Cardiovascular Pleiotropic Effects of Natriuretic Peptides
Abstract
:1. Introduction
2. Overview of ANP Metabolism
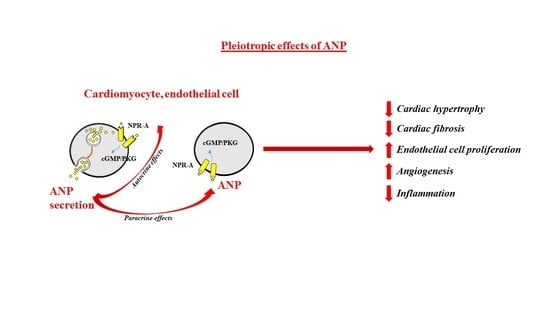
3. ANP and Cardiac Pleiotropic Effects
4. ANP and Ischemia/Riperfusion Injury
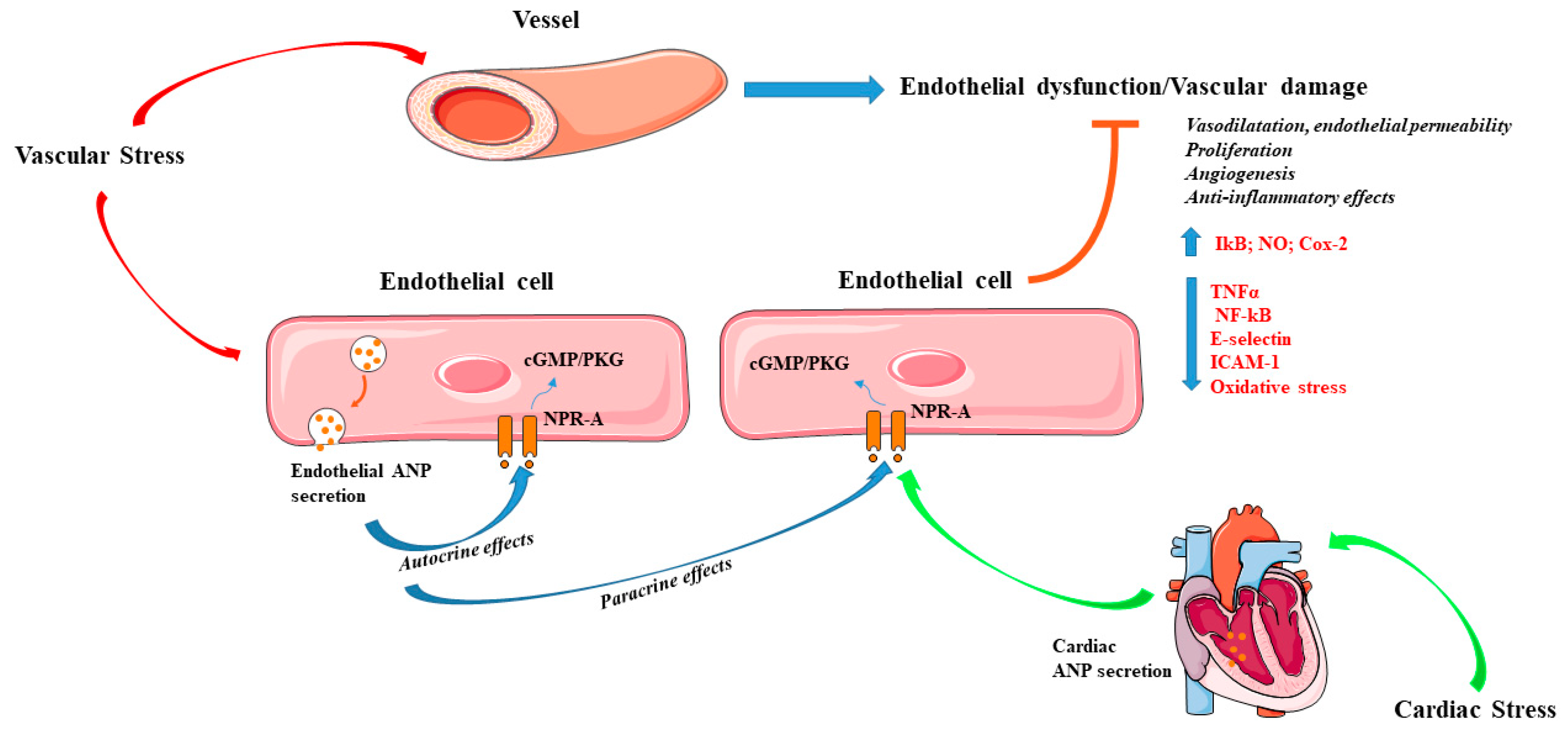
5. ANP and Vascular Pleiotropic Effects
6. Local Actions of BNP and CNP in the Cardiovascular System
7. Therapeutic Interventions Targeting NPs
8. Conclusions and Perspectives
Funding
Conflicts of Interest
References
- de Bold, A.J.; Borenstein, H.B.; Veress, A.T.; Sonnenberg, H. A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci. 1981, 28, 89–94. [Google Scholar] [CrossRef]
- Kangawa, K.; Matsuo, H. Purification and complete amino acid sequence of alpha-human atrial natriuretic polypeptide (alpha-hANP). Biochem. Biophys. Res. Commun. 1984, 118, 131–139. [Google Scholar] [CrossRef]
- Levin, E.R.; Gardner, D.G.; Samson, W.K. Natriuretic peptides. N. Engl. J. Med. 1998, 339, 321–328. [Google Scholar] [PubMed]
- Sudoh, T.; Minamino, N.; Kangawa, K.; Matsuo, H. C-type natriuretic peptide (CNP): a new member of natriuretic peptide family identified in porcine brain. Biochem. Biophys. Res. Commun. 1990, 168, 863–870. [Google Scholar] [CrossRef]
- Sudoh, T.; Kangawa, K.; Minamino, N.; Matsuo, H. A new natriuretic peptide in porcine brain. Nature 1988, 332, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Potter, L.R.; Yoder, A.R.; Flora, D.R.; Antos, L.K.; Dickey, D.M. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb. Exp. Pharmacol. 2009, 341–366. [Google Scholar]
- He, X.L.; Dukkipati, A.; Garcia, K.C. Structural determinants of natriuretic peptide receptor specificity and degeneracy. J. Mol. Biol. 2006, 361, 698–714. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, A.; Nagai-Okatani, C.; Nishigori, M.; Kangawa, K.; Minamino, N. Natriuretic peptides in human heart: Novel insight into their molecular forms, functions, and diagnostic use. Peptides 2019, 111, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Rubattu, S.; Sciarretta, S.; Valenti, V.; Stanzione, R.; Volpe, M. Natriuretic peptides: an update on bioactivity, potential therapeutic use, and implication in cardiovascular diseases. Am. J. Hypertens 2008, 21, 733–741. [Google Scholar] [CrossRef]
- Volpe, M.; Rubattu, S.; Burnett, J., Jr. Natriuretic peptides in cardiovascular diseases: current use and perspectives. Eur. Heart J. 2014, 35, 419–425. [Google Scholar] [CrossRef]
- Rubattu, S.; Forte, M.; Marchitti, S.; Volpe, M. Molecular Implications of Natriuretic Peptides in the Protection from Hypertension and Target Organ Damage Development. Int. J. Mol. Sci. 2019, 20, 798. [Google Scholar] [CrossRef] [PubMed]
- Soualmia, H.; Barthelemy, C.; Masson, F.; Maistre, G.; Eurin, J.; Carayon, A. Angiotensin II-induced phosphoinositide production and atrial natriuretic peptide release in rat atrial tissue. J. Cardiovasc Pharmacol. 1997, 29, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Stasch, J.P.; Hirth-Dietrich, C.; Kazda, S.; Neuser, D. Endothelin stimulates release of atrial natriuretic peptides in vitro and in vivo. Life Sci. 1989, 45, 869–875. [Google Scholar] [CrossRef]
- Thibault, G.; Amiri, F.; Garcia, R. Regulation of natriuretic peptide secretion by the heart. Annu. Rev. Physiol. 1999, 61, 193–217. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.Y.; Burnett, J.C., Jr. Natriuretic peptides and therapeutic applications. Heart Fail. Rev. 2007, 12, 131–142. [Google Scholar] [CrossRef] [PubMed]
