Activation of Adrenal Steroidogenesis and an Improvement of Mood Balance in Postmenopausal Females after Spa Treatment Based on Physical Activity
Abstract
:1. Introduction
2. Results
2.1. Dependent Variables and Predictors
2.2. Relevant Changes after Intervention with Physical Activity
2.3. Analysis of Relationships between Neurotic Symptoms and Predictors
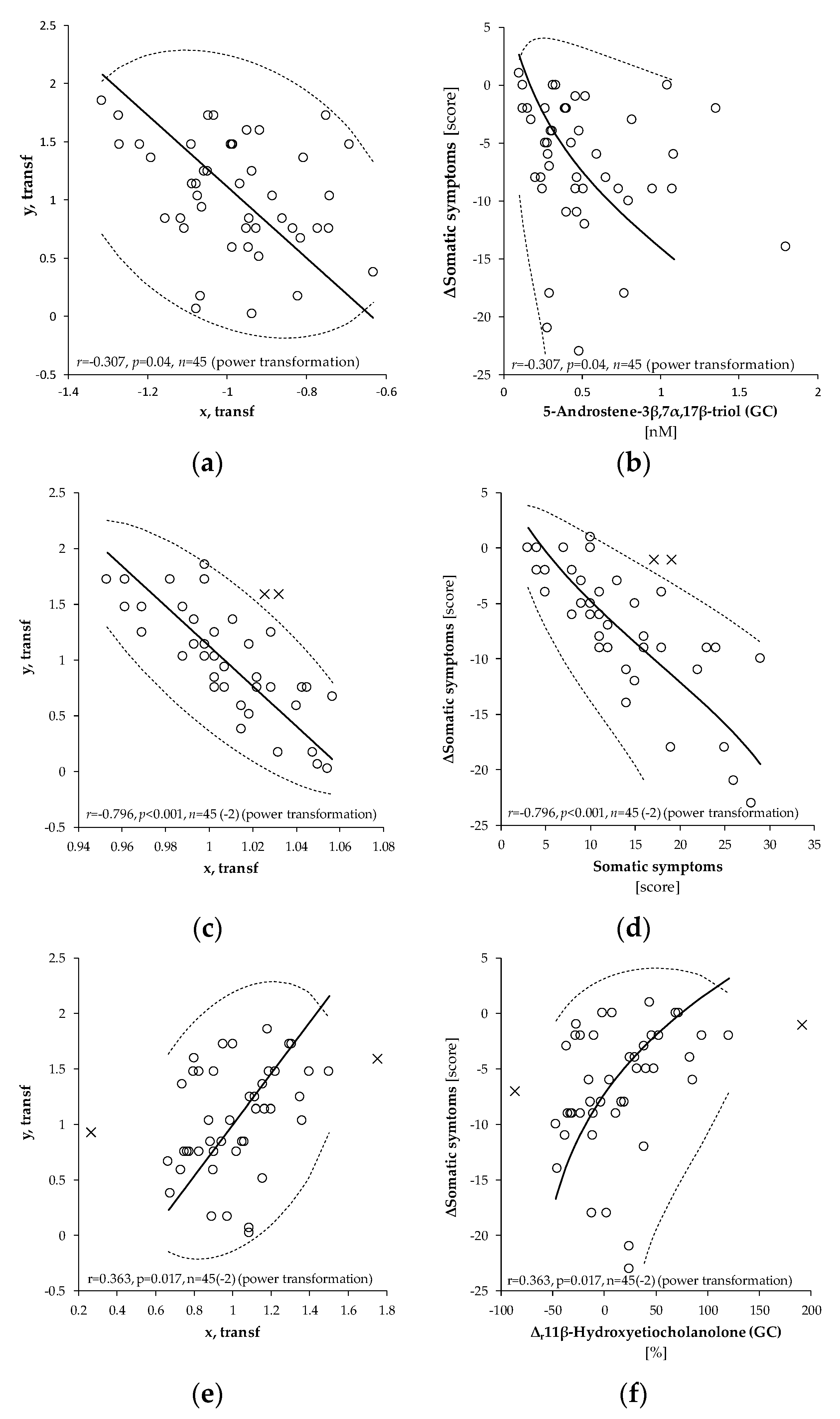
2.3.1. Somatic Symptoms
2.3.2. Psychosomatic Symptoms
2.3.3. Psychiatric Symptoms
2.3.4. Overall Neurotic Symptoms
3. Discussion
3.1. Increased Adrenal Steroidogenesis after Intervention
3.2. Positive Relationships between Initial Values of Neurotic Symptoms and Their Decline after Intervention
3.3. Positive Associations between the Initial Adrenal Activity and the Drop of Neurotic Scores after Intervention
3.4. Inverse Relationships between the Drop of Neurotic Scores and Increase in the Levels of 5α/β-Reduced C19 Steroids
3.5. Psychiatric Component of the Neurotic N-5 Questionnaire and Activity of Adrenal Cortex
3.6. The Effect of Treatment with Physical Activity on the Levels of Bioactive Steroids and Their Actions
3.6.1. Anti-Glucocorticoid Immunoprotective Steroids
3.6.2. Ergosteroids
3.6.3. Glucocorticoids
3.6.4. Progestogens
3.6.5. Glutamatergic Steroids Modulating NMDA and AMPA/Kainate Receptors (Enhancement of Mental Activity, Protection from Excitotoxicity)
3.6.6. GABAergic Steroids (Neural Inhibition and Protection, Analgesic Effects)
3.6.7. Glycinergic Steroids (Regulation of Neuronal Activity and Pain Perception)
3.6.8. Steroids Negatively Modulating L-Type Voltage Gated Calcium Channels (Antihypertensive and Analgesic Effects)
3.6.9. Steroids Modulating T-Type Voltage Gated Calcium Channels (Anti-Nociceptive Effect)
3.6.10. Multiple Positive Effects of Pregnenolone and Pregnenolone Sulfate on Transient Receptor Potential Channels
3.6.11. Steroids Affecting the Expression of Nuclear Pregnane X Receptors
4. Materials and Methods
4.1. Patients
4.2. Samples
4.3. Neurotic Scores
4.4. Analytical Methods
4.5. Terminology of Steroid Polar Conjugates
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 50+ | Older than 50 years of age |
| Δ | Difference, after treatment – before treatment |
| Δr | Relative difference, (after treatment – before treatment)/before treatment |
| a.s.l. | Above sea level |
| ACTH | Adrenocorticotropic hormone, corticotropin |
| AKR1Cs | Subfamily 1C aldoketoreductases |
| AKR1D1 | Steroid 5β-reductase |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors |
| CRH | Corticotropin releasing hormone, corticoliberin |
| CNS | Central nervous system |
| CYP3A4 | Steroid 7β- and 16α-hydroxylating enzyme |
| CYP3A7 | Steroid 7β- and 16α-hydroxylating enzyme |
| CYP11A1 | Cholesterol desmolase |
| CYP17A1 | Steroid C17-hydroxylase-C17,20-lyase |
| CYP19A1 | Aromatase |
| CYP21A2 | Steroid 21-hydroxylase |
| CYP7B1 | Steroid 7α- and 16α-hydroxylating enzyme |
| CYP11B1 | Steroid 11β-hydroxylase |
| CYP11B2 | Aldosterone synthase |
| DHEA | Dehydroepiandrosterone |
| DHEAS | Dehydroepiandrosterone sulfate |
| GABAAR | Type A γ-aminobutyric acid receptors |
| GC-MS/MS | Gas chromatography tandem mass spectrometry |
| GlyR | Glycine receptors |
| HPAA | Hypothalamic-pituitary-adrenal axis |
| HSD3B1 | Type 1 3β-hydroxysteroid dehydrogenase - Δ5/Δ4 isomerase |
| HSD3B1 | Type 2 3β-hydroxysteroid dehydrogenase - Δ5/Δ4 isomerase |
| HSD11B1 | Type 1 11β-hydroxysteroid dehydrogenase |
| HSD17Bs | 17-Hydroxysteroid dehydrogenases |
| KAR | Kainate receptors |
| L-type VGCC | Long-lasting activation voltage-gated calcium channels |
| MR | Ordinary multiple regression |
| mRNA | Messenger ribonucleic acid |
| NMDAR | N-methyl-d-aspartate receptors |
| OPLS | Multivariate regression, method of orthogonal predictions to latent structure |
| PXR | Pregnane X receptors |
| SRD5As | Steroid 5α-reductases |
| STS | Steroid sulfotransferase |
| SULT2A1 | Type 2A1 steroid sulfotransferase |
| T-type VGCC | Transient-opening voltage-gated calcium channels |
| TRPC5 | Short transient receptor potential channel 5 |
| TRPM3 | Transient receptor potential cation channel subfamily M member 3, melastatin 3 receptors |
| TRPV1 | Transient receptor potential cation channel subfamily V member 1, capsaicin receptors |
| ZF | Adrenal zona fasciculata |
| ZR | Adrenal zona reticularis |
References
- McRae, R.I. The adrenal gland. A crossroad of psychosomatic medicine. J. Am. Osteop. Assoc. 1950, 50, 193–196. [Google Scholar]
- Fava, G.A.; Sonino, N. Psychosomatic medicine. Int. J. Clin. Pract. 2010, 64, 1155–1161. [Google Scholar] [CrossRef] [PubMed]
- Oken, D. Multiaxial diagnosis and the psychosomatic model of disease. Psychosom. Med. 2000, 62, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Contoreggi, C.; Rice, K.C.; Chrousos, G. Nonpeptide corticotropin-releasing hormone receptor type 1 antagonists and their applications in psychosomatic disorders. Neuroendocrinology 2004, 80, 111–123. [Google Scholar] [CrossRef] [PubMed]
- Florio, P.; Zatelli, M.C.; Reis, F.M.; degli Uberti, E.C.; Petraglia, F. Corticotropin releasing hormone: A diagnostic marker for behavioral and reproductive disorders? Front. Biosci. 2007, 12, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Sterzl, I.; Hill, M.; Starka, L.; Velikova, M.; Kanceva, R.; Jemelkova, J.; Czernekova, L.; Kosztyu, P.; ZadraZil, J.; Matousovic, K.; et al. Patients with IgA nephropathy have altered levels of immunomodulatory C19 steroids. Glucocorticoid therapy with addition of adrenal androgens may be the choice. Physiol. Res. 2017, 66, S433–S442. [Google Scholar]
- Mastorakos, G.; Karoutsou, E.I.; Mizamtsidi, M. Corticotropin releasing hormone and the immune/inflammatory response. Eur. J. Endocrinol. 2006, 155, S77–S84. [Google Scholar] [CrossRef] [Green Version]
- Ibanez, L.; Potau, N.; Marcos, M.V.; de Zegher, F. Corticotropin-releasing hormone as adrenal androgen secretagogue. Pediatr. Res. 1999, 46, 351–353. [Google Scholar] [CrossRef]
- Dharia, S.; Parker, C.R., Jr. Adrenal androgens and aging. Semin. Reprod. Med. 2004, 22, 361–368. [Google Scholar] [CrossRef]
