Protective Effects of Curcumin Ester Prodrug, Curcumin Diethyl Disuccinate against H2O2-Induced Oxidative Stress in Human Retinal Pigment Epithelial Cells: Potential Therapeutic Avenues for Age-Related Macular Degeneration
Abstract
:1. Introduction
2. Results
2.1. Long-Term Differentiated ARPE-19 Cells Display More Native RPE Characteristics Compared to Undifferentiated ARPE-19 Cells
2.2. Evaluation of Cur and CurDD on Cell Viability of Undifferentiated and Differentiated ARPE-19 Cells.
2.3. Differentiated ARPE-19 Cells Are More Sensitive to H2O2-Induced Oxidative Stress than Undifferentiated ARPE-19 Cells
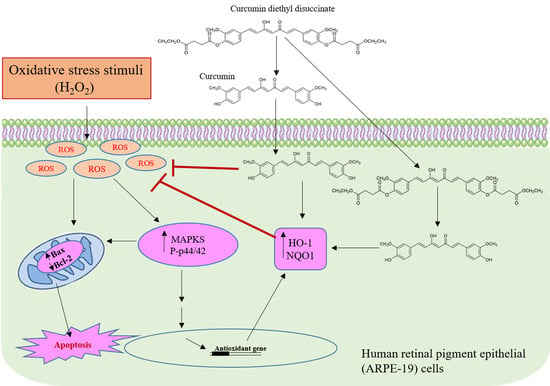
2.4. Cur and CurrDD Exert A Protective Effect against H2O2-Induced Oxidative Stress in ARPE-19 Cells
2.5. Cur and CurDD Exert Their Protective Effect against H2O2-Induced Oxidative Stress via Modulation of the p44/42 MAPK Pathway
2.6. Cur and CurDD Inhibit Apoptosis by Modulation of Bax and Bcl2 Expression in Oxidative Stressed ARPE-19 Cells
2.7. Cur and CurDD Exert Their Protective Effect against Oxidative Stress-Induced Cell Death via Modulation of Key Anti-Oxidant Enzymes in ARPE-19 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture of ARPE-19 cells
4.3. Cell viability (MTT) Assay
4.4. Evaluation of Cytotoxicity of Cur or CurDD
4.5. Evaluation of Suitable H2O2 Concentration for Cytotoxicity Induction
4.6. Evaluation of the Protective Effect of Cur and CurDD on ARPE-19 Induced Oxidative Stress
4.7. Evaluation of Reactive Oxygen Species (ROS) Production
4.8. Western Blot Analysis
4.9. Real-Time Quantitative PCR (qPCR)
4.10. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhutto, I.; Lutty, G. Understanding age-related macular degeneration (AMD): Relationships between the photoreceptor/retinal pigment epithelium/Bruch’s membrane/choriocapillaris complex. Mol. Aspects Med. 2012, 33, 295–317. [Google Scholar] [CrossRef]
- Klein, R.; Cruickshanks, K.J.; Nash, S.D.; Krantz, E.M.; Nieto, F.J.; Huang, G.H.; Pankow, J.S.; Klein, B.E.K. The prevalence of age-related macular degeneration and associated risk factors. Arch. Ophthalmol. 2010, 128, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Chappelow, A.V.; Kaiser, P.K. Neovascular age-related macular degeneration: Potential therapies. Drugs 2008, 68, 1029–1036. [Google Scholar] [CrossRef] [PubMed]
- Strauss, O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005, 85, 845–881. [Google Scholar] [CrossRef] [PubMed]
- Atienzar-Aroca, S.; Flores-Bellver, M.; Serrano-Heras, G.; Martinez-Gil, N.; Barcia, J.M.; Aparicio, S.; Perez-Cremades, D.; Garcia-Verdugo, J.M.; Diaz-Llopis, M.; Romero, F.J.; et al. Oxidative stress in retinal pigment epithelium cells increases exosome secretion and promotes angiogenesis in endothelial cells. J. Cell. Mol. Med. 2016, 20, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Patel, M.; Chan, C.-C. Molecular pathology of age-related macular degeneration. Prog. Retin. Eye Res. 2009, 28, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Beatty, S.; Koh, H.-H.; Phil, M.; Henson, D.; Boulton, M. The role of oxidative stress in the pathogenesis of age-related macular degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef]
