Comparative Analysis of the PIN Auxin Transporter Gene Family in Different Plant Species: A Focus on Structural and Expression Profiling of PINs in Solanum tuberosum
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of PIN Gene Family in Plants
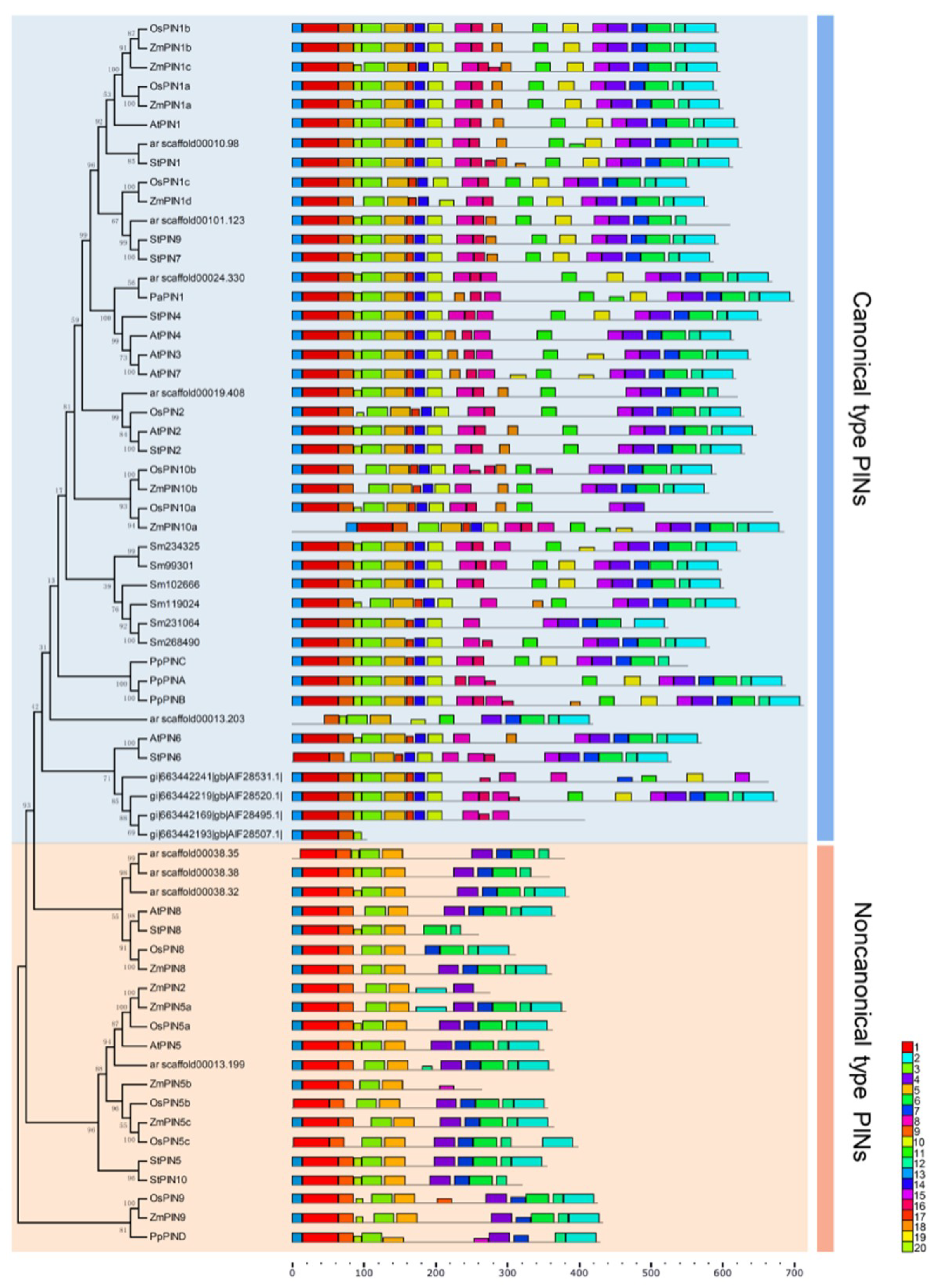
2.2. Phylogenetic Analysis of the Putative PIN Proteins
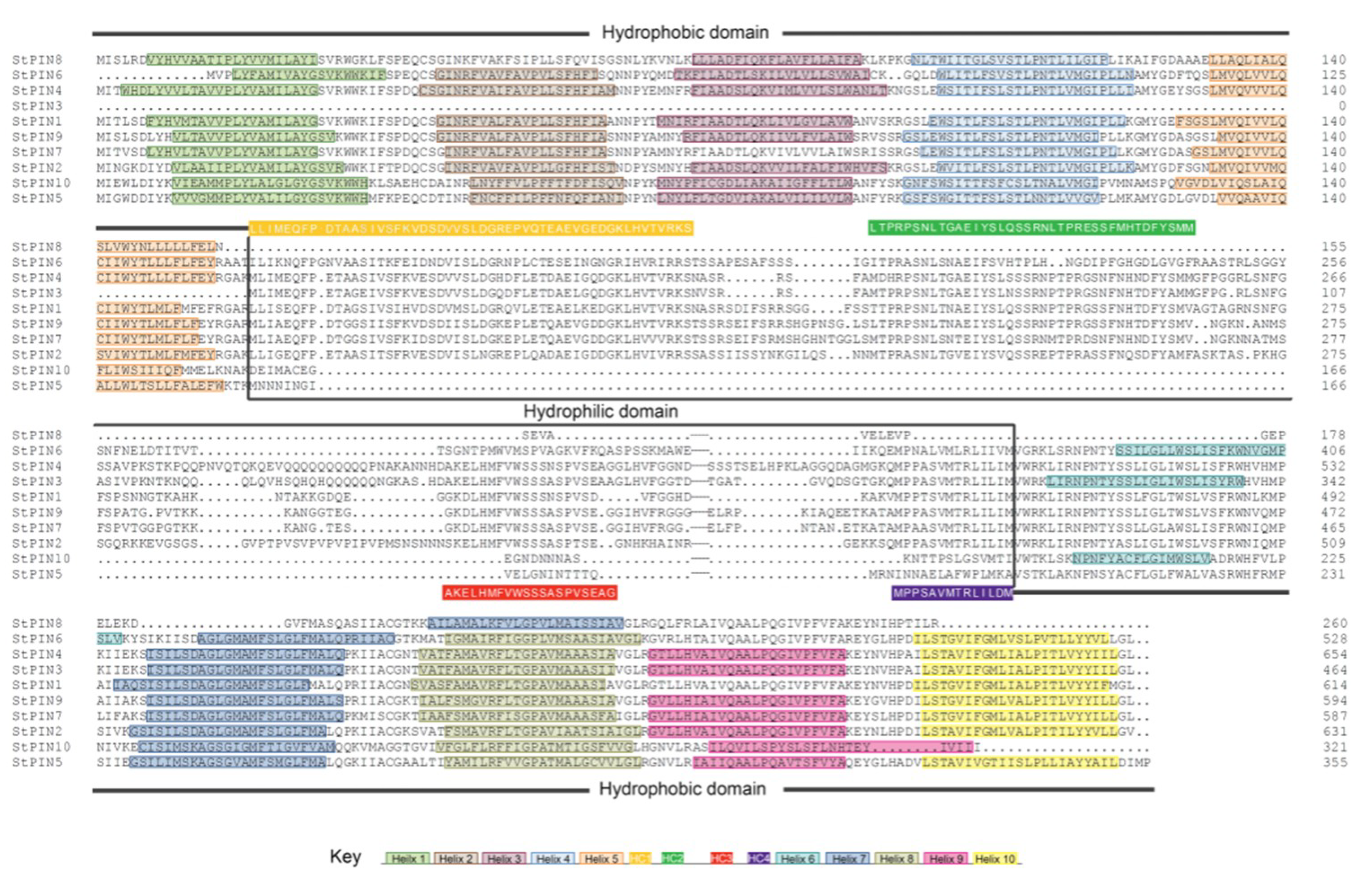
2.3. Structural Analysis of PIN Proteins
2.4. Gene Ontology Annotations of StPIN Proteins
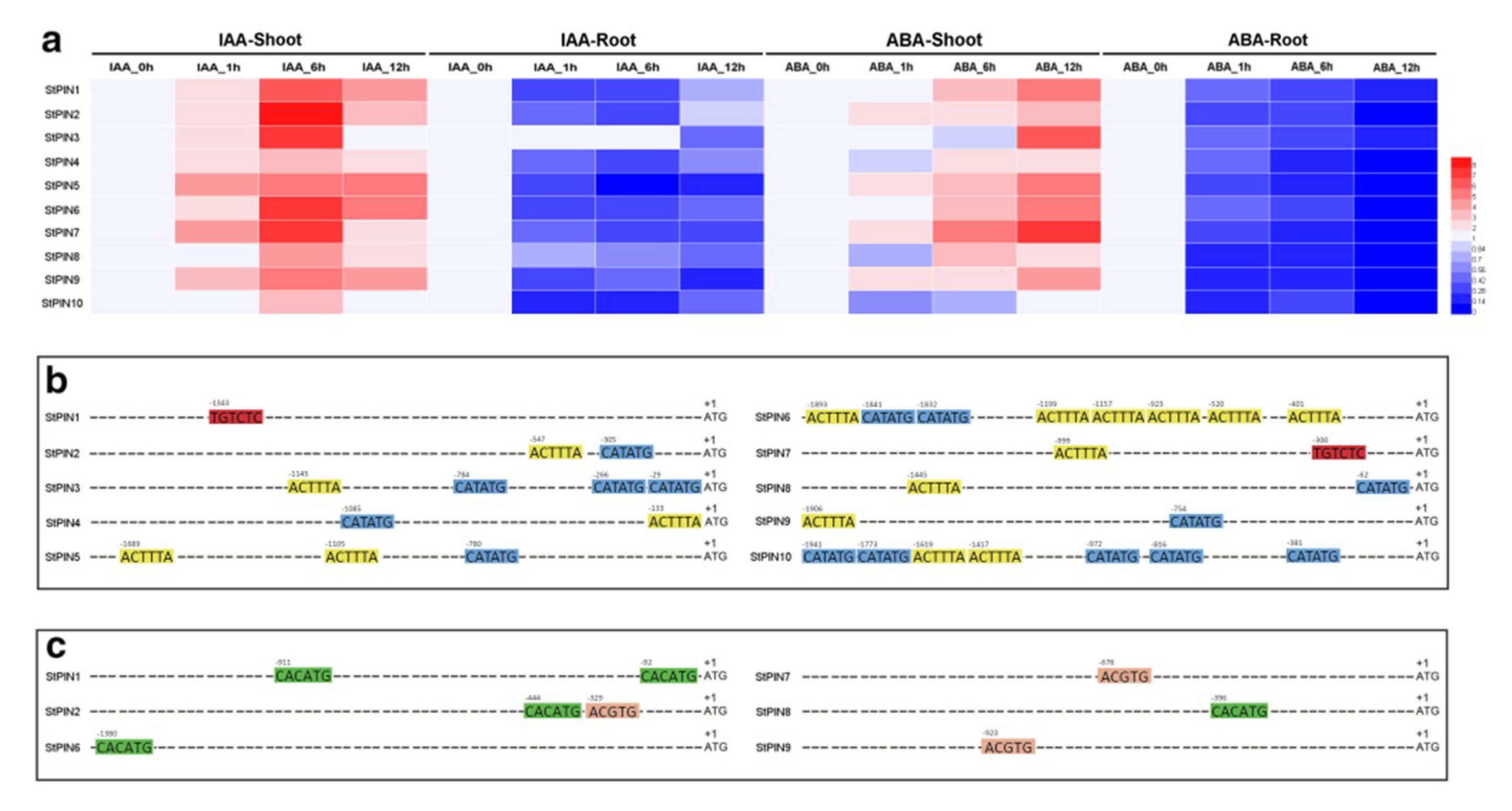
2.5. Expression Profiles of StPIN Family Genes in Response to Auxin and Abscisic acid Treatment
3. Materials and Methods
3.1. Sequence Retrieval and Identification of PIN Genes
3.2. Multiple Sequence Alignment and Phylogenetic Tree Construction
3.3. Conversed Motifs Analysis of Several Typical Species
3.4. Plant Growth, Hormone Treatments and Tissue Collection
3.5. RNA Extraction and Quantitative RT-PCR (qRT-PCR) Analysis
3.6. GO Annotation and Promoter Sequence Analysis of Potato PIN Genes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Nicola, C.; Eizabeth, T.O.T.; Matthew, C.R.; Sophie, K.A.; Rachel, S. Diversification and Expression of the PIN, AUX/LAX, and ABCB Families of Putative Auxin Transporters in Populus. Ann. Neurol. 2012, 3, 17. [Google Scholar]
- Schnabel, E.L.; Frugoli, J. The PIN and LAX families of auxin transport genes in Medicago truncatula. Mol. Genet. Genom. 2004, 272, 420–432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; He, P.; Yang, Z.; Huang, G.; Wang, L.; Pang, C.; Xiao, H.; Zhao, P.; Yu, J.; Xiao, G. A Genome-Scale Analysis of the PIN Gene Family Reveals Its Functions in Cotton Fiber Development. Front. Plant Sci. 2017, 8, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Chai, C.; Valliyodan, B.; Maupin, C.; Annen, B.; Nguyen, H.T. Genome-wide analysis and expression profiling of the PIN auxin transporter gene family in soybean (Glycine max). BMC Genom. 2015, 16, 951. [Google Scholar] [CrossRef] [PubMed]