- Swaab, D.F.; Bao, A.M.; Lucassen, P.J. The stress system in the human brain in depression and neurodegeneration. Ageing Res. Rev. 2005, 4, 141–194. [Google Scholar] [CrossRef]
- Mastorakos, G.; Pavlatou, M.G.; Mizamtsidi, M. The hypothalamic-pituitary-adrenal and the hypothalamic- pituitary-gonadal axes interplay. Pediatr. Endocrinol. Rev. 2006, 3, 172–181. [Google Scholar] [PubMed]
- Willenberg, H.S.; Haase, M.; Papewalis, C.; Schott, M.; Scherbaum, W.A.; Bornstein, S.R. Corticotropin-releasing hormone receptor expression on normal and tumorous human adrenocortical cells. Neuroendocrinology 2005, 82, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Sirianni, R.; Mayhew, B.A.; Carr, B.R.; Parker, C.R., Jr.; Rainey, W.E. Corticotropin-releasing hormone (CRH) and urocortin act through type 1 CRH receptors to stimulate dehydroepiandrosterone sulfate production in human fetal adrenal cells. J. Clin. Endocrinol. Metab. 2005, 90, 5393–5400. [Google Scholar] [CrossRef] [PubMed]
- Giatti, S.; Garcia-Segura, L.M.; Barreto, G.E.; Melcangi, R.C. Neuroactive steroids, neurosteroidogenesis and sex. Progr. Neurobiol. 2019, 176, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Bansal, R.; Singh, R. Exploring the potential of natural and synthetic neuroprotective steroids against neurodegenerative disorders: A literature review. Med. Res. Rev. 2018, 38, 1126–1158. [Google Scholar] [CrossRef] [PubMed]
- Vankova, M.; Hill, M.; Velikova, M.; Vcelak, J.; Vacinova, G.; Dvorakova, K.; Lukasova, P.; Vejrazkova, D.; Rusina, R.; Holmerova, I.; et al. Preliminary evidence of altered steroidogenesis in women with Alzheimer’s disease: Have the patients "OLDER" adrenal zona reticularis? J. Steroid. Biochem. Mol. Biol. 2016, 158, 157–177. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.; Ripova, D.; Mohr, P.; Kratochvilova, Z.; Velikova, M.; Duskova, M.; Bicikova, M.; Starka, L. Circulating C19 steroids and progesterone metabolites in women with acute depression and anxiety disorders. Horm. Mol. Biol. Clin. Investig. 2016, 26, 153–164. [Google Scholar] [CrossRef]
- Sramkova, M.; Duskova, M.; Hill, M.; Bicikova, M.; Ripova, D.; Mohr, P.; Starka, L. The role of steroids in the prediction of affective disorders in adult men. Steroids 2017, 121, 47–53. [Google Scholar] [CrossRef]
- Bicikova, M.; Macova, L.; Kolatorova, L.; Hill, M.; Novotny, J.; Jandova, D.; Starka, L. Physiological changes after spa treatment—A focus on endocrinology. Physiol. Res. 2018, 67, S525–S530. [Google Scholar]
- Engelsmann, F.; Drdkova, S. [Neurotic quastionnaires N-5 and life satisfaction]. Ceskoslovenska Psychologie 1964, 8, 340–348. [Google Scholar]
- Novotny, J.; Jandova, D.; Kubanek, J.; Vareka, J. [Possibilities in use of self-evaluation scale N-5 in diagnostic practises]. Paktick. Lékař. 2005, 85, 575–576. [Google Scholar]
- Labrie, F. Intracrinology and menopause: The science describing the cell-specific intracellular formation of estrogens and androgens from DHEA and their strictly local action and inactivation in peripheral tissues. Menopause 2019, 26, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Hill, M.; Hana, V., Jr.; Velikova, M.; Parizek, A.; Kolatorova, L.; Vitku, J.; Skodova, T.; Simkova, M.; Simjak, P.; Kancheva, R.; et al. A method for determination of one hundred endogenous steroids in human serum by gas chromatography-tandem mass spectrometry. Physiol. Res. 2019, 68, 179–207. [Google Scholar] [CrossRef] [PubMed]
- Storbeck, K.H.; Swart, P.; Africander, D.; Conradie, R.; Louw, R.; Swart, A.C. 16α-hydroxyprogesterone: Origin, biosynthesis and receptor interaction. Mol. Cell Endocrinol. 2011, 336, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Attardi, B.J.; Zeleznik, A.; Simhan, H.; Chiao, J.P.; Mattison, D.R.; Caritis, S.N.; Obstetric–Fetal Pharmacology Research Unit Network. Comparison of progesterone and glucocorticoid receptor binding and stimulation of gene expression by progesterone, 17-α hydroxyprogesterone caproate, and related progestins. Am. J. Obstet. Gynecol. 2007, 197, 599.e1–599.e7. [Google Scholar] [CrossRef]
- Rupprecht, R.; Reul, J.M.; Trapp, T.; van Steensel, B.; Wetzel, C.; Damm, K.; Zieglgansberger, W.; Holsboer, F. Progesterone receptor-mediated effects of neuroactive steroids. Neuron 1993, 11, 523–530. [Google Scholar] [CrossRef]
- Katzung, B.G. Basic and Clinical Pharmacology 14th Edition; McGraw-Hill Education: New York, NY, USA, 2017; p. 728. [Google Scholar]
- Sterzl, I.; Hampl, R.; Sterzl, J.; Votruba, J.; Starka, L. 7β-OH-DHEA counteracts dexamethasone induced suppression of primary immune response in murine spleenocytes. J. Steroid Biochem. Mol. Biol. 1999, 71, 133–137. [Google Scholar] [CrossRef]
- Hennebert, O.; Montes, M.; Favre-Reguillon, A.; Chermette, H.; Ferroud, C.; Morfin, R. Epimerase activity of the human 11β-hydroxysteroid dehydrogenase type 1 on 7-hydroxylated C19-steroids. J. Steroid Biochem. Mol. Biol. 2009, 114, 57–63. [Google Scholar] [CrossRef]
- Ahlem, C.N.; Auci, D.L.; Nicoletti, F.; Pieters, R.; Kennedy, M.R.; Page, T.M.; Reading, C.L.; Enioutina, E.Y.; Frincke, J.M. Pharmacology and immune modulating properties of 5-androstene-3β,7β,17β-triol, a DHEA metabolite in the human metabolome. J. Steroid Biochem. Mol. Biol. 2011, 126, 87–94. [Google Scholar] [CrossRef]
- Hampl, R.; Starka, L.; Jansky, L. Steroids and thermogenesis. Physiol. Res. 2006, 55, 123–131. [Google Scholar]
- Lardy, H.; Kneer, N.; Wei, Y.; Partridge, B.; Marwah, P. Ergosteroids. II: Biologically active metabolites and synthetic derivatives of dehydroepiandrosterone. Steroids 1998, 63, 158–165. [Google Scholar] [CrossRef]
- Dillard, G.M.; Bodel, P. Studies on steroid fever. II. Pyrogenic and anti-pyrogenic activity in vitro of some endogenous steroids of man. J. Clin. Investig. 1970, 49, 2418–2426. [Google Scholar] [CrossRef] [PubMed]
