Mitochondria-Targeting Antioxidant Provides Cardioprotection through Regulation of Cytosolic and Mitochondrial Zn2+ Levels with Re-Distribution of Zn2+-Transporters in Aged Rat Cardiomyocytes
Abstract
:1. Introduction
2. Results
2.1. General Parameters of the Aged Rats
2.2. MitoTEMPO Regulation of [Zn2+]i and Some Zn2+-Transporters in the Aged Left Ventricular Cardiomyocytes
2.3. Aging-Associated Redistribution of Cellular [Zn2+]i in the Aged Cardiomyocytes
2.4. Confirmation of Mitochondrial Function and ROS Level in Aging-Modeled H9c2 Cells
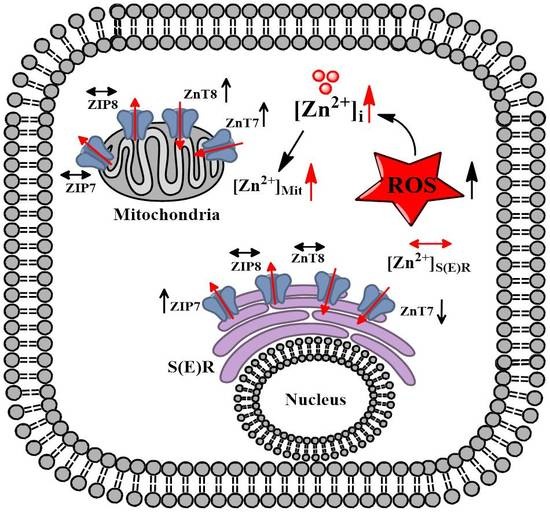
2.5. Redistribution of Zn2+-Transporters among Suborganelles in the Aged Cardiomyocytes İsolated from the Left Ventricle of 24-Month Old Rats
2.6. MitoTEMPO Preserves the Increases in Both Oxidation and K-Acetylation Levels in Left Ventricular Aged Cardiomyocytes
3. Discussion
4. Material and Methods
4.1. Experimental Animals
4.2. Fresh Cardiomyocyte İsolation from the Left Ventricle
4.3. Cell Culturing
4.4. Determination of Intracellular and Suborganelle Levels of Free Zn2+ by Confocal Microscopy Measurements
4.5. Confocal Imaging of Mitochondrial Membrane Potential and ROS Level
4.6. Isolation of Sarco(endo)Plasmic Reticulum and Mitochondria Fractions from Left Ventricular Cardiomyocytes
4.7. Western Blotting
4.8. Total Antioxidant Status (TAS) and Total Oxidant Status (TOS) Measurement in Cardiomyocytes
4.9. Determination of Oxidized Protein Thiol Level in Isolated Cardiomyocytes
4.10. Determination of Protein K-Acetylation in Cardiomyocytes
4.11. Data Analysis and Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kirkwood, T.B. Understanding the odd science of aging. Cell 2005, 120, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Shioi, T.; Inuzuka, Y. Aging as a substrate of heart failure. J. Cardiol. 2012, 60, 423–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quarles, E.K.; Dai, D.-F.; Tocchi, A.; Basisty, N.; Gitari, L.; Rabinovitch, P.S. Quality control systems in cardiac aging. Ageing Res. Rev. 2015, 23, 101–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, P.M.; Li, K.L.; Lin, Y.C. Fucoidan(-)Fucoxanthin Ameliorated Cardiac Function via IRS1/GRB2/ SOS1, GSK3beta/CREB Pathways and Metabolic Pathways in Senescent Mice. Mar. Drugs 2019, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Bihlmeyer, N.A.; Brody, J.A.; Smith, A.V.; Warren, H.R.; Lin, H.; Isaacs, A.; Liu, C.T.; Marten, J.; Radmanesh, F.; Hall, L.M.; et al. ExomeChip-Wide Analysis of 95 626 Individuals Identifies 10 Novel Loci Associated with QT and JT Intervals. Circ. Genom. Precis. Med. 2018, 11, e001758. [Google Scholar] [CrossRef]
- Olgar, Y.; Degirmenci, S.; Durak, A.; Billur, D.; Can, B.; Mutlu, G.K.; Inan, E.A.-I.; Turan, B. Aging related functional and structural changes in the heart and aorta: MitoTEMPO improves aged-cardiovascular performance. Exp. Gerontol. 2018, 110, 172–181. [Google Scholar] [CrossRef]
- Reardon, M.; Malik, M. QT interval change with age in an overtly healthy older population. Clin. Cardiol. 1996, 19, 949–952. [Google Scholar] [CrossRef]
- Arai, Y.; Kamide, K.; Hirose, N. Adipokines and Aging: Findings From Centenarians and the Very Old. Front. Endocrinol. 2019, 10, 142. [Google Scholar] [CrossRef]