- Naramoto, S. Polar transport in plants mediated by membrane transporters: Focus on mechanisms of polar auxin transport. Curr. Opin. Plant Biol. 2017, 40, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Krecek, P.; Skupa, P.; Libus, J.; Naramoto, S.; Tejos, R.; Friml, J.; Zazimalova, E. The PIN-FORMED (PIN) protein family of auxin transporters. Genomebiol 2009, 10, 249. [Google Scholar]
- Miyashita, Y.; Takasugi, T.; Ito, Y. Identification and expression analysis of PIN genes in rice. Plant Sci. (Oxford) 2010, 178, 0-428. [Google Scholar] [CrossRef]
- Pattison, R.J.; Catalá, C. Evaluating auxin distribution in tomato (Solanum lycopersicum) through ananalysis of the PIN and AUX/LAX gene families. Plant J. 2012, 70, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, E.; Kloosterman, B.; Oortwijn, M.; Visser, R.G.F.; Bachem, C.W.B. The PIN family of proteins in potato and their putative role in tuberization. Front. Plant Sci. 2013, 4, 524. [Google Scholar] [CrossRef] [PubMed]
- Adamowski, M.; Friml, J. PIN-Dependent Auxin Transport: Action, Regulation, and Evolution. Plant Cell Online 2015, 27, 20–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gälweiler, L.; Guan, C.; Müller, A.; Wisman, E.; Mendgen, K.; Yephremov, A.; Palme, K. Regulation of Polar Auxin Transport by AtPIN1 in Arabidopsis Vascular Tissue. Science 1998, 282, 2226–2230. [Google Scholar] [CrossRef] [PubMed]
- Petrasek, J. PIN Proteins Perform a Rate-Limiting Function in Cellular Auxin Efflux. Science 2006, 312, 914–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Murphy, A.S. Functional expression and characterization of Arabidopsis ABCB, AUX 1 and PIN auxin transporters in Schizosaccharomyces pombe. Plant J. 2010, 59, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Barbez, E.; Laňková, M.; Pařezová, M.; Maizel, A.; Zažímalová, E.; Petrášek, J.; Friml, J.; Kleine-Vehn, J. Single-cell-based system to monitor carrier driven cellular auxin homeostasis. BMC Plant Biol. 2013, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Wisniewska, J.; Xu, J.; Seifertova, D.; Brewer, PB.; Ruzicka, K.; Blilou, L.; Rouquie, D.; Benkova, E.; Scheres, B.; Friml, J. Polar PIN Localization Directs Auxin Flow in Plants. Science 2006, 312, 883. [Google Scholar] [CrossRef] [PubMed]