- Johansson, T.; Frandberg, P.A.; Nyberg, F.; Le Greves, P. Molecular mechanisms for nanomolar concentrations of neurosteroids at NR1/NR2B receptors. J. Pharmacol. Exp. Ther. 2008, 324, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Petrovic, M.; Sedlacek, M.; Cais, O.; Horak, M.; Chodounska, H.; Vyklicky, L., Jr. Pregnenolone sulfate modulation of N-methyl-D-aspartate receptors is phosphorylation dependent. Neuroscience 2009, 160, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Horak, M.; Vlcek, K.; Chodounska, H.; Vyklicky, L., Jr. Subtype-dependence of N-methyl-d-aspartate receptor modulation by pregnenolone sulfate. Neuroscience 2006, 137, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Adamusova, E.; Cais, O.; Vyklicky, V.; Kudova, E.; Chodounska, H.; Horak, M.; Vyklicky, L., Jr. Pregnenolone sulfate activates NMDA receptor channels. Physiol. Res. 2013, 62, 731–736. [Google Scholar]
- Park-Chung, M.; Wu, F.S.; Purdy, R.H.; Malayev, A.A.; Gibbs, T.T.; Farb, D.H. Distinct sites for inverse modulation of N-methyl-D-aspartate receptors by sulfated steroids. Mol. Pharmacol. 1997, 52, 1113–1123. [Google Scholar] [CrossRef]
- Meyer, D.A.; Carta, M.; Partridge, L.D.; Covey, D.F.; Valenzuela, C.F. Neurosteroids enhance spontaneous glutamate release in hippocampal neurons. Possible role of metabotropic sigma1-like receptors. J. Biol. Chem. 2002, 277, 28725–28732. [Google Scholar] [CrossRef]
- Irwin, R.P.; Lin, S.Z.; Rogawski, M.A.; Purdy, R.H.; Paul, S.M. Steroid potentiation and inhibition of N-methyl-d-aspartate receptor-mediated intracellular Ca++ responses: Structure-activity studies. J. Pharmacol. Exp. Ther. 1994, 271, 677–682. [Google Scholar]
- Sedlacek, M.; Korinek, M.; Petrovic, M.; Cais, O.; Adamusova, E.; Chodounska, H.; Vyklicky, L., Jr. Neurosteroid modulation of ionotropic glutamate receptors and excitatory synaptic transmission. Physiol. Res. 2008, 57, S49–S57. [Google Scholar]
- Petrovic, M.; Sedlacek, M.; Horak, M.; Chodounska, H.; Vyklicky, L., Jr. 20-oxo-5β-pregnan-3α-yl sulfate is a use-dependent NMDA receptor inhibitor. J. Neurosci. 2005, 25, 8439–8450. [Google Scholar] [CrossRef] [PubMed]
- Vales, K.; Rambousek, L.; Holubova, K.; Svoboda, J.; Bubenikova-Valesova, V.; Chodounska, H.; Vyklicky, L.; Stuchlik, A. 3α5β-Pregnanolone glutamate, a use-dependent NMDA antagonist, reversed spatial learning deficit in an animal model of schizophrenia. Behav. Brain Res. 2012, 235, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Yaghoubi, N.; Malayev, A.; Russek, S.J.; Gibbs, T.T.; Farb, D.H. Neurosteroid modulation of recombinant ionotropic glutamate receptors. Brain Res. 1998, 803, 153–160. [Google Scholar] [CrossRef]
- Yu, R.; Xu, X.H.; Sheng, M.P. Differential effects of allopregnanolone and GABA on kainate-induced lactate dehydrogenase release in cultured rat cerebral cortical cells. Acta Pharmacol. Sin. 2002, 23, 680–684. [Google Scholar] [PubMed]
- Morali, G.; Montes, P.; Hernandez-Morales, L.; Monfil, T.; Espinosa-Garcia, C.; Cervantes, M. Neuroprotective effects of progesterone and allopregnanolone on long-term cognitive outcome after global cerebral ischemia. Restorat. Neurol. Neurosci. 2011, 29, 1–15. [Google Scholar]
- Reddy, D.S. Neurosteroids: Endogenous role in the human brain and therapeutic potentials. Progr. Brain Res. 2010, 186, 113–137. [Google Scholar]
- Kokate, T.G.; Svensson, B.E.; Rogawski, M.A. Anticonvulsant activity of neurosteroids: Correlation with γ-aminobutyric acid-evoked chloride current potentiation. J. Pharmacol. Exp. Ther. 1994, 270, 1223–1229. [Google Scholar]
- Belelli, D.; Lambert, J.J.; Peters, J.A.; Gee, K.W.; Lan, N.C. Modulation of human recombinant GABAA receptors by pregnanediols. Neuropharmacology 1996, 35, 1223–1231. [Google Scholar] [CrossRef]
- Lundgren, P.; Stromberg, J.; Backstrom, T.; Wang, M. Allopregnanolone-stimulated GABA-mediated chloride ion flux is inhibited by 3β-hydroxy-5α-pregnan-20-one (isoallopregnanolone). Brain Res. 2003, 982, 45–53. [Google Scholar] [CrossRef]
- Wang, M.D.; Borra, V.B.; Stromberg, J.; Lundgren, P.; Haage, D.; Backstrom, T. Neurosteroids 3β, 20 (R/S)-pregnandiols decrease offset rate of the GABA-site activation at the recombinant GABA A receptor. Eur. J. Pharmacol. 2008, 586, 67–73. [Google Scholar] [CrossRef]
- Rahman, M.; Lindblad, C.; Johansson, I.M.; Backstrom, T.; Wang, M.D. Neurosteroid modulation of recombinant rat α5β2γ2L and α1β2γ2L GABA(A) receptors in Xenopus oocyte. Eur. J. Pharmacol. 2006, 547, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Park-Chung, M.; Malayev, A.; Purdy, R.H.; Gibbs, T.T.; Farb, D.H. Sulfated and unsulfated steroids modulate γ-aminobutyric acidA receptor function through distinct sites. Brain Res. 1999, 830, 72–87. [Google Scholar] [CrossRef]
- Mtchedlishvili, Z.; Kapur, J. A presynaptic action of the neurosteroid pregnenolone sulfate on GABAergic synaptic transmission. Mol. Pharmacol. 2003, 64, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; He, Y.; Eisenman, L.N.; Fields, C.; Zeng, C.M.; Mathews, J.; Benz, A.; Fu, T.; Zorumski, E.; Steinbach, J.H.; et al. 3β-hydroxypregnane steroids are pregnenolone sulfate-like GABAA receptor antagonists. J. Neurosci. 2002, 22, 3366–3375. [Google Scholar] [CrossRef] [PubMed]
- Maksay, G.; Laube, B.; Betz, H. Subunit-specific modulation of glycine receptors by neurosteroids. Neuropharmacology 2001, 41, 369–376. [Google Scholar] [CrossRef]
- Wu, F.S.; Chen, S.C.; Tsai, J.J. Competitive inhibition of the glycine-induced current by pregnenolone sulfate in cultured chick spinal cord neurons. Brain Res. 1997, 750, 318–320. [Google Scholar] [CrossRef]
- Weir, C.J.; Ling, A.T.; Belelli, D.; Wildsmith, J.A.; Peters, J.A.; Lambert, J.J. The interaction of anaesthetic steroids with recombinant glycine and GABAA receptors. Br. J. Anaesth. 2004, 92, 704–711. [Google Scholar] [CrossRef]
- ffrench-Mullen, J.M.; Danks, P.; Spence, K.T. Neurosteroids modulate calcium currents in hippocampal CA1 neurons via a pertussis toxin-sensitive G-protein-coupled mechanism. J. Neurosci. 1994, 14, 1963–1977. [Google Scholar] [CrossRef] [Green Version]
- Bukusoglu, C.; Sarlak, F. Pregnenolone sulfate increases intracellular Ca2+ levels in a pituitary cell line. Eur. J. Pharmacol. 1996, 298, 79–85. [Google Scholar] [CrossRef]
- Dayanithi, G.; Tapia-Arancibia, L. Rise in intracellular calcium via a nongenomic effect of allopregnanolone in fetal rat hypothalamic neurons. J. Neurosci. 1996, 16, 130–136. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.M.; Brinton, R.D. Allopregnanolone-induced rise in intracellular calcium in embryonic hippocampal neurons parallels their proliferative potential. BMC Neurosci. 2008, 9, S11. [Google Scholar] [CrossRef] [PubMed]