- Kay, P.; Yang, Y.C.; Paraoan, L. Directional protein secretion by the retinal pigment epithelium: Roles in retinal health and the development of age-related macular degeneration. J. Cell. Mol. Med. 2013, 17, 833–843. [Google Scholar] [CrossRef]
- Marazita, M.C.; Dugour, A.; Marquioni-Ramella, M.D.; Figueroa, J.M.; Suburo, A.M. Oxidative stress-induced premature senescence dysregulates VEGF and CFH expression in retinal pigment epithelial cells: Implications for Age-related Macular Degeneration. Redox Biol. 2015, 7, 78–87. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Ward, K.; Xavier, C.; Jann, J.; Clark, A.F.; Pang, I.-H.; Wu, H. The novel triterpenoid RTA 408 protects human retinal pigment epithelial cells against H2O2-induced cell injury via NF-E2-related factor 2 (Nrf2) activation. Redox Biol. 2015, 8, 98–109. [Google Scholar] [CrossRef] [Green Version]
- Winkler, B.S.; Boulton, M.E.; Gottsch, J.D.; Sternberg, P. Oxidative damage and age-related macular degeneration. Mol. Vis. 1999, 5, 32. [Google Scholar] [PubMed]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Richter, C. Reactive oxygen and DNA damage in mitochondria. Mutat. Res. 1992, 275, 249–255. [Google Scholar] [CrossRef]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal. Transduct. 2012, 2012, 646354. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Miceli, M.V.; Liles, M.R.; Newsome, D.A. Evaluation of oxidative processes in human pigment epithelial cells associated with retinal outer segment phagocytosis. Exp. Cell Res. 1994, 214, 242–249. [Google Scholar] [CrossRef]
- Kopito, R.R. Aggresomes, inclusion bodies and protein aggregation. Trends Cell Biol. 2000, 10, 524–530. [Google Scholar] [CrossRef]
- Grune, T.; Jung, T.; Merker, K.; Davies, K.J. Decreased proteolysis caused by protein aggregates, inclusion bodies, plaques, lipofuscin, ceroid, and ‘aggresomes’ during oxidative stress, aging, and disease. Int. J. Biochem. Cell Biol. 2004, 36, 2519–2530. [Google Scholar] [CrossRef] [PubMed]
- Hecquet, C.; Lefevre, G.l.; Valtink, M.; Engelmann, K.; Mascarelli, F. Activation and Role of MAP Kinase-Dependent Pathways in Retinal Pigment Epithelial Cells: ERK and RPE Cell Proliferation. Invest. Ophthalmol. Vis. Sci. 2002, 43, 3091–3098. [Google Scholar] [PubMed]
- Garg, T.K.; Chang, J.Y. Oxidative stress causes ERK phosphorylation and cell death in cultured retinal pigment epithelium: Prevention of cell death by AG126 and 15-deoxy-delta 12, 14-PGJ2. BMC Ophthalmol. 2003, 3, 5. [Google Scholar] [CrossRef]
- Giansanti, V.; Rodriguez, G.E.V.; Savoldelli, M.; Gioia, R.; Forlino, A.; Mazzini, G.; Pennati, M.; Zaffaroni, N.; Scovassi, A.I.; Torriglia, A. Characterization of stress response in human retinal epithelial cells. J. Cell. Mol. Med. 2013, 17, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Rozanowska, M.; Sarna, T.; Land, E.J.; Truscott, T.G. Free radical scavenging properties of melanin interaction of eu- and pheo-melanin models with reducing and oxidising radicals. Free Radic. Biol. Med. 1999, 26, 518–525. [Google Scholar] [PubMed]
