Stable Adult Hippocampal Neurogenesis in Cannabinoid Receptor CB2 Deficient Mice
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Preparation of Mouse Brain Sections
4.3. Immunohistochemistry of Mouse Brain Sections
4.4. Quantification of Neuronal Progenitor Cell Populations and Cell Proliferation
4.5. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AN | Adult neurogenesis |
| CB1 | Cannabinoid receptor 1 |
| CB2 | Cannabinoid receptor 2 |
| CR | Calretinin |
| DCX | Doublecortin |
| DG | Dentate gyrus |
| GCL | Granular cell layer |
| SD | Standard deviation |
| SGZ | Subgranular zone |
| NPC | Neural progenitor cells |
References
- Altman, J.; Das, G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965, 124, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.D.R.; Doan, N.B.; Imura, T.; Bush, T.G.; Sofroniew, M.V. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat. Neurosci. 2004, 7, 1233–1241. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, J.T.; Schafer, S.T.; Gage, F.H. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 2016, 167, 897–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicola, Z.; Fabel, K.; Kempermann, G. Development of the adult neurogenic niche in the hippocampus of mice. Front. Neuroanat. 2015, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Kempermann, G.; Jessberger, S.; Steiner, B.; Kronenberg, G. Milestones of neuronal development in the adult hippocampus. Trends Neurosci. 2004, 27, 447–452. [Google Scholar] [CrossRef] [PubMed]
- Bonfanti, L.; Nacher, J. New Scenarios for Neuronal Structural Plasticity in Non-Neurogenic Brain Parenchyma: The Case of Cortical Layer II Immature Neurons. Prog. Neurobiol. 2012, 98, 1–15. [Google Scholar] [CrossRef]
- Brandt, M.D.; Jessberger, S.; Steiner, B.; Kronenberg, G.; Reuter, K.; Bick-Sander, A.; Von Der Behrens, W.; Kempermann, G. Transient calretinin expression defines early postmitotic step of neuronal differentiation in adult hippocampal neurogenesis of mice. Mol. Cell. Neurosci. 2003, 24, 603–613. [Google Scholar] [CrossRef]
- Prenderville, J.A.; Kelly, Á.M.; Downer, E.J. The Role of Cannabinoids in Adult Neurogenesis. Br. J. Pharmacol. 2015, 172, 3950–3963. [Google Scholar] [CrossRef]
- Downer, E.J. High Hopes for CB2 in Neurogenesis. Br. J. Pharmacol. 2014, 171, 1345–1346. [Google Scholar] [CrossRef]
- Rubio-Araiz, A.; García-Ovejero, D.; Williams, R.J.; Moore, J.D.; Molina-Holgado, E.; Molina-Holgado, F.; Arévalo-Martín, A.; Gómez-Torres, O. CB2 cannabinoid receptors promote mouse neural stem cell proliferation. Eur. J. Neurosci. 2007, 25, 629–634. [Google Scholar]
- Palazuelos, J.; Aguado, T.; Egia, A.; Mechoulam, R.; Guzman, M.; Galve-Roperh, I. Non-psychoactive CB2cannabinoid agonists stimulate neural progenitor proliferation. FASEB J. 2006, 20, 2405–2407. [Google Scholar] [CrossRef] [PubMed]