- Perusquia, M.; Villalon, C.M. The relaxant effect of sex steroids in rat myometrium is independent of the γ-amino butyric acid system. Life Sci. 1996, 58, 913–926. [Google Scholar] [CrossRef]
- Hidalgo, A.; Suzano, R.C.; Revuelta, M.P.; Sanchez-Diaz, C.; Baamonde, A.; Cantabrana, B. Calcium and depolarization-dependent effect of pregnenolone derivatives on uterine smooth muscle. Gen. Pharmacol. 1996, 27, 879–885. [Google Scholar] [CrossRef]
- Pathirathna, S.; Brimelow, B.C.; Jagodic, M.M.; Krishnan, K.; Jiang, X.; Zorumski, C.F.; Mennerick, S.; Covey, D.F.; Todorovic, S.M.; Jevtovic-Todorovic, V. New evidence that both T-type calcium channels and GABAA channels are responsible for the potent peripheral analgesic effects of 5α-reduced neuroactive steroids. Pain 2005, 114, 429–443. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, S.M.; Pathirathna, S.; Brimelow, B.C.; Jagodic, M.M.; Ko, S.H.; Jiang, X.; Nilsson, K.R.; Zorumski, C.F.; Covey, D.F.; Jevtovic-Todorovic, V. 5β-reduced neuroactive steroids are novel voltage-dependent blockers of T-type Ca2+ channels in rat sensory neurons in vitro and potent peripheral analgesics in vivo. Mol. Pharmacol. 2004, 66, 1223–1235. [Google Scholar] [CrossRef]
- Chen, S.C.; Wu, F.S. Mechanism underlying inhibition of the capsaicin receptor-mediated current by pregnenolone sulfate in rat dorsal root ganglion neurons. Brain Res. 2004, 1027, 196–200. [Google Scholar] [CrossRef]
- Chen, S.C.; Chang, T.J.; Wu, F.S. Competitive inhibition of the capsaicin receptor-mediated current by dehydroepiandrosterone in rat dorsal root ganglion neurons. J. Pharmacol. Exp. Ther. 2004, 311, 529–536. [Google Scholar] [CrossRef]
- Majeed, Y.; Amer, M.S.; Agarwal, A.K.; McKeown, L.; Porter, K.E.; O’Regan, D.J.; Naylor, J.; Fishwick, C.W.; Muraki, K.; Beech, D.J. Stereo-selective inhibition of transient receptor potential TRPC5 cation channels by neuroactive steroids. Br. J. Pharmacol. 2011, 162, 1509–1520. [Google Scholar] [CrossRef]
- Wagner, T.F.; Loch, S.; Lambert, S.; Straub, I.; Mannebach, S.; Mathar, I.; Dufer, M.; Lis, A.; Flockerzi, V.; Philipp, S.E.; et al. Transient receptor potential M3 channels are ionotropic steroid receptors in pancreatic β cells. Nat. Cell Biol. 2008, 10, 1421–1430. [Google Scholar] [CrossRef]
- Majeed, Y.; Agarwal, A.K.; Naylor, J.; Seymour, V.A.; Jiang, S.; Muraki, K.; Fishwick, C.W.; Beech, D.J. Cis-isomerism and other chemical requirements of steroidal agonists and partial agonists acting at TRPM3 channels. Br. J. Pharmacol. 2010, 161, 430–441. [Google Scholar] [CrossRef] [Green Version]
- Naylor, J.; Li, J.; Milligan, C.J.; Zeng, F.; Sukumar, P.; Hou, B.; Sedo, A.; Yuldasheva, N.; Majeed, Y.; Beri, D.; et al. Pregnenolone sulphate- and cholesterol-regulated TRPM3 channels coupled to vascular smooth muscle secretion and contraction. Circ. Res. 2010, 106, 1507–1515. [Google Scholar] [CrossRef] [PubMed]
- Ekins, S.; Reschly, E.J.; Hagey, L.R.; Krasowski, M.D. Evolution of pharmacologic specificity in the pregnane X receptor. BMC Evol. Biol. 2008, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Bloem, L.M.; Storbeck, K.H.; Schloms, L.; Swart, A.C. 11β-hydroxyandrostenedione returns to the steroid arena: Biosynthesis, metabolism and function. Molecules 2013, 18, 13228–13244. [Google Scholar] [CrossRef] [PubMed]
- Sulcova, J.; Hill, M.; Hampl, R.; Starka, L. Age and sex related differences in serum levels of unconjugated dehydroepiandrosterone and its sulphate in normal subjects. J. Endocrinol. 1997, 154, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Hampl, R.; Hill, M.; Starka, L. 7-Hydroxydehydroepiandrosterone epimers in the life span. J. Steroid Biochem. Mol. Biol. 2001, 78, 367–372. [Google Scholar] [CrossRef]
- Staton, B.A.; Mixon, R.L.; Dharia, S.; Brissie, R.M.; Parker, C.R., Jr. Is reduced cell size the mechanism for shrinkage of the adrenal zona reticularis in aging? Endocr. Res. 2004, 30, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Maninger, N.; Wolkowitz, O.M.; Reus, V.I.; Epel, E.S.; Mellon, S.H. Neurobiological and neuropsychiatric effects of dehydroepiandrosterone (DHEA) and DHEA sulfate (DHEAS). Front. Neuroendocrinol. 2009, 30, 65–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rammouz, G.; Lecanu, L.; Aisen, P.; Papadopoulos, V. A lead study on oxidative stress-mediated dehydroepiandrosterone formation in serum: The biochemical basis for a diagnosis of Alzheimer’s disease. J. Alzheimer’s Dis. 2011, 24, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Asaba, H.; Hosoya, K.; Takanaga, H.; Ohtsuki, S.; Tamura, E.; Takizawa, T.; Terasaki, T. Blood-brain barrier is involved in the efflux transport of a neuroactive steroid, dehydroepiandrosterone sulfate, via organic anion transporting polypeptide 2. J. Neurochem. 2000, 75, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.D.; Wahlstrom, G.; Backstrom, T. The regional brain distribution of the neurosteroids pregnenolone and pregnenolone sulfate following intravenous infusion. J. Steroid Biochem. Mol. Biol. 1997, 62, 299–306. [Google Scholar] [CrossRef]
- Qaiser, M.Z.; Dolman, D.E.M.; Begley, D.J.; Abbott, N.J.; Cazacu-Davidescu, M.; Corol, D.I.; Fry, J.P. Uptake and metabolism of sulphated steroids by the blood-brain barrier in the adult male rat. J. Neurochem. 2017, 142, 672–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kriz, L.; Bicikova, M.; Mohapl, M.; Hill, M.; Cerny, I.; Hampl, R. Steroid sulfatase and sulfuryl transferase activities in human brain tumors. J. Steroid Biochem. Mol. Biol. 2008, 109, 31–39. [Google Scholar] [CrossRef]
- Kriz, L.; Bicikova, M.; Hill, M.; Hampl, R. Steroid sulfatase and sulfuryl transferase activity in monkey brain tissue. Steroids 2005, 70, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Kancheva, R.; Hill, M.; Novak, Z.; Chrastina, J.; Kancheva, L.; Starka, L. Neuroactive steroids in periphery and cerebrospinal fluid. Neuroscience 2011, 191, 22–27. [Google Scholar] [CrossRef] [PubMed]
- BioGPS. In 2011/11/13 ed.; Affymetrix: 2015. Available online: http://biogps.org/#goto=welcome (accessed on 26 July 2019).