- Ehrhardt, N.; Cui, J.; Dagdeviren, S.; Saengnipanthkul, S.; Goodridge, H.S.; Kim, J.K.; Lantier, L.; Guo, X.; Chen, Y.-D.I.; Raffel, L.J.; et al. Adiposity-Independent Effects of Aging on Insulin Sensitivity and Clearance in Mice and Humans. Obesity 2019, 27, 434–443. [Google Scholar] [CrossRef]
- Ha, M.-S.; Son, W.-M. Combined exercise is a modality for improving insulin resistance and aging-related hormone biomarkers in elderly Korean women. Exp. Gerontol. 2018, 114, 13–18. [Google Scholar] [CrossRef]
- Bhashyam, S.; Parikh, P.; Bolukoglu, H.; Shannon, A.H.; Porter, J.H.; Shen, Y.-T.; Shannon, R.P. Aging is associated with myocardial insulin resistance and mitochondrial dysfunction. Am. J. Physiol. Circ. Physiol. 2007, 293, H3063–H3071. [Google Scholar] [CrossRef] [Green Version]
- McMillin, J.B.; Taffet, G.E.; Hudson, E.K.; Tate, C.A.; Taegtmeyer, H. Mitochondrial metabolism and substrate competition in the aging Fischer rat heart. Cardiovasc. Res. 1993, 27, 2222–2228. [Google Scholar] [CrossRef]
- Sample, J.; Cleland, J.G.F.; Seymour, A.-M.L. Metabolic remodeling in the aging heart. J. Mol. Cell. Cardiol. 2006, 40, 56–63. [Google Scholar] [CrossRef]
- Levy, D.; Reichert, C.O.; Bydlowski, S.P. Paraoxonases Activities and Polymorphisms in Elderly and Old-Age Diseases: An Overview. Antioxidants 2019, 8, 118. [Google Scholar] [CrossRef]
- Qian, X.; Asad, S.B.; Li, J.; Wang, J.; Wei, D.; Zhao, Y.; Wang, Y.; Zhu, H. Link between cardiac function and the antioxidative defense mechanism in aged rats. Biochem. Biophys. Res. Commun. 2019, 513, 1100–1105. [Google Scholar] [CrossRef]
- Shaheen, A.A.; El-Fattah, A.A.A. Effect of dietary zinc on lipid peroxidation, glutathione, protein thiols levels and superoxide dismutase activity in rat tissues. Int. J. Biochem. Cell Boil. 1995, 27, 89–95. [Google Scholar] [CrossRef]
- Farinati, F.; Cardin, R.; D’Incà, R.; Naccarato, R.; Sturniolo, G.C. Zinc treatment prevents lipid peroxidation and increases glutathione availability in Wilson’s disease. J. Lab. Clin. Med. 2003, 141, 372–377. [Google Scholar] [CrossRef]
- Anversa, P.; Palackal, T.; Sonnenblick, E.H.; Olivetti, G.; Meggs, L.G.; Capasso, J.M. Myocyte cell loss and myocyte cellular hyperplasia in the hypertrophied aging rat heart. Circ. Res. 1990, 67, 871–885. [Google Scholar] [CrossRef]
- Lakatta, E.G.; Sollott, S.J.; Pepe, S. The old heart: Operating on the edge. Novartis Found. Symp. 2001, 235, 172–196. [Google Scholar]
- Yang, X.; Sreejayan, N.; Ren, J. Views from within and beyond: Narratives of cardiac contractile dysfunction under senescence. Endocrine 2005, 26, 127–137. [Google Scholar] [CrossRef]
- Hacker, T.A.; McKiernan, S.H.; Douglas, P.S.; Wanagat, J.; Aiken, J.M. Age-related changes in cardiac structure and function in Fischer 344 × Brown Norway hybrid rats. Am. J. Physiol. Circ. Physiol. 2006, 290, H304–H311. [Google Scholar] [CrossRef]
- Preston, C.C.; Oberlin, A.S.; Holmuhamedov, E.L.; Gupta, A.; Sagar, S.; Syed, R.H.K.; Siddiqui, S.A.; Raghavakaimal, S.; Terzic, A.; Jahangir, A. Aging-induced alterations in gene transcripts and functional activity of mitochondrial oxidative phosphorylation complexes in the heart. Mech. Ageing Dev. 2008, 129, 304–312. [Google Scholar] [CrossRef] [Green Version]
- Beal, M.F. Oxidatively modified proteins in aging and disease. Free Radic. Boil. Med. 2002, 32, 797–803. [Google Scholar] [CrossRef]
- Ristow, M. Interview with Michael Ristow. Aging 2012, 4, 2. [Google Scholar] [CrossRef]