- John, S.W.; Krege, J.H.; Oliver, P.M.; Hagaman, J.R.; Hodgin, J.B.; Pang, S.C.; Flynn, T.G.; Smithies, O. Genetic decreases in atrial natriuretic peptide and salt-sensitive hypertension. Science 1995, 267, 679–681. [Google Scholar] [CrossRef] [PubMed]
- Seronde, M.F.; Gayat, E.; Logeart, D.; Lassus, J.; Laribi, S.; Boukef, R.; Sibellas, F.; Launay, J.M.; Manivet, P.; Sadoune, M.; et al. Comparison of the diagnostic and prognostic values of B-type and atrial-type natriuretic peptides in acute heart failure. Int. J. Cardiol. 2013, 168, 3404–3411. [Google Scholar] [CrossRef] [PubMed]
- Paget, V.; Legedz, L.; Gaudebout, N.; Girerd, N.; Bricca, G.; Milon, H.; Vincent, M.; Lantelme, P. N-terminal pro-brain natriuretic peptide: a powerful predictor of mortality in hypertension. Hypertension 2011, 57, 702–709. [Google Scholar] [CrossRef] [PubMed]
- Sabatine, M.S.; Morrow, D.A.; de Lemos, J.A.; Omland, T.; Sloan, S.; Jarolim, P.; Solomon, S.D.; Pfeffer, M.A.; Braunwald, E. Evaluation of multiple biomarkers of cardiovascular stress for risk prediction and guiding medical therapy in patients with stable coronary disease. Circulation 2012, 125, 233–240. [Google Scholar] [CrossRef]
- Daniels, L.B. Natriuretic Peptides and Assessment of Cardiovascular Disease Risk in Asymptomatic Persons. Curr. Cardiovasc. Risk Rep. 2010, 4, 120–127. [Google Scholar] [CrossRef] [Green Version]
- Volpe, M.; Battistoni, A.; Rubattu, S. Natriuretic peptides in heart failure: Current achievements and future perspectives. Int. J. Cardiol. 2019, 281, 186–189. [Google Scholar] [CrossRef] [PubMed]
- Clerico, A.; Passino, C.; Franzini, M.; Emdin, M. Cardiac biomarker testing in the clinical laboratory: where do we stand? General overview of the methodology with special emphasis on natriuretic peptides. Clin. Chim. Acta. 2015, 443, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.; McDonald, K.; de Boer, R.A.; Maisel, A.; Cleland, J.G.F.; Kozhuharov, N.; Coats, A.J.S.; Metra, M.; Mebazaa, A.; Ruschitzka, F.; et al. Heart Failure Association of the European Society of, C. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur. J. Heart Fail. 2019, 21, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Franco, V.; Chen, Y.F.; Oparil, S.; Feng, J.A.; Wang, D.; Hage, F.; Perry, G. Atrial natriuretic peptide dose-dependently inhibits pressure overload-induced cardiac remodeling. Hypertension 2004, 44, 746–750. [Google Scholar] [CrossRef] [PubMed]
- Molkentin, J.D. A friend within the heart: natriuretic peptide receptor signaling. J. Clin. Investig. 2003, 111, 1275–1277. [Google Scholar] [CrossRef] [PubMed]
- Holtwick, R.; van Eickels, M.; Skryabin, B.V.; Baba, H.A.; Bubikat, A.; Begrow, F.; Schneider, M.D.; Garbers, D.L.; Kuhn, M. Pressure-independent cardiac hypertrophy in mice with cardiomyocyte-restricted inactivation of the atrial natriuretic peptide receptor guanylyl cyclase-A. J. Clin. Investig. 2003, 111, 1399–1407. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.; Chen, Y.F.; Feng, J.A.; Hayashi, T.; Oparil, S.; Perry, G.J. Volume overload results in exaggerated cardiac hypertrophy in the atrial natriuretic peptide knockout mouse. Cardiovasc. Res. 2004, 61, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Calvieri, C.; Rubattu, S.; Volpe, M. Molecular mechanisms underlying cardiac antihypertrophic and antifibrotic effects of natriuretic peptides. J. Mol. Med. (Berl.) 2012, 90, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Kook, H.; Itoh, H.; Choi, B.S.; Sawada, N.; Doi, K.; Hwang, T.J.; Kim, K.K.; Arai, H.; Baik, Y.H.; Nakao, K. Physiological concentration of atrial natriuretic peptide induces endothelial regeneration in vitro. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1388–H1397. [Google Scholar] [CrossRef] [Green Version]