- Uno, Y.; Hosaka, S.; Yamazaki, H. Identifcation and Analysis of CYP7A1, CYP17A1, CYP20A1, CYP27A1 and CYP51A1 in Cynomolgus Macaques. Jpn. Soc. Vet. Sci. 2014, 76, 1647–1650. [Google Scholar]
- Scotney, H.; Symonds, M.E.; Law, J.; Budge, H.; Sharkey, D.; Manolopoulos, K.N. Glucocorticoids modulate human brown adipose tissue thermogenesis in vivo. Metab. Clin. Exp. 2017, 70, 125–132. [Google Scholar] [CrossRef] [Green Version]
- De Kloet, E.R.; Meijer, O.C.; de Nicola, A.F.; de Rijk, R.H.; Joels, M. Importance of the brain corticosteroid receptor balance in metaplasticity, cognitive performance and neuro-inflammation. Front. Neuroendocrinol. 2018, 49, 124–145. [Google Scholar] [CrossRef]
- Yiallouris, A.; Tsioutis, C.; Agapidaki, E.; Zafeiri, M.; Agouridis, A.P.; Ntourakis, D.; Johnson, E.O. Adrenal Aging and Its Implications on Stress Responsiveness in Humans. Front. Endocrinol. 2019, 10, 54. [Google Scholar] [CrossRef]
- Roelfsema, F.; van Heemst, D.; Iranmanesh, A.; Takahashi, P.; Yang, R.; Veldhuis, J.D. Impact of age, sex and body mass index on cortisol secretion in 143 healthy adults. Endocr. Connect. 2017, 6, 500–509. [Google Scholar] [CrossRef] [Green Version]
- Herbert, J. Cortisol and depression: Three questions for psychiatry. Psychol. Med. 2013, 43, 449–469. [Google Scholar] [CrossRef]
- Borges, S.; Gayer-Anderson, C.; Mondelli, V. A systematic review of the activity of the hypothalamic-pituitary-adrenal axis in first episode psychosis. Psychoneuroendocrinology 2013, 38, 603–611. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, A.S.; Cleare, A.J. Hypothalamic-pituitary-adrenal axis dysfunction in chronic fatigue syndrome. Nat. Rev. Endocrinol. 2011, 8, 22–32. [Google Scholar] [CrossRef]
- Traustadottir, T.; Bosch, P.R.; Matt, K.S. The HPA axis response to stress in women: Effects of aging and fitness. Psychoneuroendocrinology 2005, 30, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, M.; Denier, C.; Oudinet, J.P.; Adams, D.; Guennoun, R. Progesterone neuroprotection: The background of clinical trial failure. J. Steroid Biochem. Mol. Biol. 2016, 160, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Monnet, F.P.; Mahe, V.; Robel, P.; Baulieu, E.E. Neurosteroids, via sigma receptors, modulate the [3H]norepinephrine release evoked by N-methyl-d-aspartate in the rat hippocampus. Proc. Natl. Acad. Sci. USA 1995, 92, 3774–3778. [Google Scholar] [CrossRef] [PubMed]
- Shirakawa, H.; Katsuki, H.; Kume, T.; Kaneko, S.; Akaike, A. Pregnenolone sulphate attenuates AMPA cytotoxicity on rat cortical neurons. Eur. J. Neurosci. 2005, 21, 2329–2335. [Google Scholar] [CrossRef] [PubMed]
- Bender, C.; Rassetto, M.; de Olmos, J.S.; de Olmos, S.; Lorenzo, A. Involvement of AMPA/kainate-excitotoxicity in MK801-induced neuronal death in the retrosplenial cortex. Neuroscience 2010, 169, 720–732. [Google Scholar] [CrossRef]
- Egbenya, D.L.; Hussain, S.; Lai, Y.C.; Xia, J.; Anderson, A.E.; Davanger, S. Changes in synaptic AMPA receptor concentration and composition in chronic temporal lobe epilepsy. Mol. Cell. Neurosci. 2018, 92, 93–103. [Google Scholar] [CrossRef]
- Weiss, J.H. Ca permeable AMPA channels in diseases of the nervous system. Front. Mol. Neurosci. 2011, 4, 42. [Google Scholar] [CrossRef]
- Kuhse, J.; Betz, H.; Kirsch, J. The inhibitory glycine receptor: Architecture, synaptic localization and molecular pathology of a postsynaptic ion-channel complex. Curr. Opin. Neurobiol. 1995, 5, 318–323. [Google Scholar] [CrossRef]
- Lynch, J.W.; Callister, R.J. Glycine receptors: A new therapeutic target in pain pathways. Curr. Opin. Investig. Drugs 2006, 7, 48–53. [Google Scholar] [PubMed]
- Felizola, S.J.; Maekawa, T.; Nakamura, Y.; Satoh, F.; Ono, Y.; Kikuchi, K.; Aritomi, S.; Ikeda, K.; Yoshimura, M.; Tojo, K.; et al. Voltage-gated calcium channels in the human adrenal and primary aldosteronism. J. Steroid Biochem. Mol. Biol. 2014, 144 Pt B, 410–416. [Google Scholar] [CrossRef]
- Caterina, M.J.; Julius, D. The vanilloid receptor: A molecular gateway to the pain pathway. Annu. Rev. Neurosci. 2001, 24, 487–517. [Google Scholar] [CrossRef] [PubMed]
- Riccio, A.; Li, Y.; Moon, J.; Kim, K.S.; Smith, K.S.; Rudolph, U.; Gapon, S.; Yao, G.L.; Tsvetkov, E.; Rodig, S.J.; et al. Essential role for TRPC5 in amygdala function and fear-related behavior. Cell 2009, 137, 761–772. [Google Scholar] [CrossRef] [PubMed]
- Kliewer, S.A.; Goodwin, B.; Willson, T.M. The nuclear pregnane X receptor: A key regulator of xenobiotic metabolism. Endocr. Rev. 2002, 23, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Cheng, Q.; Ou, Z.; Lee, J.H.; Xu, M.; Kochhar, U.; Ren, S.; Huang, M.; Pflug, B.R.; Xie, W. Pregnane X receptor as a therapeutic target to inhibit androgen activity. Endocrinology 2010, 151, 5721–5729. [Google Scholar] [CrossRef]
- Garg, A.; Zhao, A.; Erickson, S.L.; Mukherjee, S.; Lau, A.J.; Alston, L.; Chang, T.K.; Mani, S.; Hirota, S.A. Pregnane X Receptor Activation Attenuates Inflammation-Associated Intestinal Epithelial Barrier Dysfunction by Inhibiting Cytokine-Induced Myosin Light-Chain Kinase Expression and c-Jun N-Terminal Kinase 1/2 Activation. J. Pharmacol. Exp. Ther. 2016, 359, 91–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bicikova, M.; Kolatorova, L.; Macova, L.; Bestak, J.; Hill, M.; Formanova, P.; Jandova, D.; Moravek, O.; Novotny, J. [Steroidal metabolomic biomarkers as an indicator of the effect of spa therapy and balneotherapy]. Rehabilitace Fyzikální Lékařství 2018, 25, 99–108. [Google Scholar]
- Brochu, M.; Belanger, A. Comparative study of plasma steroid and steroid glucuronide levels in normal men and in men with benign prostatic hyperplasia. Prostate 1987, 11, 33–40. [Google Scholar] [CrossRef]