- Lee, H.Y.; Oh, B.H. Aging and arterial stiffness. Circ. J. 2010, 74, 2257–2262. [Google Scholar] [CrossRef]
- Turan, B.; Fliss, H.; Desilets, M. Oxidants increase intracellular free Zn2+ concentration in rabbit ventricular myocytes. Am. J. Physiol. 1997, 272, H2095–H2106. [Google Scholar] [CrossRef]
- Degirmenci, S.; Olgar, Y.; Durak, A.; Tuncay, E.; Turan, B. Cytosolic increased labile Zn2+ contributes to arrhythmogenic action potentials in left ventricular cardiomyocytes through protein thiol oxidation and cellular ATP depletion. J. Trace Elem. Med. Biol. 2018, 48, 202–212. [Google Scholar] [CrossRef]
- Durak, A.; Olgar, Y.; Degirmenci, S.; Akkus, E.; Tuncay, E.; Turan, B. A SGLT2 inhibitor dapagliflozin suppresses prolonged ventricular-repolarization through augmentation of mitochondrial function in insulin-resistant metabolic syndrome rats. Cardiovasc. Diabetol. 2018, 17, 144. [Google Scholar] [CrossRef]
- Cortopassi, G.A.; Arnheim, N. Detection of a specific mitochondrial DNA deletion in tissues of older humans. Nucleic Acids Res. 1990, 18, 6927–6933. [Google Scholar] [CrossRef] [Green Version]
- Pikó, L.; Hougham, A.J.; Bulpitt, K.J. Studies of sequence heterogeneity of mitochondrial DNA from rat and mouse tissues: Evidence for an increased frequency of deletions/additions with aging. Mech. Ageing Dev. 1988, 43, 279–293. [Google Scholar] [CrossRef]
- Pivovarova, N.B.; Stanika, R.I.; Kazanina, G.; Villanueva, I.; Andrews, S.B. The interactive roles of zinc and calcium in mitochondrial dysfunction and neurodegeneration. J. Neurochem. 2014, 128, 592–602. [Google Scholar] [CrossRef]
- Dai, D.-F.; Chiao, Y.A.; Marcinek, D.J.; Szeto, H.H.; Rabinovitch, P.S. Mitochondrial oxidative stress in aging and healthspan. Longev. Heal. 2014, 3, 6. [Google Scholar] [CrossRef]
- Jang, Y.; Wang, H.; Xi, J.; Mueller, R.A.; Norfleet, E.A.; Xu, Z. NO mobilizes intracellular Zn2+ via cGMP/PKG signaling pathway and prevents mitochondrial oxidant damage in cardiomyocytes. Cardiovasc. Res. 2007, 75, 426–433. [Google Scholar] [CrossRef]
- Stefanidou, M.; Maravelias, C.; Dona, A.; Spiliopoulou, C. Zinc: A multipurpose trace element. Arch. Toxicol. 2006, 80, 1–9. [Google Scholar] [CrossRef]
- Raza, M.; Deshpande, L.S.; Blair, R.E.; Carter, D.S.; Sombati, S.; DeLorenzo, R.J. Aging is associated with elevated intracellular calcium levels and altered calcium homeostatic mechanisms in hippocampal neurons. Neurosci. Lett. 2007, 418, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Giorgi, C.; Baldassari, F.; Bononi, A.; Bonora, M.; De Marchi, E.; Marchi, S.; Missiroli, S.; Patergnani, S.; Rimessi, A.; Suski, J.M.; et al. Mitochondrial Ca2+ and apoptosis. Cell Calcium 2012, 52, 36–43. [Google Scholar] [CrossRef]
- Tuncay, E.; Bitirim, V.C.; Durak, A.; Carrat, G.R.J.; Taylor, K.M.; Rutter, G.A.; Turan, B. Hyperglycemia-Induced Changes in ZIP7 and ZnT7 Expression Cause Zn2+ Release From the Sarco(endo)plasmic Reticulum and Mediate ER Stress in the Heart. Diabetes 2017, 66, 1346–1358. [Google Scholar] [CrossRef]
- Turan, B.; Tuncay, E. Impact of Labile Zinc on Heart Function: From Physiology to Pathophysiology. Int. J. Mol. Sci. 2017, 18, 2395. [Google Scholar] [CrossRef]
- Tuncay, E.; Bitirim, C.V.; Olgar, Y.; Durak, A.; Rutter, G.A.; Turan, B. Zn2+-transporters ZIP7 and ZnT7 play important role in progression of cardiac dysfunction via affecting sarco(endo)plasmic reticulum-mitochondria coupling in hyperglycemic cardiomyocytes. Mitochondrion 2019, 44, 41–52. [Google Scholar] [CrossRef]
