2004, 240, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Seki, S.; Habu, Y.; Kawamura, T.; Takeda, K.; Dobashi, H.; Ohkawa, T.; Hiraide, H. The liver as a crucial organ in the first line of host defense: The roles of Kupffer cells, natural killer (NK) cells and NK1.1 Ag+ T cells in T helper 1 immune responses. Immunol. Rev. 2000, 174, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Eberl, G.; Colonna, M.; Di Santo, J.P.; McKenzie, A.N. Innate lymphoid cells. Innate lymphoid cells: A new paradigm in immunology. Science 2015, 348, aaa6566. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, M.; Miyazaki, H.; Ono, S.; Seki, S. Immunoenhancing therapy with interleukin-18 against bacterial infection in immunocompromised hosts after severe surgical stress. J. Leukoc. Biol. 2013, 93, 689–698. [Google Scholar] [CrossRef] [PubMed]
- Cambier, J. Immunosenescence: A problem of lymphopoiesis, homeostasis, microenvironment, and signaling. Immunol. Rev. 2005, 205, 5–6. [Google Scholar] [CrossRef]
- Miller, R.A.; Berger, S.B.; Burke, D.T.; Galecki, A.; Garcia, G.G.; Harper, J.M.; Sadighi Akha, A.A. T cells in aging mice: Genetic, developmental, and biochemical analyses. Immunol. Rev. 2005, 205, 94–103. [Google Scholar] [CrossRef]
- Berzins, S.P.; Boyd, R.L.; Miller, J.F. The role of the thymus and recent thymic migrants in the maintenance of the adult peripheral lymphocyte pool. J. Exp. Med. 1998, 187, 1839–1848. [Google Scholar] [CrossRef] [PubMed]
- Abo, T. Extrathymic pathways of T cell differentiation. Arch. Immunol. Ther. Exp. 2001, 49, 81–90. [Google Scholar]
- Sato, K.; Ohtsuka, K.; Hasegawa, K.; Yamagiwa, S.; Watanabe, H.; Asakura, H.; Abo, T. Evidence for extrathymic generation of intermediate T cell receptor cells in the liver revealed in thymectomized, irradiated mice subjected to bone marrow transplantation. J. Exp. Med. 1995, 182, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Seki, S.; Abo, T.; Ohteki, T.; Sugiura, K.; Kumagai, K. Unusual alpha beta-T cells expanded in autoimmune lpr mice are probably a counterpart of normal T cells in the liver. J. Immunol. 1991, 147, 1214–1221. [Google Scholar] [PubMed]
- Fei, F.; Lee, K.M.; McCarry, B.E.; Bowdish, D.M. Age-associated metabolic dysregulation in bone marrow-derived macrophages stimulated with lipopolysaccharide. Sci. Rep. 2016, 6, 22637. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Motegi, A.; Kinoshita, M.; Inatsu, A.; Habu, Y.; Saitoh, D.; Seki, S. IL-15-induced CD8+ CD122+ T cells increase antibacterial and anti-tumor immune responses: Implications for immune function in aged mice. J. Leukoc. Biol. 2008, 84, 1047–1056. [Google Scholar] [CrossRef]
- Ikemizu, S.; Chirifu, M.; Davis, S.J. IL-2 and IL-15 signaling complexes: Different but the same. Nat. Immunol. 2012, 13, 1141–1142. [Google Scholar] [CrossRef]
- Waldmann, T.A. The biology of interleukin-2 and interleukin-15: Implications for cancer therapy and vaccine design. Nat. Rev. Immunol. 2006, 6, 595–601. [Google Scholar] [CrossRef]
- Rosmaraki, E.E.; Douagi, I.; Roth, C.; Colucci, F.; Cumano, A.; Di Santo, J.P. Identification of committed NK cell progenitors in adult murine bone marrow. Eur. J. Immunol. 2001, 31, 1900–1909. [Google Scholar] [CrossRef]
- Yoshizawa, K.; Nakajima, S.; Notake, T.; Miyagawa, S.; Hida, S.; Taki, S. IL-15-high-responder developing NK cells bearing Ly49 receptors in IL-15-/-mice. J. Immunol. 2011, 187, 5162–5169. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Miyaji, C.; Kawachi, Y.; Iiai, T.; Ohtsuka, K.; Iwanage, T.; Takahashi-Iwanaga, H.; Abo, T. Relationships between intermediate TCR cells and NK1.1+ T cells in various immune organs. NK1.1+ T cells are present within a population of intermediate TCR cells. J. Immunol. 1995, 155, 2972–2983. [Google Scholar] [PubMed]