- Sanchez-Guijo, A.; Oji, V.; Hartmann, M.F.; Traupe, H.; Wudy, S.A. Simultaneous quantification of cholesterol sulfate, androgen sulfates, and progestagen sulfates in human serum by LC-MS/MS. J. Lipid Res. 2015, 56, 1843–1851. [Google Scholar] [CrossRef] [Green Version]
- Labrie, F.; Belanger, A.; Cusan, L.; Gomez, J.L.; Candas, B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J. Clin. Endocrinol. Metab. 1997, 82, 2396–2402. [Google Scholar] [CrossRef] [PubMed]
- Brochu, M.; Belanger, A.; Dupont, A.; Cusan, L.; Labrie, F. Effects of flutamide and aminoglutethimide on plasma 5α-reduced steroid glucuronide concentrations in castrated patients with cancer of the prostate. J. Steroid Biochem. 1987, 28, 619–622. [Google Scholar] [CrossRef]
- Abu-Hayyeh, S.; Papacleovoulou, G.; Lovgren-Sandblom, A.; Tahir, M.; Oduwole, O.; Jamaludin, N.A.; Ravat, S.; Nikolova, V.; Chambers, J.; Selden, C.; et al. Intrahepatic cholestasis of pregnancy levels of sulfated progesterone metabolites inhibit farnesoid X receptor resulting in a cholestatic phenotype. Hepatology 2013, 57, 716–726. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.J.; Reyes, H.; Axelson, M.; Palma, J.; Hernandez, I.; Ribalta, J.; Sjovall, J. Progesterone metabolites and bile acids in serum of patients with intrahepatic cholestasis of pregnancy: Effect of ursodeoxycholic acid therapy. Hepatology 1997, 26, 1573–1579. [Google Scholar] [CrossRef] [PubMed]
- Tokushige, K.; Hashimoto, E.; Kodama, K.; Tobari, M.; Matsushita, N.; Kogiso, T.; Taniai, M.; Torii, N.; Shiratori, K.; Nishizaki, Y.; et al. Serum metabolomic profile and potential biomarkers for severity of fibrosis in nonalcoholic fatty liver disease. J. Gastroenterol. 2013, 48, 1392–1400. [Google Scholar] [CrossRef] [Green Version]
- Trygg, J.; Holmes, E.; Lundstedt, T. Chemometrics in metabonomics. J. Proteome Res. 2007, 6, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Trygg, J.; Wold, S. Orthogonal projections to latent structure. J. Chemometr. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Madsen, R.; Lundstedt, T.; Trygg, J. Chemometrics in metabolomics—A review in human disease diagnosis. Anal. Chim. Acta 2010, 659, 23–33. [Google Scholar] [CrossRef]
- Meloun, M.; Hill, M.; Militky, J.; Kupka, K. Transformation in the PC-aided biochemical data analysis. Clin. Chem. Lab. Med. 2000, 38, 553–559. [Google Scholar] [CrossRef]
| Variable | Basal Values | Δr [%] a | p-Value c |
| Age [years] | 58 (55, 61) | --- | --- |
| Pregnenolone [nM] | 0.935 (0.717, 1.18) | 27 (−10.5, 58) | <0.001 |
| Pregnenolone sulfate [nM] | 79.6 (53.1, 106) | 10.8 (−7.23, 25.4) | 0.011 |
| 17-Hydroxypregnenolone [nM] | 1.62 (1.09, 3.02) | 52.4 (−4.44, 151) | <0.001 |
| 17-Hydroxypregnenolone sulfate [nM] | 3.21 (2.18, 5.68) | 41.2 (−9.57, 78.4) | <0.001 |
| 16α-Hydroxypregnenolone [nM] | 0.31 (0.191, 0.493) | 28.8 (−14.6, 88.5) | 0.003 |
| 20α-Dihydropregnenolone [nM] | 1.3 (0.949, 1.93) | 10.6 (−11.8, 21.5) | 0.038 |
| Dehydroepiandrosterone (DHEA) [nM] | 6.75 (4.3, 8) | 24.4 (−3.65, 66) | <0.001 |
| 7α-Hydroxy-DHEA [nM] | 1.2 (0.841, 1.92) | 22.7 (−20.2, 53.7) | 0.003 |
| Androstenediol [nM] | 1.29 (1.02, 1.97) | 11.1 (−3.87, 34.2) | 0.005 |
| 17-Hydroxyprogesterone [nM] | 0.77 (0.502, 1.22) | 43.1 (−23.5, 110) | 0.001 |
| 16α-Hydroxyprogesterone [nM] | 0.458 (0.287, 1.01) | 32.7 (−25.6, 195) | 0.002 |
| Androstenedione [nM] | 3.11 (1.98, 4.16) | 25.6 (−12.6, 76.4) | 0.003 |
| Isopregnanolone sulfate [nM] | 6.04 (4.52, 8.59) | 5.58 (−9.3, 28) | 0.031 |
| Conjugated epipregnanolone [nM] | 1.56 (0.837, 2.43) | 8.84 (−11.6, 40.3) | 0.013 |
| 5α-Pregnane-3α,20α-diol [nM] | 0.0574 (0.0278, 0.114) | 24.3 (−41, 87.6) | 0.034 |
| 17-Hydroxyallopregnanolone [nM] | 0.00813 (0.00426, 0.018) | 30.5 (−55.1, 183) | 0.046 |
| 17-Hydroxyallopregnanolone sulfate [nM] | 0.928 (0.638, 1.53) | 17.6 (−10.7, 40.2) | 0.032 |
| 17-Hydroxypregnanolone [nM] | 0.0365 (0.0132, 0.0743) | 7.65 (−62.3, 135) | 0.035 |
| 17-Hydroxypregnanolone sulfate [nM] | 6.69 (4.56, 9.11) | 18.5 (−7.25, 37.2) | 0.022 |
| Conjugated 5α-pregnane-3α,17α,20α-triol [nM] | 388 (201, 740) | 198 (40.2, 416) | <0.001 |
| Epiandrosterone [nM] | 1.18 (0.838, 1.52) | 21.9 (−16.2, 43.7) | 0.006 |
| Epietiocholanolone sulfate [nM] | 43.6 (23.5, 65.3) | 19.6 (−1.59, 35.5) | <0.001 |
| Conjugated 5β-androstane-3α,17β-diol [nM] | 0.721 (0.389, 1.14) | 15.5 (−13.9, 55.8) | 0.015 |
| Cortisol [nM] | 413 (291, 472) | 24.2 (−5.81, 61.6) | 0.004 |
| Cortisol/DHEAS | 0.183 (0.0928, 0.416) | 20.2 (−12.8, 71.7) | 0.008 |
| Corticosterone [nM] | 12.6 (6.72, 17.5) | 21.9 (−13.7, 129) | <0.001 |
| 5α,20α-Tetrahydrocorticosterone [nM] | 0.0848 (0.0442, 0.193) | 50.8 (−37.8, 254) | 0.001 |
| 5β,20α-Tetrahydrocorticosterone [nM] | 0.433 (0.218, 0.84) | 22.6 (−35.4, 111) | 0.013 |
| 11β-Hydroxyandrostenedione [nM] | 136 (84, 201) | 12.9 (−12.6, 53.7) | 0.008 |
| Somatic symptoms score | 11 (8, 17) | −50 (−72.7, −25) | <0.001 |
| Psychosomatic symptoms score | 15 (11, 23) | −71.4 (−80, −50) | <0.001 |
| Psychiatric symptoms score | 12.5 (5, 18) | −57.3 (−86, −36.1) | <0.001 |
| Overall symptoms score | 39 (28, 57) | −56.8 (−76, −42.1) | <0.001 |
| Absolute Changes of Neurotic Symptoms | Basal Values | Δ b | p-Value a |
| Somatic symptoms score | 11 (8, 17) | −6 (−9, −2) | <0.001 |
| Psychosomatic symptoms score | 15 (11, 23) | −10 (−15, −6) | <0.001 |
| Psychiatric symptoms score | 12.5 (5, 18) | −6 (−10.3, −3) | <0.001 |
| Overall symptoms score | 39 (28, 57) | −21 (−33, −13) | <0.001 |
| Data Type | Variable | OPLS (Predictive Component) | ||||
|---|---|---|---|---|---|---|
| Component Loading | t-Statistic | R c | Regression Coefficient | t-Statistic | ||