- McCord, M.C.; Aizenman, E. The role of intracellular zinc release in aging, oxidative stress, and Alzheimer’s disease. Front. Aging Neurosci. 2014, 6, 77. [Google Scholar] [CrossRef]
- Aydemir, T.B.; Troche, C.; Kim, J.; Kim, M.H.; Teran, O.Y.; Leeuwenburgh, C.; Cousins, R.J. Aging amplifies multiple phenotypic defects in mice with zinc transporter Zip14 (Slc39a14) deletion. Exp. Gerontol. 2016, 85, 88–94. [Google Scholar] [CrossRef] [Green Version]
- Chabosseau, P.; Tuncay, E.; Meur, G.; Bellomo, E.A.; Hessels, A.; Hughes, S.; Johnson, P.R.; Bugliani, M.; Marchetti, P.; Turan, B.; et al. Mitochondrial and ER-targeted eCALWY probes reveal high levels of free Zn2+. ACS Chem. Biol. 2014, 9, 2111–2120. [Google Scholar] [CrossRef]
- Tuncay, E.; Okatan, E.N.; Vassort, G.; Turan, B. ss-blocker timolol prevents arrhythmogenic Ca2+ release and normalizes Ca2+ and Zn2+ dyshomeostasis in hyperglycemic rat heart. PLoS ONE 2013, 8, e71014. [Google Scholar] [CrossRef]
- Tuncay, E.; Bilginoglu, A.; Sozmen, N.N.; Zeydanli, E.N.; Ugur, M.; Vassort, G.; Turan, B. Intracellular free zinc during cardiac excitation-contraction cycle: Calcium and redox dependencies. Cardiovasc. Res. 2011, 89, 634–642. [Google Scholar] [CrossRef]
- Tuncay, E.; Turan, B. Intracellular Zn2+ Increase in Cardiomyocytes Induces both Electrical and Mechanical Dysfunction in Heart via Endogenous Generation of Reactive Nitrogen Species. Biol. Trace Elem. Res. 2016, 169, 294–302. [Google Scholar] [CrossRef]
- Ayaz, M.; Turan, B. Selenium prevents diabetes-induced alterations in [Zn2+]i and metallothionein level of rat heart via restoration of cell redox cycle. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H1071–H1080. [Google Scholar] [CrossRef]
- Kamalov, G.; Deshmukh, P.A.; Baburyan, N.Y.; Gandhi, M.S.; Johnson, P.L.; Ahokas, R.A.; Bhattacharya, S.K.; Sun, Y.; Gerling, I.C.; Weber, K.T. Coupled Calcium and Zinc Dyshomeostasis and Oxidative Stress in cardiac myocytes and mitochondria of Rats with Chronic Aldosteronism. J. Cardiovasc. Pharmacol. 2009, 53, 414–423. [Google Scholar] [CrossRef]
- Maret, W. Zinc and Human Disease. Met. Ions Role Life Sci. 2013, 13, 389–414. [Google Scholar] [CrossRef]
- Maret, W. Molecular aspects of human cellular zinc homeostasis: Redox control of zinc potentials and zinc signals. BioMetals 2009, 22, 149–157. [Google Scholar] [CrossRef]
- Dineley, K.E.; Richards, L.L.; Votyakova, T.V.; Reynolds, I.J. Zinc causes loss of membrane potential and elevates reactive oxygen species in rat brain mitochondria. Mitochondrion 2005, 5, 55–65. [Google Scholar] [CrossRef]
- Kamalov, G.; Ahokas, R.A.; Zhao, W.; Shahbaz, A.U.; Bhattacharya, S.K.; Sun, Y.; Gerling, I.C.; Weber, K.T. Temporal responses to intrinsically coupled calcium and zinc dyshomeostasis in cardiac myocytes and mitochondria during aldosteronism. Am. J. Physiol. Circ. Physiol. 2010, 298, H385–H394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierce, G.N.; Dhalla, N.S. Heart mitochondrial function in chronic experimental diabetes in rats. Can. J. Cardiol. 1985, 1, 48–54. [Google Scholar] [PubMed]