- Sarna, T.; Burke, J.M.; Korytowski, W.; Rozanowska, M.; Skumatz, C.M.; Zareba, A.; Zareba, M. Loss of melanin from human RPE with aging: Possible role of melanin photooxidation. Exp. Eye Res. 2003, 76, 89–98. [Google Scholar] [CrossRef]
- Cai, J.; Nelson, K.C.; Wu, M.; Sternberg, P.; Jones, D.P. Oxidative damage and protection of the RPE. Prog. Retin. Eye Res. 2000, 19, 205–221. [Google Scholar] [CrossRef]
- Zhu, C.; Dong, Y.; Liu, H.; Ren, H.; Cui, Z. Hesperetin protects against H2O2-triggered oxidative damage via upregulation of the Keap1-Nrf2/HO-1 signal pathway in ARPE-19 cells. Biomed. Pharmacother. 2017, 88, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Costa, V.; Moradas-Ferreira, P. Oxidative stress and signal transduction in Saccharomyces cerevisiae: Insights into ageing, apoptosis and diseases. Mol. Aspects Med. 2001, 22, 217–246. [Google Scholar] [CrossRef]
- Feeney-Burns, L.; Hilderbrand, E.S.; Eldridge, S. Aging human RPE: Morphometric analysis of macular, equatorial, and peripheral cells. Invest. Ophthalmol. Vis. Sci. 1984, 25, 195–200. [Google Scholar] [PubMed]
- Weiter, J.J.; Delori, F.C.; Wing, G.L.; Fitch, K.A. Retinal pigment epithelial lipofuscin and melanin and choroidal melanin in human eyes. Invest. Ophthalmol. Vis. Sci. 1986, 27, 145–152. [Google Scholar] [PubMed]
- Liles, M.R.; Newsome, D.A.; Oliver, P.D. Antioxidant enzymes in the aging human retinal pigment epithelium. Arch. Ophthalmol. 1991, 109, 1285–1288. [Google Scholar] [CrossRef]
- Kalt, W.; Hanneken, A.; Milbury, P.; Tremblay, F. Recent research on polyphenolics in vision and eye Health. J. Agric. Food Chem. 2010, 58, 4001–4007. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.-C.; Nandrot, E.F.; Dun, Y.; Finnemann, S.C. Dietary antioxidants prevent age-related retinal pigment epithelium actin damage and blindness in mice lacking αvβ5 integrin. Free Radic. Biol. Med. 2012, 52, 660–670. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, Y.-y.; Jiang, Q.; Li, K.-r.; Zhao, Y.-x.; Cao, C.; Yao, J. Salvianolic acid A protects RPE cells against oxidative stress through activation of Nrf2/HO-1 signaling. Free Radic. Biol. Med. 2014, 69, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Ak, T.; Gülçin, İ. Antioxidant and radical scavenging properties of curcumin. Chem. Biol. Interac. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Akiko, J.-M.; Aya, F.; Toshiya, M. Curcumin: From chemistry to chemistry-based functions. Curr. Pharm. Des. 2013, 19, 2084–2092. [Google Scholar]
- Lee, J.C.; Kinniry, P.A.; Arguiri, E.; Serota, M.; Kanterakis, S.; Chatterjee, S.; Solomides, C.C.; Javvadi, P.; Koumenis, C.; Cengel, K.A.; et al. Dietary curcumin increases antioxidant defenses in lung, ameliorates radiation-induced pulmonary fibrosis, and improves survival in mice. Radiat. Res. 2010, 173, 590–601. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Du, Z.-Y.; Zheng, X.; Li, D.-L.; Zhou, R.-P.; Zhang, K. Use of curcumin in diagnosis, prevention, and treatment of Alzheimer’s disease. Neural Regen. Res. 2018, 13, 742–752. [Google Scholar]