| Relevant predictors (matrix X) | 7α-Hydroxy-DHEA | 0.168 | 2.80 | 0.302 * | −0.051 | −0.52 |
| 7-oxo-DHEA | 0.117 | 1.70 | 0.210 | −0.011 | −0.18 | |
| 5-Androstene-3β,7α,17β-triol | 0.215 | 3.64 | 0.385 ** | 0.163 | 1.84 | |
| 20α-Dihydroprogesterone | 0.223 | 6.62 | 0.400 ** | 0.125 | 1.87 | |
| Conjugated pregnanolone | 0.241 | 4.00 | 0.432 ** | 0.080 | 0.77 | |
| Conjugated 5β,20α-tetrahydroprogesterone | 0.239 | 5.43 | 0.429 ** | 0.094 | 1.25 | |
| 5β-Pregnane-3α,20α-diol | 0.130 | 3.36 | 0.233 ** | −0.080 | −0.88 | |
| Conjugated 5β-pregnane-3α,20α-diol | 0.198 | 3.49 | 0.356 ** | −0.089 | −1.22 | |
| Conjugated 17-hydroxypregnanolone | 0.265 | 5.20 | 0.476 ** | 0.120 | 1.10 | |
| Conjugated 5β-pregnane-3α,17α,20α-triol | 0.223 | 3.03 | 0.400 ** | 0.136 | 1.78 | |
| 5α-Androstane-3,17-dione | 0.095 | 3.06 | 0.170 ** | −0.148 | −2.16 * | |
| 5α-Androstane-3α,17β-diol | 0.093 | 1.59 | 0.166 | 0.070 | 1.31 | |
| 11β-Hydroxyandrostenedione | 0.281 | 4.81 | 0.505 ** | 0.057 | 0.89 | |
| 11β-Hydroxyandrosterone sulfate | 0.197 | 2.36 | 0.353 * | 0.095 | 0.77 | |
| 11β-Hydroxyetiocholanolone | 0.280 | 3.33 | 0.502 ** | 0.032 | 0.34 | |
| Somatic symptoms | 0.476 | 6.13 | 0.855 ** | 0.704 | 15.75 ** | |
| Δr5α-Androstane-3,17-dione a | −0.090 | −3.17 | −0.162 ** | −0.026 | −0.22 | |
| ΔrAndrosterone | −0.028 | −0.63 | −0.051 | 0.107 | 0.88 | |
| ΔrEpiandrosterone | −0.111 | −1.65 | −0.198 | −0.010 | −0.18 | |
| ΔrEtiocholanolone | −0.197 | −2.46 | −0.353 * | −0.224 | −1.65 | |
| ΔrEpietiocholanolone | −0.066 | −1.18 | −0.119 | 0.144 | 2.48 * | |
| Δr11β-Hydroxyandrosterone | −0.176 | −2.93 | −0.317 * | 0.155 | 2.94 * | |
| Δr11β-Hydroxyetiocholanolone | −0.203 | −7.20 | −0.364 ** | −0.064 | −0.99 | |
| (matrix Y) | ΔSomatic symptoms b | −1.000 | −14.53 | −0.935 ** | ||
| Explained variability of dependent variable | 87.4% (76.2% after cross-validation) | |||||
| Data Type | Variable | OPLS (Predictive Component) | ||||
|---|---|---|---|---|---|---|
| Component Loading | t-Statistic | R c | Regression Coefficient | t-Statistic | ||
| Relevant predictors (matrix X) | 16α-Hydroxypregnenolone | 0.208 | 2.88 | 0.341 * | −0.098 | −1.35 |
| Dehydroepiandrosterone | 0.205 | 2.28 | 0.337 * | −0.002 | −0.02 | |
| 7α-Hydroxy-DHEA | 0.194 | 2.24 | 0.318 * | −0.132 | −1.01 | |
| Androstenediol | 0.225 | 2.68 | 0.368 * | 0.023 | 0.24 | |
| Androstenedione | 0.252 | 3.20 | 0.412 ** | 0.086 | 1.15 | |
| Allopregnanolone sulfate | 0.192 | 8.04 | 0.314 ** | 0.028 | 0.33 | |
| Isopregnanolone sulfate | 0.189 | 3.74 | 0.309 ** | −0.059 | −1.08 | |
| Conjugated pregnanolone | 0.244 | 2.34 | 0.399 * | 0.101 | 1.50 | |
| Conjugated 5α-pregnane-3α,20α-diol | 0.147 | 2.94 | 0.241 * | −0.047 | −0.68 | |
| Conjugated 5β,20α-tetrahydroprogesterone | 0.219 | 3.23 | 0.359 ** | 0.157 | 3.19 ** | |
| Conjugated 5β-pregnane-3α,20α-diol | 0.191 | 2.50 | 0.312 * | −0.033 | −0.56 | |
| 17-Hydroxyallopregnanolone sulfate | 0.233 | 3.64 | 0.382 ** | 0.144 | 2.10 * | |
| Androsterone | 0.188 | 3.40 | 0.309 ** | 0.027 | 0.67 | |
| Epietiocholanolone sulfate | 0.150 | 2.26 | 0.246 * | 0.018 | 0.19 | |
| Conjugated 5β-androstane-3α,17β-diol | 0.128 | 1.34 | 0.210 | −0.015 | −0.19 | |
| Psychosomatic symptoms | 0.573 | 5.33 | 0.939 ** | 0.804 | 8.97 ** | |
| Δr11β-Hydroxyandrostenedione a | −0.269 | −2.66 | −0.440 * | −0.153 | −1.91 * | |
| (matrix Y) | ΔPsychosomatic symptoms b | −1.000 | −9.07 | −0.920 ** | ||
| Explained variability of dependent variable | 84.7% (72.9% after cross-validation) | |||||
| Data Type | Variable | OPLS (Predictive Component) | ||||
|---|---|---|---|---|---|---|
| Component Loading | t-Statistics | R c | Regression Coefficient | t-Statistics | ||
| Relevant predictors (matrix X) | Psychiatric symptoms | 0.355 | 5.91 | 0.658 ** | 0.195 | 3.42 ** |
| Δr16α-Hydroxypregnenolone a | −0.288 | −3.12 | −0.535 ** | −0.077 | −2.22 * | |
| ΔrConjugated androstenediol | 0.301 | 3.38 | 0.559 ** | 0.086 | 2.38 * | |
| ΔrConjugated 5α,20α-tetrahydroprogesterone | −0.192 | −5.49 | −0.355 ** | −0.092 | −2.43 * | |
| ΔrConjugated 5β,20α-tetrahydroprogesterone | −0.238 | −2.25 | −0.442 * | −0.086 | −2.74 * | |
| ΔrAndrosterone sulfate | 0.356 | 3.97 | 0.661 ** | 0.110 | 3.20 ** | |
| ΔrEpiandrosterone sulfate | 0.320 | 3.54 | 0.594 ** | 0.099 | 4.41 ** | |
| ΔrConjugated 5α-androstane-3α,17β-diol | 0.296 | 3.99 | 0.549 ** | 0.068 | 2.77 * | |
| Δr11β-Hydroxyandrostenedione | −0.234 | −1.52 | −0.434 | −0.078 | −2.06 * | |
| Δr11β-Hydroxyandrosterone | −0.363 | −5.97 | −0.674 ** | −0.085 | −4.00 ** | |
| Δr11β-Hydroxyepiandrosterone | −0.148 | −0.90 | −0.274 | −0.065 | −2.69 * | |
| Δr11β-Hydroxyetiocholanolone | −0.410 | −9.39 | −0.760 ** | −0.123 | −5.37 ** | |
| (matrix Y) | ΔPsychiatric symptoms b | −1.000 | −5.12 | −0.659 ** | ||
| Explained variability | 43.5% (38.6% after cross-validation) | |||||
| Data Type | Variable | OPLS (Predictive Component) | ||||
|---|---|---|---|---|---|---|
| Component Loading | t-Statistic | R c | Regression Coefficient | t-Statistic | ||
| Relevant predictors (matrix X) | 16α-Hydroxypregnenolone | 0.146 | 3.79 | 0.303 ** | −0.160 | −1.28 |
| Dehydroepiandrosterone | 0.157 | 3.18 | 0.327 ** | 0.022 | 0.42 | |
| 7α-Hydroxy-DHEA | 0.161 | 3.68 | 0.333 ** | −0.124 | −1.03 | |
| Androstenediol | 0.225 | 10.26 | 0.467 ** | 0.057 | 1.16 | |
| 5-Androstene-3β,7α,17β-triol | 0.214 | 3.63 | 0.444 ** | 0.016 | 0.20 | |
| Androstenedione | 0.184 | 3.56 | 0.382 ** | 0.066 | 0.69 | |
| Allopregnanolone sulfate | 0.163 | 4.69 | 0.337 ** | −0.045 | −0.73 | |
| Conjugated pregnanolone | 0.242 | 3.72 | 0.502 ** | 0.066 | 0.64 | |