- Tsukahara, A.; Seki, S.; Iiai, T.; Moroda, T.; Watanabe, H.; Suzuki, S.; Tada, T.; Hiraide, H.; Hatakeyama, K.; Abo, T. Mouse liver T cells: Their change with aging and in comparison with peripheral T cells. Hepatology 1997, 26, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Takayama, E.; Seki, S.; Ohkawa, T.; Ami, K.; Habu, Y.; Yamaguchi, T.; Tadakuma, T.; Hiraide, H. Mouse CD8+ CD122+ T cells with intermediate TCR increasing with age provide a source of early IFN-gamma production. J. Immunol. 2000, 164, 5652–5658. [Google Scholar] [CrossRef] [PubMed]
- Mathews, D.V.; Dong, Y.; Higginbotham, L.B.; Kim, S.C.; Breeden, C.P.; Stobert, E.A.; Jenkins, J.; Tso, J.Y.; Larsen, C.P.; Adams, A.B. CD122 signaling in CD8+ memory T cells drives costimulation-independent rejection. J. Clin. Investig. 2018, 128, 4557–4572. [Google Scholar] [CrossRef]
- Judge, A.D.; Zhang, X.; Fujii, H.; Surh, C.D.; Sprent, J. Interleukin 15 controls both proliferation and survival of a subset of memory-phenotype CD8(+) T cells. J. Exp. Med. 2002, 196, 935–946. [Google Scholar] [CrossRef]
- Yamada, H.; Matsuzaki, G.; Chen, Q.; Iwamoto, Y.; Nomoto, K. Reevaluation of the origin of CD44(high) “memory phenotype” CD8 T cells: Comparison between memory CD8 T cells and thymus-independent CD8 T cells. Eur. J. Immunol. 2001, 31, 1917–1926. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, S.; Hwang, I.; Tough, D.F.; Sprent, J. Potent and selective stimulation of memory-phenotype CD8+ T cells in vivo by IL-15. Immunity 1998, 8, 591–599. [Google Scholar] [CrossRef]
- Nakagawa, R.; Inui, T.; Nagafune, I.; Tazunoki, Y.; Motoki, K.; Yamauchi, A.; Hirashima, M.; Habu, Y.; Nakashima, H.; Seki, S. Essential role of bystander cytotoxic CD122+CD8+ T cells for the antitumor immunity induced in the liver of mice by alpha-galactosylceramide. J. Immunol. 2004, 172, 6550–6557. [Google Scholar] [CrossRef]
- Starzl, T.E.; Lerner, R.A.; Dixon, F.J.; Groth, C.G.; Brettschneider, L.; Terasaki, P.I. Shwartzman reaction after human renal homotransplantation. N. Engl. J. Med. 1968, 278, 642–648. [Google Scholar] [CrossRef]
- Takayama, E.; Koike, Y.; Ohkawa, T.; Majima, T.; Fukasawa, M.; Shinomiya, N.; Yamaguchi, T.; Konishi, M.; Hiraide, H.; Tadakuma, T.; et al. Functional and Vbeta repertoire characterization of human CD8+ T-cell subsets with natural killer cell markers, CD56+ CD57− T cells, CD56+ CD57+ T cells and CD56− CD57+ T cells. Immunology 2003, 108, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, H.; Yang, M.F.; Shu, W.; Sun, M.J.; Xu, Y. Effects of aging on endotoxin tolerance induced by lipopolysaccharides derived from Porphyromonas gingivalis and Escherichia coli. PLoS ONE 2012, 7, e39224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ji, M.; Liao, Y.; Yang, J.; Gao, J. Endotoxin tolerance induced by lipopolysaccharide preconditioning protects against surgeryinduced cognitive impairment in aging mice. Mol. Med. Rep. 2018, 17, 3845–3852. [Google Scholar] [CrossRef] [PubMed]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinoshita, M.; Nakashima, M.; Nakashima, H.; Seki, S. Immune Mechanisms Underlying Susceptibility to Endotoxin Shock in Aged Hosts: Implication in Age-Augmented Generalized Shwartzman Reaction. Int. J. Mol. Sci. 2019, 20, 3260. https://doi.org/10.3390/ijms20133260
Kinoshita M, Nakashima M, Nakashima H, Seki S. Immune Mechanisms Underlying Susceptibility to Endotoxin Shock in Aged Hosts: Implication in Age-Augmented Generalized Shwartzman Reaction. International Journal of Molecular Sciences. 2019; 20(13):3260. https://doi.org/10.3390/ijms20133260
Chicago/Turabian StyleKinoshita, Manabu, Masahiro Nakashima, Hiroyuki Nakashima, and Shuhji Seki. 2019. "Immune Mechanisms Underlying Susceptibility to Endotoxin Shock in Aged Hosts: Implication in Age-Augmented Generalized Shwartzman Reaction" International Journal of Molecular Sciences 20, no. 13: 3260. https://doi.org/10.3390/ijms20133260