- Woo, J.M.; Shin, D.-Y.; Lee, S.J.; Joe, Y.; Zheng, M.; Yim, J.H.; Callaway, Z.; Chung, H.T. Curcumin protects retinal pigment epithelial cells against oxidative stress via induction of heme oxygenase-1 expression and reduction of reactive oxygen. Mol. Vis. 2012, 18, 901–908. [Google Scholar]
- Hollborn, M.; Chen, R.; Wiedemann, P.; Reichenbach, A.; Bringmann, A.; Kohen, L. Cytotoxic effects of curcumin in human retinal pigment epithelial cells. PLoS ONE 2013, 8, e59603. [Google Scholar] [CrossRef]
- Zhu, W.; Wu, Y.; Meng, Y.-F.; Wang, J.-Y.; Xu, M.; Tao, J.-J.; Lu, J. Effect of curcumin on aging retinal pigment epithelial cells. Drug Des. Devel. Ther. 2015, 9, 5337–5344. [Google Scholar] [Green Version]
- Mandal, M.N.; Patlolla, J.M.; Zheng, L.; Agbaga, M.P.; Tran, J.T.; Wicker, L.; Kasus-Jacobi, A.; Elliott, M.H.; Rao, C.V.; Anderson, R.E. Curcumin protects retinal cells from light-and oxidant stress-induced cell death. Free. Radic. Biol. Med. 2009, 46, 672–679. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Rautio, J.; Kumpulainen, H.; Heimbach, T.; Oliyai, R.; Oh, D.; Järvinen, T.; Savolainen, J. Prodrugs: Design and clinical applications. Nat. Rev. Drug Discov. 2008, 7, 255. [Google Scholar] [CrossRef] [PubMed]
- Abet, V.; Filace, F.; Recio, J.; Alvarez-Builla, J.; Burgos, C. Prodrug approach: An overview of recent cases. Eur. J. Med. Chem. 2017, 127, 810–827. [Google Scholar] [CrossRef] [PubMed]
- Wichitnithad, W.; Nimmannit, U.; Wacharasindhu, S.; Rojsitthisak, P. Synthesis, characterization and biological evaluation of succinate prodrugs of curcuminoids for colon cancer treatment. Molecules 2011, 16, 1888–1900. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, B.; Palanisamy, U.D.; Chua, K.H.; Kuppusamy, U.R. Protective effect of myricetin derivatives from Syzygium malaccense against hydrogen peroxide-induced stress in ARPE-19 cells. Mol. Vis. 2019, 25, 47–59. [Google Scholar] [PubMed]
- Dunn, K.C.; Aotaki-Keen, A.E.; Putkey, F.R.; Hjelmeland, L.M. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp. Eye Res. 1996, 62, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Samuel, W.; Jaworski, C.; Postnikova, O.A.; Kutty, R.K.; Duncan, T.; Tan, L.X.; Poliakov, E.; Lakkaraju, A.; Redmond, T.M. Appropriately differentiated ARPE-19 cells regain phenotype and gene expression profiles similar to those of native RPE cells. Mol. Vis. 2017, 23, 60–89. [Google Scholar] [PubMed]
- Ahmado, A.; Carr, A.J.; Vugler, A.A.; Semo, M.; Gias, C.; Lawrence, J.M.; Chen, L.L.; Chen, F.K.; Turowski, P.; da Cruz, L.; et al. Induction of differentiation by pyruvate and DMEM in the human retinal pigment epithelium cell line ARPE-19. Invest. Ophthalmol. Vis. Sci. 2011, 52, 7148–7159. [Google Scholar] [CrossRef] [PubMed]
- Premanand, C.; Rema, M.; Sameer, M.Z.; Sujatha, M.; Balasubramanyam, M. Effect of curcumin on proliferation of human retinal endothelial cells under in vitro conditions. Invest. Ophthalmol. Vis. Sci. 2006, 47, 2179–2184. [Google Scholar] [CrossRef]
- Ambegaokar, S.S.; Wu, L.; Alamshahi, K.; Lau, J.; Jazayeri, L.; Chan, S.; Khanna, P.; Hsieh, E.; Timiras, P.S. Curcumin inhibits dose-dependently and time-dependently neuroglial cell proliferation and growth. Neuro. Endocrinol. Lett. 2003, 24, 469–473. [Google Scholar]