- Fukushima, A.; Zhang, L.; Huqi, A.; Rawat, S.; Altamimi, T.; Lam, V.H.; Wagg, C.S.; Dhaliwal, K.K.; Hornberger, L.K.; Kantor, P.F.; et al. Acetylation contributes to hypertrophy-caused maturational delay of cardiac energy metabolism. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Parodi-Rullán, R.M.; Chapa-Dubocq, X.R.; Javadov, S. Acetylation of Mitochondrial Proteins in the Heart: The Role of SIRT3. Front. Physiol. 2018, 9, 1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yogev, O.; Pines, O. Dual targeting of mitochondrial proteins: Mechanism, regulation and function. Biochim. Biophys. Acta 2011, 1808, 1012–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, S.; Molina-López, J.; Parsons, D.; Corpe, C.; Maret, W.; Hogstrand, C. Differential cytolocation and functional assays of the two major human SLC30A8 (ZnT8) isoforms. J. Trace Elements Med. Biol. 2017, 44, 116–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornton, J.K.; Taylor, K.M.; Ford, D.; Valentine, R.A. Differential Subcellular Localization of the Splice Variants of the Zinc Transporter ZnT5 Is Dictated by the Different C-Terminal Regions. PLoS ONE 2011, 6, e23878. [Google Scholar] [CrossRef]
- Tuncay, E.; Hafez, G.; Okatan, E.N.; Turan, B. Profiling of cardiac β-adrenoceptor subtypes in the cardiac left ventricle of rats with metabolic syndrome: Comparison with streptozotocin-induced diabetic rats. Can. J. Physiol. Pharmacol. 2015, 93, 517–525. [Google Scholar] [CrossRef]
- Yaras, N.; Ugur, M.; Ozdemir, S.; Gurdal, H.; Purali, N.; Lacampagne, A.; Vassort, G.; Turan, B. Effects of diabetes on ryanodine receptor Ca release channel (RyR2) and Ca2+ homeostasis in rat heart. Diabetes 2005, 54, 3082–3088. [Google Scholar] [CrossRef]
- Chanoit, G.; Lee, S.; Xi, J.; Zhu, M.; McIntosh, R.A.; Mueller, R.A.; Norfleet, E.A.; Xu, Z. Exogenous zinc protects cardiac cells from reperfusion injury by targeting mitochondrial permeability transition pore through inactivation of glycogen synthase kinase-3β. Am. J. Physiol. Circ. Physiol. 2008, 295, H1227–H1233. [Google Scholar] [CrossRef]
- Billur, D.; Tuncay, E.; Okatan, E.N.; Olgar, Y.; Durak, A.T.; Degirmenci, S.; Can, B.; Turan, B. Interplay between Cytosolic Free Zn2+ and Mitochondrion Morphological Changes in Rat Ventricular Cardiomyocytes. Biol. Trace Elem. Res. 2016, 174, 177–188. [Google Scholar] [CrossRef]
- Erel, O. A novel automated method to measure total antioxidant response against potent free radical reactions. Clin. Biochem. 2004, 37, 112–119. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olgar, Y.; Tuncay, E.; Turan, B. Mitochondria-Targeting Antioxidant Provides Cardioprotection through Regulation of Cytosolic and Mitochondrial Zn2+ Levels with Re-Distribution of Zn2+-Transporters in Aged Rat Cardiomyocytes. Int. J. Mol. Sci. 2019, 20, 3783. https://doi.org/10.3390/ijms20153783
Olgar Y, Tuncay E, Turan B. Mitochondria-Targeting Antioxidant Provides Cardioprotection through Regulation of Cytosolic and Mitochondrial Zn2+ Levels with Re-Distribution of Zn2+-Transporters in Aged Rat Cardiomyocytes. International Journal of Molecular Sciences. 2019; 20(15):3783. https://doi.org/10.3390/ijms20153783
Chicago/Turabian StyleOlgar, Yusuf, Erkan Tuncay, and Belma Turan. 2019. "Mitochondria-Targeting Antioxidant Provides Cardioprotection through Regulation of Cytosolic and Mitochondrial Zn2+ Levels with Re-Distribution of Zn2+-Transporters in Aged Rat Cardiomyocytes" International Journal of Molecular Sciences 20, no. 15: 3783. https://doi.org/10.3390/ijms20153783