- Vieten, A.; Vanneste, S.; Wisniewska, J.; Benkova, E.; Benjamins, R.; Beeckman, T.; Luschniq, C.; Friml, J. Functional redundancy of PIN proteins is accompanied by auxin-dependentcross-regulation of PIN expression. Development 2005, 132, 4521–4531. [Google Scholar] [CrossRef] [PubMed]
- Paponov, I.A.; Teale, W.D.; Trebar, M.; Blilou, I.; Palme, K. The PIN auxin efflux facilitators: Evolutionary and functional perspectives. Trends Plant Sci. 2005, 10, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Jásik, J.; Bokor, B.; Stuchlík, S.; Mičieta, K.; Turna, J.; Schmelzer, E. Effects of auxins on PIN-FORMED2 (PIN2) dynamics are not mediated by inhibiting PIN2 endocytosis. Plant Physiol. 2016, 172, 1019–1031. [Google Scholar] [CrossRef]
- Yuting, L.; Qingkun, D.; Daniel, K.; Jiabao, H.; Guolan, L.; Xiaowei, W.; Xiaoyue, Z.; Alice, Y.C.; Henming, W.; Lizhen, T. RopGEF1 Plays a Critical Role in Polar Auxin Transport in Early Development. Plant Physiol. Preview 2017. [Google Scholar] [CrossRef]
- Li, K.; Kamiya, T.; Fujiwara, T. Differential roles of PIN1 and PIN2 in root meristem maintenance under low-B conditions in Arabidopsis thaliana. Plant Cell Physiol. 2015, 56, 1205–1214. [Google Scholar] [CrossRef]
- Mravec, J.; Skupa, P.; Bailly, A.; Hoyerova, K.; Krecek, P.; Bielach, A.; Petrasek, J.; Zhang, J.; Gaykova, V.; Stierhof, Y.D.; et al. Subcellular homeostasis of phytohormone auxin is mediated by the ER localized PIN5 transporter. Nature 2009, 459, 1136–1140. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Wang, B.; Moreno, I.; Duplakova, N.; Simon, S.; Carraro, N.; Reemmer, J.; Pencik, A.; Chen, X.; Tejos, R.; et al. ER localized auxin transporter PIN8 regulates auxin homeostasis and male gametophyte development in Arabidopsis. Nat. Commun. 