- Moro, C.; Klimcakova, E.; Lolmede, K.; Berlan, M.; Lafontan, M.; Stich, V.; Bouloumie, A.; Galitzky, J.; Arner, P.; Langin, D. Atrial natriuretic peptide inhibits the production of adipokines and cytokines linked to inflammation and insulin resistance in human subcutaneous adipose tissue. Diabetologia 2007, 50, 1038–1047. [Google Scholar] [CrossRef] [Green Version]
- Alexander, M.R.; Knowles, J.W.; Nishikimi, T.; Maeda, N. Increased atherosclerosis and smooth muscle cell hypertrophy in natriuretic peptide receptor A-/-apolipoprotein E-/- mice. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1077–1082. [Google Scholar] [CrossRef] [PubMed]
- Horio, T.; Tokudome, T.; Maki, T.; Yoshihara, F.; Suga, S.; Nishikimi, T.; Kojima, M.; Kawano, Y.; Kangawa, K. Gene expression, secretion, and autocrine action of C-type natriuretic peptide in cultured adult rat cardiac fibroblasts. Endocrinology 2003, 144, 2279–2284. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.; Knudson, O.; Wu, F.; Morser, J.; Dole, W.P.; Wu, Q. Hypertension in mice lacking the proatrial natriuretic peptide convertase corin. Proc. Natl. Acad. Sci. USA 2005, 102, 785–790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Cao, P.; Dong, N.; Peng, J.; Zhang, C.; Wang, H.; Zhou, T.; Yang, J.; Zhang, Y.; Martelli, E.E.; et al. PCSK6-mediated corin activation is essential for normal blood pressure. Nat. Med. 2015, 21, 1048–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Zhou, T.; Niu, Y.; He, M.; Wang, C.; Liu, M.; Yang, J.; Zhang, Y.; Zhou, J.; Fukuda, K.; et al. Identification and functional analysis of CORIN variants in hypertensive patients. Hum. Mutat. 2017, 38, 1700–1710. [Google Scholar] [CrossRef] [PubMed]
- Rame, J.E.; Drazner, M.H.; Post, W.; Peshock, R.; Lima, J.; Cooper, R.S.; Dries, D.L. Corin I555(P568) allele is associated with enhanced cardiac hypertrophic response to increased systemic afterload. Hypertension 2007, 49, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Rame, J.E.; Tam, S.W.; McNamara, D.; Worcel, M.; Sabolinski, M.L.; Wu, A.H.; Dries, D.L. Dysfunctional corin i555(p568) allele is associated with impaired brain natriuretic peptide processing and adverse outcomes in blacks with systolic heart failure: results from the Genetic Risk Assessment in Heart Failure substudy. Circ. Heart Fail. 2009, 2, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Suga, S.; Nakao, K.; Hosoda, K.; Mukoyama, M.; Ogawa, Y.; Shirakami, G.; Arai, H.; Saito, Y.; Kambayashi, Y.; Inouye, K.; et al. Receptor selectivity of natriuretic peptide family, atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide. Endocrinology 1992, 130, 229–239. [Google Scholar] [CrossRef]
- Vanderheyden, M.; Bartunek, J.; Goethals, M. Brain and other natriuretic peptides: molecular aspects. Eur. J. Heart Fail. 2004, 6, 261–268. [Google Scholar] [CrossRef]
- Zois, N.E.; Bartels, E.D.; Hunter, I.; Kousholt, B.S.; Olsen, L.H.; Goetze, J.P. Natriuretic peptides in cardiometabolic regulation and disease. Nat. Rev. Cardiol. 2014, 11, 403–412. [Google Scholar] [CrossRef]
- Sengenes, C.; Berlan, M.; De Glisezinski, I.; Lafontan, M.; Galitzky, J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J. 2000, 14, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Dessi-Fulgheri, P.; Sarzani, R.; Rappelli, A. Role of the natriuretic peptide system in lipogenesis/lipolysis. Nutr. Metab. Cardiovasc. Dis. 2003, 13, 244–249. [Google Scholar] [CrossRef]