- Palazuelos, J.; Ortega, Z.; Díaz-Alonso, J.; Guzmán, M.; Galve-Roperh, I. CB2 Cannabinoid Receptors Promote Neural Progenitor Cell Proliferation via MTORC1 Signaling. J. Biol. Chem. 2012, 287, 1198–1209. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.S.; Ribeiro, F.F.; Ferreira, F.; Vaz, S.H.; Sebastião, A.M.; Xapelli, S. Interaction between Cannabinoid Type 1 and Type 2 Receptors in the Modulation of Subventricular Zone and Dentate Gyrus Neurogenesis. Front. Pharmacol. 2017, 8, 516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avraham, H.K.; Jiang, S.; Fu, Y.; Rockenstein, E.; Makriyannis, A.; Zvonok, A.; Masliah, E.; Avraham, S. The Cannabinoid CB2 Receptor Agonist AM1241 Enhances Neurogenesis in GFAP/Gp120 Transgenic Mice Displaying Deficits in Neurogenesis. Br. J. Pharmacol. 2014, 171, 468–479. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Ferrer, I.; Cuartero, M.I.; Zarruk, J.G.; Pradillo, J.M.; Hurtado, O.; Romera, V.G.; Díaz-Alonso, J.; García-Segura, J.M.; Guzmán, M.; Lizasoain, I.; et al. Cannabinoid Type-2 Receptor Drives Neurogenesis and Improves Functional Outcome After Stroke. Stroke 2017, 48, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Rom, S.; Persidsky, Y. Cannabinoid receptor 2: Potential role in immunomodulation and neuroinflammation Review. J. Neuroimmune Pharmacol. 2013, 8, 608–620. [Google Scholar] [CrossRef] [PubMed]
- Horgusluoglu, E.; Nudelman, K.; Nho, K.; Saykin, A.J. Adult Neurogenesis and Neurodegenerative Diseases: A Systems Biology Perspective. Am. J. Med. Genet. 2017, 174, 93–112. [Google Scholar] [CrossRef]
- Bakker, R.; Tiesinga, P.; Kötter, R. The Scalable Brain Atlas: Instant Web-Based Access to Public Brain Atlases and Related Content. Neuroinformatics 2015, 13, 353–366. [Google Scholar] [CrossRef] [Green Version]
- Badhwar, A.; Lerch, J.P.; Hamel, E.; Sled, J.G. Impaired structural correlates of memory in Alzheimer’s disease mice. NeuroImage Clin. 2013, 3, 290–300. [Google Scholar] [CrossRef]
- Ben Abdallah, N.M.B.; Slomianka, L.; Vyssotski, A.L.; Lipp, H.-P. Early age-related changes in adult hippocampal neurogenesis in C57 mice. Neurobiol. Aging 2010, 31, 151–161. [Google Scholar] [CrossRef]
- Plümpe, T.; Ehninger, D.; Steiner, B.; Klempin, F.; Jessberger, S.; Brandt, M.; Römer, B.; Rodriguez, G.; Kronenberg, G.; Kempermann, G. Variability of Doublecortin-Associated Dendrite Maturation in Adult Hippocampal Neurogenesis Is Independent of the Regulation of Precursor Cell Proliferation. BMC Neurosci. 2006, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Lipp, H.P.; Bonfanti, L. Adult Neurogenesis in Mammals: Variations and Confusions. Brain Behav. Evol. 2016, 87, 205–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sisay, S.; Pryce, G.; Jackson, S.J.; Tanner, C.; Ross, R.A.; Michael, G.J.; Selwood, D.L.; Giovannoni, G.; Baker, D. Genetic Background Can Result in a Marked or Minimal Effect of Gene Knockout (GPR55 and CB2 Receptor) in Experimental Autoimmune Encephalomyelitis Models of Multiple Sclerosis. PLoS ONE 2013, 8, e76907. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.S.; Bick-Sander, A.; Fabel, K.; Leal-Galicia, P.; Tauber, S.; Ramírez-Rodríguez, G.; Müller, A.; Melnik, A.; Waltinger, T.P.; Ullrich, O.; et al. Cannabinoid receptor CB1 mediates baseline and activity-induced survival of new neurons in adult hippocampal neurogenesis. Cell Commun. Signal. 2010, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Aguado, T.; Palazuelos, J.; Monory, K.; Stella, N.; Cravatt, B.; Lutz, B.; Marsicano, G.; Kokaia, Z.; Guzman, M.; Galve-Roperh, I. The Endocannabinoid System Promotes Astroglial Differentiation by Acting on Neural Progenitor Cells. J. Neurosci. 2006, 26, 1551–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sellner, S.; Paricio-Montesinos, R.; Spieß, A.; Masuch, A.; Erny, D.; Harsan, L.A.; Elverfeldt, D.V.; Schwabenland, M.; Biber, K.; Staszewski, O.; et al. Microglial CX3CR1 Promotes Adult Neurogenesis by Inhibiting Sirt 1/P65 Signaling Independent of CX3CL1. Acta Neuropathol. Commun. 2016, 4, 102. [Google Scholar] [CrossRef]
- Morrens, J.; Van Den Broeck, W.; Kempermann, G. Glial Cells in Adult Neurogenesis. Glia 2012, 60, 159–174. [Google Scholar] [CrossRef]
- Rueden, C.T.; Schindelin, J.; Hiner, M.C.; DeZonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. Imagej2: Imagej for the Next Generation of Scientific Image Data. BMC Bioinform. 2017, 8, 529. [Google Scholar] [CrossRef]

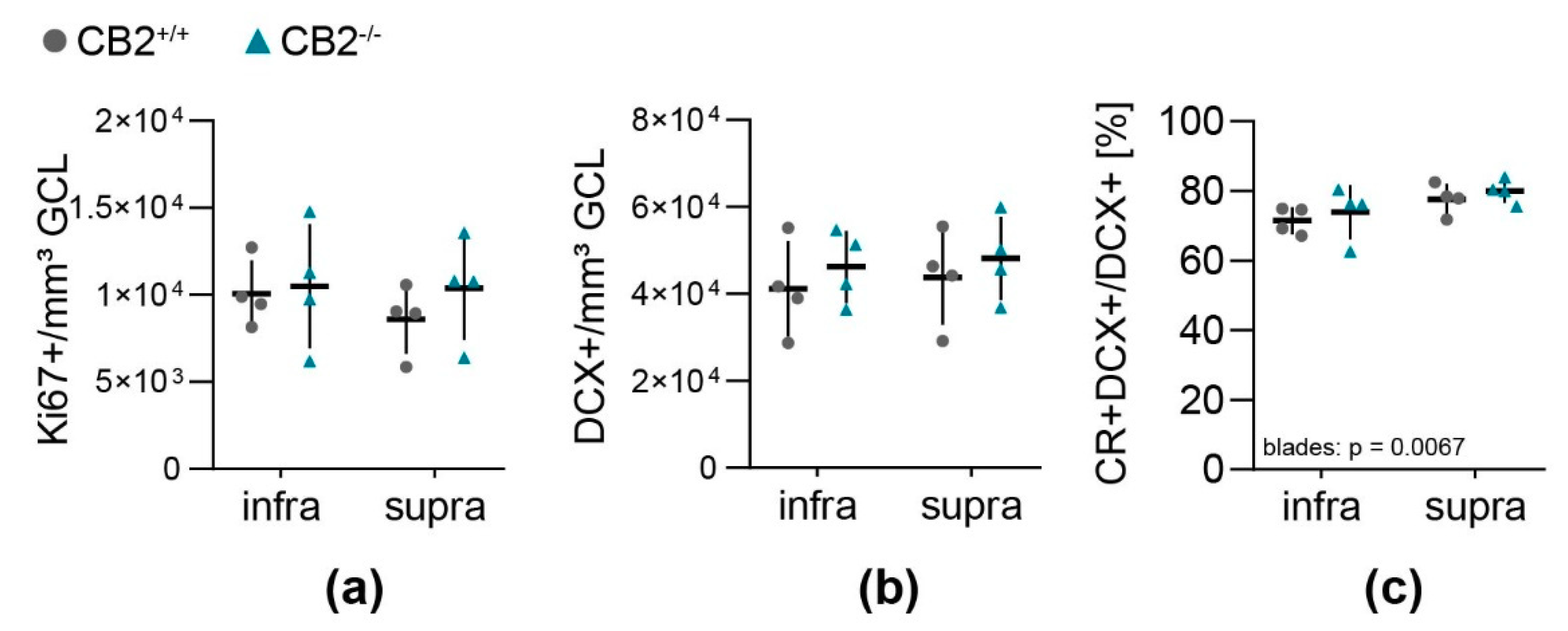
| Genotype | Ki67+ per mm3 | DCX+ per mm3 | CR+DCX+/DCX+ [%] | ||||||
|---|---|---|---|---|---|---|---|---|---|
| inf. | sup. | both | inf. | sup. | both | inf. | sup. | both | |
| CB2+/+ | 10,060 ± 1921 | 8604 ± 1971 | 9174 ± 1737 | 41,188 ± 10,908 | 46,220 ± 8353 | 42,739 ± 10,794 | 71.49 ± 3.86 | 77.68 ± 4.4 | 75.25 ± 3.69 |
| CB2−/− | 10,504 ± 3564 | 10,388 ± 2972 | 10,443 ± 3192 | 43,837 ± 10,917 | 48,191 ± 9600 | 47,390 ± 9028 | 73.92 ± 7.8 | 80.00 ± 3.38 | 77.63 ± 5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mensching, L.; Djogo, N.; Keller, C.; Rading, S.; Karsak, M. Stable Adult Hippocampal Neurogenesis in Cannabinoid Receptor CB2 Deficient Mice. Int. J. Mol. Sci. 2019, 20, 3759. https://doi.org/10.3390/ijms20153759
Mensching L, Djogo N, Keller C, Rading S, Karsak M. Stable Adult Hippocampal Neurogenesis in Cannabinoid Receptor CB2 Deficient Mice. International Journal of Molecular Sciences. 2019; 20(15):3759. https://doi.org/10.3390/ijms20153759
Chicago/Turabian StyleMensching, Leonore, Nevena Djogo, Christina Keller, Sebastian Rading, and Meliha Karsak. 2019. "Stable Adult Hippocampal Neurogenesis in Cannabinoid Receptor CB2 Deficient Mice" International Journal of Molecular Sciences 20, no. 15: 3759. https://doi.org/10.3390/ijms20153759