2012, 3, 941. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Andrés, G.; Soriano-Ortega, E.; Gao, C.; Bernabe-Orts, J.; Narasimhan, M.; Muller, A.; Tejos, R.; Jiang, L.; Friml, J.; Aniento, F.; et al. Sorting Motifs Involved in the Trafficking and Localization of the PIN1 Auxin Efflux Carrier. Plant Physiol. 2016, 171, 1965. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Peng, Z.; Limin, W.; Yuzhou, Z.; Xiaosi, W.; Hui, X.; Jianing, Y.; Guanghui, X. The PIN, gene family in cotton (Gossypium hirsutum): Genome-wide identification and gene expression analyses during root development and abiotic stress responses. BMC Genom. 2017, 18, 507. [Google Scholar]
- Bennett, T.; Brockington, S.F.; Rothfels, C.; Graham, S.W.; Stevenson, D.; Kutchan, T.; Rolf, M.; Thomas, P.; Wong, GK.; et al. Paralogous Radiations of PIN Proteins with Multiple Origins of Noncanonical PIN Structure. Mol. Biol. Evol. 2014, 31, 2042–2060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, S.; Skupa, P.; Viaene, T.; Zwiewka, M.; Tejos, R.; Klíma, P.; Carna, M.; Rolcik, J.; De Rycke, R.; Moreno, I.; et al. PIN6 auxin transporter at endoplasmic reticulum and plasma membrane mediates auxin homeostasis and organogenesis in Arabidopsis. New Phytol. 2016, 211, 65–74. [Google Scholar] [CrossRef]
- Huang, F.; Zago, M.K.; Abas, L.; van Marion, A.; Galvan-Ampudia, C.S.; Offringa, R. Phosphorylation of conserved PIN motifs directs Arabidopsis PIN1 polarity and auxin transport. Plant Cell 2010, 22, 1129–1142. [Google Scholar] [CrossRef]
- Zhang, J.; Nodzynski, T.; Pencık, A.; Rolcık, J.; Friml, J. PIN phosphorylation is sufficient to mediate PIN polarity and direct auxin transport. Proc. Natl. Acad. Sci. USA 2010, 107, 918–922. [Google Scholar] [CrossRef]
- Viaene, T.; Delwiche, C.F.; Rensing, S.A.; Friml, J. Origin and evolution of PIN auxin transporters in the green lineage. Trends Plant Sci. 2013, 18, 5–10. [Google Scholar] [CrossRef]