- Bordicchia, M.; Spannella, F.; Ferretti, G.; Bacchetti, T.; Vignini, A.; Di Pentima, C.; Mazzanti, L.; Sarzani, R. PCSK9 is Expressed in Human Visceral Adipose Tissue and Regulated by Insulin and Cardiac Natriuretic Peptides. Int. J. Mol. Sci. 2019, 20, 245. [Google Scholar] [CrossRef] [PubMed]
- Bordicchia, M.; Ceresiani, M.; Pavani, M.; Minardi, D.; Polito, M.; Wabitsch, M.; Cannone, V.; Burnett, J.C., Jr.; Dessi-Fulgheri, P.; Sarzani, R. Insulin/glucose induces natriuretic peptide clearance receptor in human adipocytes: a metabolic link with the cardiac natriuretic pathway. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R104–R114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarzani, R.; Marcucci, P.; Salvi, F.; Bordicchia, M.; Espinosa, E.; Mucci, L.; Lorenzetti, B.; Minardi, D.; Muzzonigro, G.; Dessi-Fulgheri, P.; et al. Angiotensin II stimulates and atrial natriuretic peptide inhibits human visceral adipocyte growth. Int. J. Obes. (Lond.) 2008, 32, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.; Birkenfeld, A.L.; Melander, O.; Moro, C. Natriuretic Peptides in Cardiovascular and Metabolic Crosstalk: Implications for Hypertension Management. Hypertension 2018, 72, 270–276. [Google Scholar] [CrossRef]
- Kuhn, M. Molecular Physiology of Membrane Guanylyl Cyclase Receptors. Physiol. Rev. 2016, 96, 751–804. [Google Scholar] [CrossRef] [PubMed]
- Potter, L.R. Natriuretic peptide metabolism, clearance and degradation. FEBS J. 2011, 278, 1808–1817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubattu, S.; Sciarretta, S.; Morriello, A.; Calvieri, C.; Battistoni, A.; Volpe, M. NPR-C: a component of the natriuretic peptide family with implications in human diseases. J. Mol. Med. (Berl.) 2010, 88, 889–897. [Google Scholar] [CrossRef]
- Hollister, A.S.; Rodeheffer, R.J.; White, F.J.; Potts, J.R.; Imada, T.; Inagami, T. Clearance of atrial natriuretic factor by lung, liver, and kidney in human subjects and the dog. J. Clin. Investig. 1989, 83, 623–628. [Google Scholar] [CrossRef]
- Nakao, K.; Sugawara, A.; Morii, N.; Sakamoto, M.; Yamada, T.; Itoh, H.; Shiono, S.; Saito, Y.; Nishimura, K.; Ban, T.; et al. The pharmacokinetics of alpha-human atrial natriuretic polypeptide in healthy subjects. Eur. J. Clin. Pharmacol. 1986, 31, 101–103. [Google Scholar] [CrossRef] [PubMed]
- Yandle, T.G.; Richards, A.M.; Nicholls, M.G.; Cuneo, R.; Espiner, E.A.; Livesey, J.H. Metabolic clearance rate and plasma half life of alpha-human atrial natriuretic peptide in man. Life Sci. 1986, 38, 1827–1833. [Google Scholar] [CrossRef]
- Mukoyama, M.; Nakao, K.; Hosoda, K.; Suga, S.; Saito, Y.; Ogawa, Y.; Shirakami, G.; Jougasaki, M.; Obata, K.; Yasue, H.; et al. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. J. Clin. Investig. 1991, 87, 1402–1412. [Google Scholar] [CrossRef] [PubMed]
- Holmes, S.J.; Espiner, E.A.; Richards, A.M.; Yandle, T.G.; Frampton, C. Renal, endocrine, and hemodynamic effects of human brain natriuretic peptide in normal man. J. Clin. Endocrinol. Metab. 1993, 76, 91–96. [Google Scholar] [PubMed]
- Hunt, P.J.; Richards, A.M.; Espiner, E.A.; Nicholls, M.G.; Yandle, T.G. Bioactivity and metabolism of C-type natriuretic peptide in normal man. J. Clin. Endocrinol. Metab. 1994, 78, 1428–1435. [Google Scholar] [PubMed]