- Zhuang, S.; Yan, Y.; Daubert, R.A.; Han, J.; Schnellmann, R.G. ERK promotes hydrogen peroxide-induced apoptosis through caspase-3 activation and inhibition of Akt in renal epithelial cells. Am. J. Physiol. Renal Physiol. 2007, 292, F440–F447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.K.; Kim, H.J.; Kwon, C.H.; Kim, J.H.; Woo, J.S.; Jung, J.S.; Kim, J.M. Role of ERK activation in cisplatin-induced apoptosis in OK renal epithelial cells. J. Appl. Toxicol. 2005, 25, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Yang, R.; Zheng, Z.; Zhou, Y.; Geng, Y.; Hu, Y.; Wu, S.; Wu, W. Sulforaphane-cysteine-induced apoptosis via phosphorylated ERK1/2-mediated maspin pathway in human non-small cell lung cancer cells. Cell Death Discov. 2017, 3, 17025. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Jung, J.S.; Jeong, Y.H.; Hyun, J.W.; Le, T.K.; Kim, D.H.; Choi, E.C.; Kim, H.S. Antioxidant mechanism of isoflavone metabolites in hydrogen peroxide-stimulated rat primary astrocytes: Critical role of hemeoxygenase-1 and NQO1 expression. J. Neurochem. 2011, 119, 909–919. [Google Scholar] [CrossRef] [PubMed]
- Jian, Z.; Li, K.; Liu, L.; Zhang, Y.; Zhou, Z.; Li, C.; Gao, T. Heme oxygenase-1 protects human melanocytes from H2O2-induced oxidative stress via the Nrf2-ARE pathway. J. Invest. Dermatol. 2011, 131, 1420–1427. [Google Scholar] [CrossRef]
- Dai, W.; Wang, H.; Fang, J.; Zhu, Y.; Zhou, J.; Wang, X.; Zhou, Y.; Zhou, M. Curcumin provides neuroprotection in model of traumatic brain injury via the Nrf2-ARE signaling pathway. Brain Res. Bull. 2018, 140, 65–71. [Google Scholar] [CrossRef]
- Voisin, A.; Monville, C.; Plancheron, A.; Balbous, A.; Gaillard, A.; Leveziel, N. hRPE cells derived from induced pluripotent stem cells are more sensitive to oxidative stress than ARPE-19 cells. Exp. Eye Res. 2018, 177, 76–86. [Google Scholar] [CrossRef]
- Wang, X.J.; Gao, J.X.; Wang, Y.C.; Zhao, B.; Zhang, Y.J.; Han, F.; Zheng, Z.; Hu, D.H. Curcumin pretreatment prevents hydrogen peroxide-induced oxidative stress through enhanced mitochondrial function and deactivation of Akt/Erk signaling pathways in rat bone marrow mesenchymal stem cells. Mol. Cell Biochem. 2018, 443, 37–45. [Google Scholar] [CrossRef]
- Dai, P.P.; Mao, Y.X.; Sun, X.Y.; Li, X.M.; Muhammad, I.; Gu, W.Y.; Zhang, D.F.; Zhou, Y.; Ni, Z.Y.; Ma, J.F.; et al. Attenuation of oxidative stress-induced osteoblast apoptosis by curcumin is associated with preservation of mitochondrial functions and increased Akt-GSK3 beta signaling. Cell Physiol. Biochem. 2017, 41, 661–677. [Google Scholar] [CrossRef]
- Patel, A.; Patel, J.K.; Pathak, Y.V. Injectable pro-drugs approach for retina and posterior segment disease. In Drug Delivery for the Retina and Posterior Segment Disease; Patel, J.K., Sutariya, V., Kanwar, J.R., Pathak, Y.V., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 327–349. [Google Scholar] [CrossRef]
- Barot, M.; Bagui, M.; Gokulgandhi, M.R.; Mitra, A.K. Prodrug strategies in ocular drug delivery. Med. Chem. 2012, 8, 753–768. [Google Scholar] [CrossRef]