- Bennett, T.A.; Liu, M.M.; Aoyama, T.; Bierfreund, N.M.; Harrison, C.J.; Coudert, Y.; Dennis, R.J.; O’Connor, D.; Wang, X.Y.; White, C.D.; et al. Plasma membrane-targeted PIN proteins drive shoot development in a moss. Curr. Biol. 2014, 24, 2776. [Google Scholar] [CrossRef]
- Cristian, F.; Silvia, F.; Serena, V. The Maize PIN Gene Family of Auxin Transporters. Front. Plant Sci. 2012, 3, 16. [Google Scholar] [Green Version]
- Palovaara, J.; Hallberg, H.; Stasolla, C.; Luit, B.; Hakman, I. Expression of a gymnosperm PIN homologous gene correlates with auxin immunolocalization pattern at cotyledon formation and in demarcation of the procambium during Picea abies somatic embryo development and in seedling tissues. Tree Physiol. 2010, 30, 479–489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloosterman, B.; DeKoeyer, D.; Griffiths, R.; Flinn, B.; Steuernagel, B.; Scholz, U.; Sonnewald, S.; Sonnewald, U.; Bryan, GJ.; Prat, S.; et al. Genes driving potato tuber initiation and growth: Identification based on transcriptional changes using the POCI array. Funct. Integr. Genom. 2008, 8, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Li, X.; Lv, Y.; Ding, L. Comparative analysis of the phytocyanin gene family in 10 plant species: A focus on Zea mays. Front. Plant Sci. 2015, 6, 515. [Google Scholar] [CrossRef] [PubMed]
- Kent, W.J.; Baertsch, R.; Hinrichs, A.; Miller, W.; Haussler, D. Evolution’s cauldron: Duplication, deletion, and rearrangement in the mouse and human genomes. Proc. Natl. Acad. Sci. USA 2003, 100, 11484–11489. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Landherr, L.L.; Frohlich, M.W.; Leebens-Mack, J.; Ma, H.; Depamphilis, C.W. Patterns of gene duplication in the plant SKP1 gene family in angiosperms: Evidence for multiple mechanisms of rapid gene birth. Plant J. 2007, 50, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Tang, H.; Wang, X.; Paterson, A.H. PGDD: A database of gene and genome duplication in plants. Nucleic Acids Res. 2013, 41, D1152–D1158. [Google Scholar] [CrossRef]