- Kerr, M.A.; Kenny, A.J. The purification and specificity of a neutral endopeptidase from rabbit kidney brush border. Biochem. J. 1974, 137, 477–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanneste, Y.; Michel, A.; Dimaline, R.; Najdovski, T.; Deschodt-Lanckman, M. Hydrolysis of alpha-human atrial natriuretic peptide in vitro by human kidney membranes and purified endopeptidase-24.11. Evidence for a novel cleavage site. Biochem. J. 1988, 254, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Yandle, T.G.; Brennan, S.O.; Espiner, E.A.; Nicholls, M.G.; Richards, A.M. Endopeptidase-24.11 in human plasma degrades atrial natriuretic factor (ANF) to ANF(99-105/106-126). Peptides 1989, 10, 891–894. [Google Scholar] [CrossRef]
- Bayes-Genis, A. Neprilysin in Heart Failure: From Oblivion to Center Stage. JACC Heart Fail. 2015, 3, 637–640. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Nakao, K.; Hama, N.; Imura, H.; Mori, S.; Yamaguchi, M.; Yasuhara, M.; Hori, R. Clearance mechanisms of atrial and brain natriuretic peptides in rats. Pharm. Res. 1994, 11, 60–64. [Google Scholar] [CrossRef]
- Volpe, M.; Rubattu, S.; Battistoni, A. ARNi: A Novel Approach to Counteract Cardiovascular Diseases. Int. J. Mol. Sci. 2019, 20, 2092. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, U.M.; Gong, J.; Jhund, P.S.; Shen, L.; Kober, L.; Desai, A.S.; Lefkowitz, M.P.; Packer, M.; Rouleau, J.L.; Solomon, S.D.; et al. Effect of sacubitril/valsartan on recurrent events in the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur. J. Heart Fail. 2018, 20, 760–768. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Hanze, J.; Heese, F.; Sodmann, R.; Lang, R.E. Gene expression of natriuretic peptide receptors in myocardial cells. Circ. Res. 1995, 77, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kuc, R.E.; Maguire, J.J.; Fidock, M.; Davenport, A.P. Novel snake venom ligand dendroaspis natriuretic peptide is selective for natriuretic peptide receptor-A in human heart: downregulation of natriuretic peptide receptor-A in heart failure. Circ. Res. 2006, 99, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Horio, T.; Nishikimi, T.; Yoshihara, F.; Matsuo, H.; Takishita, S.; Kangawa, K. Inhibitory regulation of hypertrophy by endogenous atrial natriuretic peptide in cultured cardiac myocytes. Hypertension 2000, 35, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.F.; Bishopric, N.H.; Pratt, R.E. Atrial natriuretic peptide induces apoptosis in neonatal rat cardiac myocytes. J. Biol. Chem. 1997, 272, 14860–14866. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Gardner, D.G. Natriuretic peptides inhibit DNA synthesis in cardiac fibroblasts. Hypertension 1995, 25, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Z.; Chen, S.J.; Majid-Hasan, E.; Oparil, S.; Chen, Y.F. Dietary salt supplementation selectively downregulates NPR-C receptor expression in kidney independently of ANP. Am. J. Physiol. Renal. Physiol. 2002, 282, F220–F227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klinger, J.R.; Warburton, R.R.; Pietras, L.A.; Smithies, O.; Swift, R.; Hill, N.S. Genetic disruption of atrial natriuretic peptide causes pulmonary hypertension in normoxic and hypoxic mice. Am. J. Physiol. 1999, 276, L868–L874. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.Z.; Chen, S.J.; Li, G.; Chen, Y.F. Hypoxia reduces atrial natriuretic peptide clearance receptor gene expression in ANP knockout mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 279, L511–L519. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.A.; Perry, G.; Mori, T.; Hayashi, T.; Oparil, S.; Chen, Y.F. Pressure-independent enhancement of cardiac hypertrophy in atrial natriuretic peptide-deficient mice. Clin. Exp. Pharmacol. Physiol. 2003, 30, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Kasama, S.; Furuya, M.; Toyama, T.; Ichikawa, S.; Kurabayashi, M. Effect of atrial natriuretic peptide on left ventricular remodelling in patients with acute myocardial infarction. Eur. Heart J. 2008, 29, 1485–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinoshita, H.; Kuwahara, K.; Nishida, M.; Jian, Z.; Rong, X.; Kiyonaka, S.; Kuwabara, Y.; Kurose, H.; Inoue, R.; Mori, Y.; et al. Inhibition of TRPC6 channel activity contributes to the antihypertrophic effects of natriuretic peptides-guanylyl cyclase-A signaling in the heart. Circ. Res. 2010, 106, 1849–1860. [Google Scholar] [CrossRef] [PubMed]
- Oliver, P.M.; Fox, J.E.; Kim, R.; Rockman, H.A.; Kim, H.S.; Reddick, R.L.; Pandey, K.N.; Milgram, S.L.; Smithies, O.; Maeda, N. Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc. Natl. Acad. Sci. USA 1997, 94, 14730–14735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knowles, J.W.; Esposito, G.; Mao, L.; Hagaman, J.R.; Fox, J.E.; Smithies, O.; Rockman, H.A.; Maeda, N. Pressure-independent enhancement of cardiac hypertrophy in natriuretic peptide receptor A-deficient mice. J. Clin. Investig. 2001, 107, 975–984. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, I.; Rossi, K.; Garbers, D.L. A genetic model provides evidence that the receptor for atrial natriuretic peptide (guanylyl cyclase-A) inhibits cardiac ventricular myocyte hypertrophy. Proc. Natl. Acad. Sci. USA 2001, 98, 2703–2706. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, D.; Kudoh, S.; Shiojima, I.; Zou, Y.; Harada, K.; Shimoyama, M.; Imai, Y.; Monzen, K.; Yamazaki, T.; Yazaki, Y.; et al. Atrial natriuretic peptide inhibits cardiomyocyte hypertrophy through mitogen-activated protein kinase phosphatase-1. Biochem. Biophys. Res. Commun. 2004, 322, 310–319. [Google Scholar] [CrossRef]
- Nemoto, S.; Sheng, Z.; Lin, A. Opposing effects of Jun kinase and p38 mitogen-activated protein kinases on cardiomyocyte hypertrophy. Mol. Cell Biol. 1998, 18, 3518–3526. [Google Scholar] [CrossRef]
- Calderone, A.; Thaik, C.M.; Takahashi, N.; Chang, D.L.; Colucci, W.S. Nitric oxide, atrial natriuretic peptide, and cyclic GMP inhibit the growth-promoting effects of norepinephrine in cardiac myocytes and fibroblasts. J. Clin. Investig. 1998, 101, 812–818. [Google Scholar] [CrossRef]
- Tokudome, T.; Horio, T.; Kishimoto, I.; Soeki, T.; Mori, K.; Kawano, Y.; Kohno, M.; Garbers, D.L.; Nakao, K.; Kangawa, K. Calcineurin-nuclear factor of activated T cells pathway-dependent cardiac remodeling in mice deficient in guanylyl cyclase A, a receptor for atrial and brain natriuretic peptides. Circulation 2005, 111, 3095–3104. [Google Scholar] [CrossRef]
- Laskowski, A.; Woodman, O.L.; Cao, A.H.; Drummond, G.R.; Marshall, T.; Kaye, D.M.; Ritchie, R.H. Antioxidant actions contribute to the antihypertrophic effects of atrial natriuretic peptide in neonatal rat cardiomyocytes. Cardiovasc. Res. 2006, 72, 112–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubattu, S.; Bigatti, G.; Evangelista, A.; Lanzani, C.; Stanzione, R.; Zagato, L.; Manunta, P.; Marchitti, S.; Venturelli, V.; Bianchi, G.; et al. Association of atrial natriuretic peptide and type a natriuretic peptide receptor gene polymorphisms with left ventricular mass in human essential hypertension. J. Am. Coll. Cardiol. 2006, 48, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, T.; Soma, M.; Takahashi, Y.; Rehemudula, D.; Kanmatsuse, K.; Furuya, K. Functional deletion mutation of the 5’-flanking region of type A human natriuretic peptide receptor gene and its association with essential hypertension and left ventricular hypertrophy in the Japanese. Circ. Res. 2000, 86, 841–845. [Google Scholar] [CrossRef] [PubMed]