| Conjugated 5β,20α-tetrahydroprogesterone | 0.161 | 2.70 | 0.334 * | 0.103 | 0.77 | |
| 5β-Pregnane-3α,20α-diol | 0.136 | 2.46 | 0.282 * | −0.030 | −0.31 | |
| Conjugated 5β-pregnane-3α,20α-diol | 0.198 | 4.94 | 0.410 ** | −0.080 | −1.20 | |
| 17-Hydroxyallopregnanolone sulfate | 0.200 | 2.40 | 0.415 * | 0.092 | 1.35 | |
| Conjugated 17-hydroxypregnanolone | 0.191 | 2.37 | 0.396 * | 0.081 | 0.69 | |
| 5β-Pregnane-3α,17α,20α-triol | 0.190 | 2.98 | 0.394 * | 0.058 | 1.76 | |
| Androsterone | 0.128 | 3.19 | 0.265 ** | 0.044 | 0.68 | |
| Epietiocholanolone sulfate | 0.230 | 3.10 | 0.477 ** | 0.116 | 1.03 | |
| 5α-Androstane-3α,17β-diol | 0.159 | 2.78 | 0.330 * | 0.057 | 1.00 | |
| 5β-Androstane-3β,17β-diol | 0.277 | 4.32 | 0.575 ** | 0.152 | 1.82 | |
| 11β-Hydroxyandrostenedione | 0.191 | 2.99 | 0.397 * | 0.028 | 0.28 | |
| 11β-Hydroxyetiocholanolone | 0.197 | 2.52 | 0.409 * | 0.103 | 0.88 | |
| Overall neurotic symptoms | 0.401 | 3.72 | 0.833 ** | 0.589 | 3.56 ** | |
| Δr16α-Hydroxypregnenolone a | −0.142 | −2.69 | −0.295 * | −0.007 | −0.07 | |
| Δr11β-Hydroxyandrostenedione | −0.133 | −2.54 | −0.277 * | 0.050 | 1.45 | |
| Δr11β-Hydroxyandrosterone | −0.172 | −2.89 | −0.356 * | 0.107 | 1.14 | |
| Δr11β-Hydroxyepiandrosterone | −0.125 | −2.20 | −0.260 * | −0.017 | −0.29 | |
| Δr11β-Hydroxyetiocholanolone | −0.187 | −3.69 | −0.387 ** | −0.147 | −1.62 | |
| (matrix Y) | ΔOverall neurotic symptoms b | −1.000 | −7.22 | −0.809 ** | ||
| Explained variability of dependent variable | 65.5% (31% after cross-validation) | |||||
| Steroid | Active Progestogens [24,25,26,27] | Active Glucocorticoids [25] | Immunoprotective Steroids [6,28,29,30,31] | Ergosteroids [31,32,33] | NMDAR Modulators [34,35,36,37,38,39,40,41,42,43] | AMPAR/KAR Modulators [41,44,45] | GABAAR Modulators [46,47,48,49,50,51,52,53,54,55] | GlyR Modulators [56,57,58] | L-type VGCCs Modulators [59,60,61,62,63,64] | T-type VGCCs Modulators [65,66] | TRPV1 Modulators [67,68] | TRPC5 Modulators [69] | TRPM3 Modulators [70,71,72] | PXR Modulators [73] |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pregnenolone | C+ | C− | C− | C− | U−C− | C− | C− | (U+)C+ | U+ | |||||
| 20α-Dihydropregnenolone | C+ | U− | U+ | |||||||||||
| 17-Hydroxypregnenolone | C+ | U+ | ||||||||||||
| Dehydroepiandrosterone | U+ | U+ | C+ | C− | C− | C− | U−C− | C− | (U+)C+ | U+C+ | ||||
| 7α-Hydroxy-DHEA | U+ | U+ | ||||||||||||
| 7-oxo-DHEA | U+ | U+ | ||||||||||||
| Androstenediol | U+ | U+ | ||||||||||||
| 5-Androstene-3β,7α,17β-triol | U+ | U+ | ||||||||||||
| 20α-Dihydroprogesterone | (U+) | |||||||||||||
| 17-Hydroxyprogesterone | (U+) | (U+) | U+ | |||||||||||
| 16α-Hydroxyprogesterone | U+ | |||||||||||||
| Androstenedione | U+ | |||||||||||||
| Allopregnanolone | U+ | C+ | U− | U+ | U− | U+ | ?U− | U+ | ||||||
| 17-Hydroxyallopregnanolone | ?U− | |||||||||||||
| Isopregnanolone | U−, C− | ?U− | ||||||||||||
| Pregnanolone | U+ | C− | C− | U+ | U− | U+ | ?U− | (U−)C− | U+ | |||||
| Epipregnanolone | U− | C− | ?U− | U+ | ||||||||||
| 17-Hydroxypregnanolone | ?U− | |||||||||||||
| 17-Hydroxypregnanolone conjugated | ?U− | |||||||||||||
| 5α,20α-Tetrahydroprogesterone | ?U− | |||||||||||||
| 5α-Pregnane-3α,20α-diol | U+ | ?U− | U+C+ | |||||||||||
| 5α-Pregnane-3α,17α,20α-triol | ?U− | |||||||||||||
| 5β,20α-Tetrahydroprogesterone | ?U− | |||||||||||||
| 5β-Pregnane-3α,20α-diol | U+ | U+ | ?U− | U+ | ||||||||||
| 5β-Pregnane-3α,17α,20α-triol | ?U− | |||||||||||||
| 5α-Androstane-3,17-dione | ?U− | U+ | ||||||||||||
| Androsterone | U+C− | C− | ?U− | U+ | ||||||||||
| Epiandrosterone | C− | C− | ?U− | C+ | U+ | |||||||||
| Etiocholanolone | U+ | U+ | ?U− | U− | U+ | |||||||||
| Epietiocholanolone | ?U− | |||||||||||||
| 5α-Androstane-3α,17β-diol | U+ | ?U− | U+ | |||||||||||
| 5β-Androstane-3α,17β-diol | U+ | ?U− | ||||||||||||
| Cortisol | U+ | U+ | ||||||||||||
| Corticosterone | U+ | U+ | U+ | |||||||||||
| 5α,20α-Tetrahydrocorticosterone | ?U− | U+ | ||||||||||||
| 5β,20α-Tetrahydrocorticosterone | ?U− | |||||||||||||
| 11β-Hydroxyetiocholanolone | U+ | ?U− |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honců, P.; Hill, M.; Bičíková, M.; Jandová, D.; Velíková, M.; Kajzar, J.; Kolátorová, L.; Bešťák, J.; Máčová, L.; Kancheva, R.; et al. Activation of Adrenal Steroidogenesis and an Improvement of Mood Balance in Postmenopausal Females after Spa Treatment Based on Physical Activity. Int. J. Mol. Sci. 2019, 20, 3687. https://doi.org/10.3390/ijms20153687
Honců P, Hill M, Bičíková M, Jandová D, Velíková M, Kajzar J, Kolátorová L, Bešťák J, Máčová L, Kancheva R, et al. Activation of Adrenal Steroidogenesis and an Improvement of Mood Balance in Postmenopausal Females after Spa Treatment Based on Physical Activity. International Journal of Molecular Sciences. 2019; 20(15):3687. https://doi.org/10.3390/ijms20153687
Chicago/Turabian StyleHonců, Pavla, Martin Hill, Marie Bičíková, Dobroslava Jandová, Marta Velíková, Jiří Kajzar, Lucie Kolátorová, Jiří Bešťák, Ludmila Máčová, Radmila Kancheva, and et al. 2019. "Activation of Adrenal Steroidogenesis and an Improvement of Mood Balance in Postmenopausal Females after Spa Treatment Based on Physical Activity" International Journal of Molecular Sciences 20, no. 15: 3687. https://doi.org/10.3390/ijms20153687
APA StyleHonců, P., Hill, M., Bičíková, M., Jandová, D., Velíková, M., Kajzar, J., Kolátorová, L., Bešťák, J., Máčová, L., Kancheva, R., Krejčí, M., Novotný, J., & Stárka, Ľ. (2019). Activation of Adrenal Steroidogenesis and an Improvement of Mood Balance in Postmenopausal Females after Spa Treatment Based on Physical Activity. International Journal of Molecular Sciences, 20(15), 3687. https://doi.org/10.3390/ijms20153687