- Cholkar, K.; Trinh, H.M.; Vadlapudi, A.D.; Mitra, A.K. Synthesis and characterization of ganciclovir long chain lipid prodrugs. Adv. Ophthalmol. Vis. Syst. 2014, 1, 19–25. [Google Scholar]
- Ratnatilaka Na Bhuket, P.; Jithavech, P.; Ongpipattanakul, B.; Rojsitthisak, P. Interspecies differences in stability kinetics and plasma esterases involved in hydrolytic activation of curcumin diethyl disuccinate, a prodrug of curcumin. RSC Adv. 2019, 9, 4626–4634. [Google Scholar] [CrossRef] [Green Version]
- Tong, W.G.; Ding, X.Z.; Talamonti, M.S.; Bell, R.H.; Adrian, T.E. LTB4 stimulates growth of human pancreatic cancer cells via MAPK and PI-3 kinase pathways. Biochem. Biophys. Res. Commun. 2005, 335, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Hsiao, M.; Chang, J.L.; Yang, S.F.; Tseng, T.H.; Cheng, C.W.; Chow, J.M.; Lin, K.H.; Lin, Y.W.; Liu, C.C.; et al. Quercetin induces mitochondrial-derived apoptosis via reactive oxygen species-mediated ERK activation in HL-60 leukemia cells and xenograft. Arch. Toxicol. 2015, 89, 1103–1117. [Google Scholar] [CrossRef] [PubMed]
- Bhujade, A.; Gupta, G.; Talmale, S.; Das, S.K.; Patil, M.B. Induction of apoptosis in A431 skin cancer cells by Cissus quadrangularis Linn stem extract by altering Bax-Bcl-2 ratio, release of cytochrome c from mitochondria and PARP cleavage. Food Funct. 2013, 4, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Li, X.D.; Feng, K.; Li, J.; Yu, D.G.; Fan, Q.M.; Tang, T.T.; Yao, X.; Wang, X.Q. Curcumin inhibits apoptosis of chondrocytes through activation ERK1/2 signaling pathways induced autophagy. Nutrients 2017, 9, 414. [Google Scholar] [CrossRef]
- Zhao, L.; Gu, Q.; Xiang, L.; Dong, X.; Li, H.; Ni, J.; Wan, L.; Cai, G.; Chen, G. Curcumin inhibits apoptosis by modulating Bax/Bcl-2 expression and alleviates oxidative stress in testes of streptozotocin-induced diabetic rats. Ther. Clin. Risk Manag. 2017, 13, 1099–1105. [Google Scholar] [CrossRef]
- Sreejayan; Rao, M.N. Nitric oxide scavenging by curcuminoids. J. Pharm. Pharmacol. 1997, 49, 105–107. [Google Scholar] [CrossRef]
- Cui, Q.; Li, X.; Zhu, H. Curcumin ameliorates dopaminergic neuronal oxidative damage via activation of the Akt/Nrf2 pathway. Mol. Med. Rep. 2016, 13, 1381–1388. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, R.; Ye, M.; Zhang, L. Genipin protects against H2O2-induced oxidative damage in retinal pigment epithelial cells by promoting Nrf2 signaling. Int. J. Mol. Med. 2019, 43, 936–944. [Google Scholar] [CrossRef]
- Ishii, T.; Itoh, K.; Yamamoto, M. Roles of Nrf2 in activation of antioxidant enzyme genes via antioxidant responsive elements. Methods Enzymol. 2002, 348, 182–190. [Google Scholar] [PubMed]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.S.; Woo, I.S.; Kim, H.J.; Eun, S.Y.; Paek, K.S.; Kim, H.J.; Chang, K.C.; Lee, J.H.; Lee, H.T.; Kim, J.H.; et al. Up-regulation of aldose reductase expression mediated by phosphatidylinositol 3-kinase/Akt and Nrf2 is involved in the protective effect of curcumin against oxidative damage. Free Radic. Biol. Med. 2007, 43, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.H.; Zhang, X.J.; Fan, H.G.; Liu, Y. Curcumin upregulates transcription factor Nrf2, HO-1 expression and protects rat brains against focal ischemia. Brain Res. 2009, 1282, 133–141. [Google Scholar] [CrossRef]