- Bartels, E.D.; Nielsen, J.M.; Bisgaard, L.S.; Goetze, J.P.; Nielsen, L.B. Decreased expression of natriuretic peptides associated with lipid accumulation in cardiac ventricle of obese mice. Endocrinology 2010, 151, 5218–5225. [Google Scholar] [CrossRef] [PubMed]
- Rubattu, S.; Sciarretta, S.; Ciavarella, G.M.; Venturelli, V.; De Paolis, P.; Tocci, G.; De Biase, L.; Ferrucci, A.; Volpe, M. Reduced levels of N-terminal-proatrial natriuretic peptide in hypertensive patients with metabolic syndrome and their relationship with left ventricular mass. J. Hypertens. 2007, 25, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Cuspidi, C.; Meani, S.; Fusi, V.; Severgnini, B.; Valerio, C.; Catini, E.; Leonetti, G.; Magrini, F.; Zanchetti, A. Metabolic syndrome and target organ damage in untreated essential hypertensives. J. Hypertens. 2004, 22, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Sangawa, K.; Nakanishi, K.; Ishino, K.; Inoue, M.; Kawada, M.; Sano, S. Atrial natriuretic peptide protects against ischemia-reperfusion injury in the isolated rat heart. Ann. Thorac. Surg. 2004, 77, 233–237. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.M.; Philipp, S.; Downey, J.M.; Cohen, M.V. Atrial natriuretic peptide administered just prior to reperfusion limits infarction in rabbit hearts. Basic Res. Cardiol. 2006, 101, 311–318. [Google Scholar] [CrossRef]
- Okawa, H.; Horimoto, H.; Mieno, S.; Nomura, Y.; Yoshida, M.; Shinjiro, S. Preischemic infusion of alpha-human atrial natriuretic peptide elicits myoprotective effects against ischemia reperfusion in isolated rat hearts. Mol. Cell. Biochem. 2003, 248, 171–177. [Google Scholar] [CrossRef]
- Takata, Y.; Hirayama, Y.; Kiyomi, S.; Ogawa, T.; Iga, K.; Ishii, T.; Nagai, Y.; Ibukiyama, C. The beneficial effects of atrial natriuretic peptide on arrhythmias and myocardial high-energy phosphates after reperfusion. Cardiovasc. Res. 1996, 32, 286–293. [Google Scholar] [CrossRef] [Green Version]
- Wakui, S.; Sezai, A.; Tenderich, G.; Hata, M.; Osaka, S.; Taniguchi, Y.; Koerfer, R.; Minami, K. Experimental investigation of direct myocardial protective effect of atrial natriuretic peptide in cardiac surgery. J. Thorac. Cardiovasc. Surg. 2010, 139, 918–925. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Saiki, Y.; Horii, A.; Fukushige, S.; Kawamoto, S.; Adachi, O.; Akiyama, M.; Ito, K.; Masaki, N.; Saiki, Y. Atrial natriuretic peptide induces peroxisome proliferator activated receptor gamma during cardiac ischemia-reperfusion in swine heart. Gen. Thorac Cardiovasc. Surg. 2017, 65, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, M.; Sivarajah, A.; Thiemermann, C. Beneficial effects of PPAR-gamma ligands in ischemia-reperfusion injury, inflammation and shock. Cardiovasc. Res. 2005, 65, 772–781. [Google Scholar] [CrossRef] [PubMed]