- Decker, E.L.; Frank, W.; Sarnighausen, E.; Reski, R. Moss Systems Biology en Route: Phytohormones in Physcomitrella, Development. Plant Biol. 2006, 8, 397–405. [Google Scholar] [CrossRef]
- Jordan, I.K.; Makarova, K.S.; Spouge, J.L.; Wolf, Y.I.; Koonin, E.V. Lineage-specific gene expansions in bacterial and archaeal genomes. Genome Res. 2001, 11, 555–565. [Google Scholar] [CrossRef]
- Lespinet, O.; Wolf, Y.I.; Koonin, E.V.; Aravind, L. The Role of Lineage-Specific Gene Family Expansion in the Evolution of Eukaryotes. Genome Res. 2002, 12, 1048–1059. [Google Scholar] [CrossRef] [Green Version]
- Vanneste, S.; Friml, J. Auxin: A Trigger for Change in Plant Development. Cell 2009, 136, 1005–1016. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A. Auxin: A regulator of cold stress response. Physiol. Plant. 2012, 147, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Kazan, K. Auxin and the integration of environmental signals into plant root development. Ann. Bot. 2013, 112, 1655–1665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, H.; Dhonukshe, P.; Brewer, P.B.; Friml, J. Spatiotemporal asymmetric auxin distribution: A means to coordinate plant development. Cell. Mol. Life Sci. 2006, 63, 2738–2754. [Google Scholar] [CrossRef] [PubMed]
- Michniewicz, M.; Brewer, P.B.; Friml, J.I. Polar Auxin Transport and Asymmetric Auxin Distribution. Arab. Book 2007, 5, e0108. [Google Scholar]
- Busk, P.K.; Montserrat, P. Regulation of abscisic acid-induced transcription. Plant Mol. Biol. 1998, 37, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Yue., R.; Tie, S.; Sun, T.; Zhang, L.; Yang, Y.; Qi, J.; Yan, S.; Han, X.; Wang, H.; Shen, C. Genome-Wide Identification and Expression Profiling Analysis of ZmPIN, ZmPILS, ZmLAX and ZmABCB Auxin Transporter Gene Families in Maize (Zea mays L.) under Various Abiotic Stresses. PLoS ONE 2015, 10, e0118751. [Google Scholar] [CrossRef] [PubMed]