- Paraoan, L.; Ratnayaka, A.; Spiller, D.G.; Hiscott, P.; White, M.R.; Grierson, I. Unexpected intracellular localization of the AMD-associated cystatin C variant. Traffic 2004, 5, 884–895. [Google Scholar] [CrossRef]







| Antibodies | Dilution |
|---|---|
| Anti-RDH5 (Abcam) Anti-CRALBP (Abcam) Anti-Phospho-p44/42 (Cell Signalling, Hertfordshire, UK) Anti-Bax (Cell Signalling, Hertfordshire, UK) Anti-Bcl-2 (Cell Signalling, Hertfordshire, UK) Anti-HO-1 (Cell Signalling, Hertfordshire, UK) Anti-NQO1 (Cell Signalling, Hertfordshire, UK) Anti-GAPDH (Abcam) Secondary horseradish peroxidase (HRP)-conjugated anti-rabbit (Sigma-Aldrich, Dorset, UK) Secondary horseradish peroxidase (HRP)-conjugated anti--mouse (Sigma--Aldrich, Dorset, UK) | 1:500 1:500 1:1000 1:1000 1:1000 1:1000 1:1000 1:500 1:2000 1:2000 |
| Bax | Forward 5′TCAGGATGCGTCCACCAAGAAG3′ Reverse 5′TGTGTCCACGGCGGCAATCATC3′ |
| Bcl-2 | Forward 5′ATCGCCCTGTGGATGACTGAGT3′ Reverse 5′GCCAGGAGAAATCAAACAGAGGC3′ |
| P-p44/42 | Forward 5′ACACCAACCTCTCGTACATCGG3′ Reverse 5′TGGCAGTAGGTCTGGTGCTCAA3′ |
| HO-1 | Forward 5′CCAGGCAGAGAATGCTGAGTTC3′ Reverse 5′AAGACTGGGCTCTCCTTGTTGC3′ |
| NQO1 | Forward 5′CCTGCCATTCTGAAAGGCTGGT3′ Reverse 5′GTGGTGATGGAAAGCACTGCCT3′ |
| p62 | Forward 5′TGTGTAGCGTCTGCGAGGGAAA3′ Reverse 5′AGTGTCCGTGTTTCACCTTCCG3′ |
| LC3-II | Forward 5′GAGAAGCAGCTTCCTGTTCTGG3′ Reverse 5′GTGTCCGTTCACCAACAGGAAG3′ |
| Beta tubulin | Forward 5′CTGGACCGCATCTCTGTGTACT3′ Reverse 5′GCCAAAAGGACCTGAGCGAACA3′ |
| GAPDH | Forward 5′TTGCCCTCAACGACCACTTT3′ Reverse 5′TGGTCCAGGGGTCTTACTCC3′ |
| ACTB | Forward 5′CACCATTGGCAATGAGCGGTTC3′ Reverse 5′AGGTCTTTGCGGATGTCCACGT3′ |
| RPL-5 | Forward 5′ATGCTCGGAAACGCTTGGT3′ Reverse 5′GCGCAGACTATCATATCCCCC3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muangnoi, C.; Sharif, U.; Ratnatilaka Na Bhuket, P.; Rojsitthisak, P.; Paraoan, L. Protective Effects of Curcumin Ester Prodrug, Curcumin Diethyl Disuccinate against H2O2-Induced Oxidative Stress in Human Retinal Pigment Epithelial Cells: Potential Therapeutic Avenues for Age-Related Macular Degeneration. Int. J. Mol. Sci. 2019, 20, 3367. https://doi.org/10.3390/ijms20133367
Muangnoi C, Sharif U, Ratnatilaka Na Bhuket P, Rojsitthisak P, Paraoan L. Protective Effects of Curcumin Ester Prodrug, Curcumin Diethyl Disuccinate against H2O2-Induced Oxidative Stress in Human Retinal Pigment Epithelial Cells: Potential Therapeutic Avenues for Age-Related Macular Degeneration. International Journal of Molecular Sciences. 2019; 20(13):3367. https://doi.org/10.3390/ijms20133367
Chicago/Turabian StyleMuangnoi, Chawanphat, Umar Sharif, Pahweenvaj Ratnatilaka Na Bhuket, Pornchai Rojsitthisak, and Luminita Paraoan. 2019. "Protective Effects of Curcumin Ester Prodrug, Curcumin Diethyl Disuccinate against H2O2-Induced Oxidative Stress in Human Retinal Pigment Epithelial Cells: Potential Therapeutic Avenues for Age-Related Macular Degeneration" International Journal of Molecular Sciences 20, no. 13: 3367. https://doi.org/10.3390/ijms20133367