- Higo, K.; Ugawa, Y.; Iwamoto, M.; Korenaqa, T. Plant cis-acting regulatory DNA elements (PLACE) database: 1999. Nucleic Acids Res. 1999, 27, 297–300. [Google Scholar] [CrossRef] [Green Version]
- Ruoqiu, W.; Peng, Z.; Nana, K.; Ruize, L.; Yue, P.; Chenxi, H.; Haoli, M.; Qin, C. Genome-Wide Identification and Characterization of the Potato bHLH Transcription Factor Family. Genes 2018, 9, 54. [Google Scholar] [Green Version]
- Bailey, T.L.; Elkan, C. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. UCSD Tech. Rep. 1994, 2, 28–36. [Google Scholar]
- Nicot, N.; Hausman, J.F.; Hoffmann, L.; Evers, D. Housekeeping gene selection for real-time RT-PCR normalization in potato during biotic and abiotic stress. J. Exp. Bot. 2005, 56, 2907–2914. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, G.; Murphy, A.; De, K.D.; Tai, H.; Bizimungu, B.; Si, H.; Li, X.Q. Differences between the bud end and stem end of potatoes in dry matter content, starch granule size, and carbohydrate metabolic gene expression at the growing and sprouting stages. J. Agric. Food Chem. 2016, 64, 176. [Google Scholar] [CrossRef] [PubMed]


| Lineage | Organism | Number of Predicted Genes (Loci) 1 | Number of PIN Genes | Number of Long PIN Genes | Number of Short PIN Genes | Tandem Duplication (Pairs) 2,3 | Segmental Duplication (Pairs) 2,3 | References |
|---|---|---|---|---|---|---|---|---|
| Algaes | Charophyceae | 300 | 0 | 0 | 0 | 0 | 0 | This study |
| Chlamydomonas reinhardtii | 17,741 | 0 | 0 | 0 | 0 | 0 | Viaene T., et al. [29] | |
| Klebsormidiophyceae | 55,298 | 1 | 0 | 1 | 0 | 0 | Viaene T., et al. [29] | |
| Moss | Physcomitrella patens | 32,926 | 4 | 3 | 1 | 0 | 0 | Bennett, Tom A., et al. [25] |
| Lycophytes | Selaginella moellendorffii | 22,273 | 6 | 6 | 0 | 0 | 1 | Cristian, F., et al. [31] |
| Ferns | Cystopteris fragilis | 7194 | 4 | 0 | 0 | 0 | 0 | This study |
| Gymnosperm | Picea abies | 28,354 | 1 | 1 | 0 | 1 | 0 | Viaene T., et al. [29] |
| Amborellales | Amborella trichopoda | 26,846 | 9 | 5 | 4 | 1 | 0 | This study |
| Dicots | Arabidopsis thaliana | 27,416 | 8 | 5 | 2 | 0 | 0 | Paponov, I.A. et al. [17] |
| Brassica oleracea | 35,400 | 16 | 11 | 5 | 0 | 10 | This study | |
| Glycine max | 56,044 | 23 | 11 | 6 | 0 | 13 | Wang, Y., et al. [4] | |
| Populus trichocarpa | 41,335 | 16 | 7 | 6 | 0 | 9 | Nicola, C. et al. [1] | |
| Solanum tuberosum | 39,028 | 10 | 7 | 3 | 2 | 1 | Roumeliotis, E. et al. [9] | |
| Gossypium raimondii | 37,505 | 10 | 6 | 3 | 0 | 4 | Zhang Y., et al. [3] | |
| Utricularia gibba | 28,494 | 7 | 6 | 1 | 0 | 0 | This study | |
| Monocots | Brachypodium distachyon | 34,310 | 10 | 5 | 5 | 0 | 5 | Cristian, F., et al. [31] |
| Oryza sativa | 42,189 | 12 | 7 | 4 | 0 | 6 | Wang, Y., et al. [4] | |
| Phalaenopsis equestris | 31,384 | 6 | 4 | 2 | 0 | 0 | This study | |
| Zea mays | 40,557 | 12 | 7 | 4 | 0 | 5 | Cristian, F., et al. [31] | |
| Total | 155 | 91 | 47 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Wang, D.; Zhang, C.; Kong, N.; Ma, H.; Chen, Q. Comparative Analysis of the PIN Auxin Transporter Gene Family in Different Plant Species: A Focus on Structural and Expression Profiling of PINs in Solanum tuberosum. Int. J. Mol. Sci. 2019, 20, 3270. https://doi.org/10.3390/ijms20133270
Yang C, Wang D, Zhang C, Kong N, Ma H, Chen Q. Comparative Analysis of the PIN Auxin Transporter Gene Family in Different Plant Species: A Focus on Structural and Expression Profiling of PINs in Solanum tuberosum. International Journal of Molecular Sciences. 2019; 20(13):3270. https://doi.org/10.3390/ijms20133270
Chicago/Turabian StyleYang, Chenghui, Dongdong Wang, Chao Zhang, Nana Kong, Haoli Ma, and Qin Chen. 2019. "Comparative Analysis of the PIN Auxin Transporter Gene Family in Different Plant Species: A Focus on Structural and Expression Profiling of PINs in Solanum tuberosum" International Journal of Molecular Sciences 20, no. 13: 3270. https://doi.org/10.3390/ijms20133270