- Charan, K.; Goyal, A.; Gupta, J.K.; Yadav, H.N. Role of atrial natriuretic peptide in ischemic preconditioning-induced cardioprotection in the diabetic rat heart. J. Surg. Res. 2016, 201, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, M.; Saito, Y.; Kishimoto, I.; Harada, M.; Kuwahara, K.; Takahashi, N.; Kawakami, R.; Nakagawa, Y.; Tanimoto, K.; Yasuno, S.; et al. Role of natriuretic peptide receptor guanylyl cyclase-A in myocardial infarction evaluated using genetically engineered mice. Hypertension 2005, 46, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Tsutamoto, T.; Wada, A.; Maeda, K.; Mabuchi, N.; Tsutsui, T.; Horie, H.; Ohnishi, M.; Kinoshita, M. Intravenous atrial natriuretic peptide prevents left ventricular remodeling in patients with first anterior acute myocardial infarction. J. Am. Coll. Cardiol. 2001, 37, 1820–1826. [Google Scholar] [CrossRef] [Green Version]
- Kuga, H.; Ogawa, K.; Oida, A.; Taguchi, I.; Nakatsugawa, M.; Hoshi, T.; Sugimura, H.; Abe, S.; Kaneko, N. Administration of atrial natriuretic peptide attenuates reperfusion phenomena and preserves left ventricular regional wall motion after direct coronary angioplasty for acute myocardial infarction. Circ. J. 2003, 67, 443–448. [Google Scholar] [CrossRef]
- Kasama, S.; Toyama, T.; Hatori, T.; Sumino, H.; Kumakura, H.; Takayama, Y.; Ichikawa, S.; Suzuki, T.; Kurabayashi, M. Effects of intravenous atrial natriuretic peptide on cardiac sympathetic nerve activity and left ventricular remodeling in patients with first anterior acute myocardial infarction. J. Am. Coll. Cardiol. 2007, 49, 667–674. [Google Scholar] [CrossRef]
- Kitakaze, M.; Asakura, M.; Kim, J.; Shintani, Y.; Asanuma, H.; Hamasaki, T.; Seguchi, O.; Myoishi, M.; Minamino, T.; Ohara, T.; et al. Human atrial natriuretic peptide and nicorandil as adjuncts to reperfusion treatment for acute myocardial infarction (J-WIND): two randomised trials. Lancet 2007, 370, 1483–1493. [Google Scholar] [CrossRef]
- Brandt, R.R.; Heublein, D.M.; Mattingly, M.T.; Pittelkow, M.R.; Burnett, J.C., Jr. Presence and secretion of atrial natriuretic peptide from cultured human aortic endothelial cells. Am. J. Physiol. 1995, 268, H921–H925. [Google Scholar] [CrossRef]
- Kuhn, M.; Volker, K.; Schwarz, K.; Carbajo-Lozoya, J.; Flogel, U.; Jacoby, C.; Stypmann, J.; van Eickels, M.; Gambaryan, S.; Hartmann, M.; et al. The natriuretic peptide/guanylyl cyclase—A system functions as a stress-responsive regulator of angiogenesis in mice. J. Clin. Investig. 2009, 119, 2019–2030. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, S.; Marchitti, S.; Bianchi, F.; Moyes, A.; Barbato, E.; Di Castro, S.; Stanzione, R.; Cotugno, M.; Castello, L.; Calvieri, C.; et al. C2238 atrial natriuretic peptide molecular variant is associated with endothelial damage and dysfunction through natriuretic peptide receptor C signaling. Circ. Res. 2013, 112, 1355–1364. [Google Scholar] [CrossRef]
- Barbato, E.; Bartunek, J.; Mangiacapra, F.; Sciarretta, S.; Stanzione, R.; Delrue, L.; Cotugno, M.; Marchitti, S.; Iaccarino, G.; Sirico, G.; et al. Influence of rs5065 atrial natriuretic peptide gene variant on coronary artery disease. J. Am. Coll. Cardiol. 2012, 59, 1763–1770. [Google Scholar] [CrossRef] [PubMed]
- Scarpino, S.; Marchitti, S.; Stanzione, R.; Evangelista, A.; Di Castro, S.; Savoia, C.; Quarta, G.; Sciarretta, S.; Ruco, L.; Volpe, M.; et al. Reactive oxygen species-mediated effects on vascular remodeling induced by human atrial natriuretic peptide T2238C molecular variant in endothelial cells in vitro. J. Hypertens. 2009, 27, 1804–1813. [Google Scholar] [CrossRef] [PubMed]
- Rubattu, S.; Sciarretta, S.; Marchitti, S.; Bianchi, F.; Forte, M.; Volpe, M. The T2238C Human Atrial Natriuretic Peptide Molecular Variant and the Risk of Cardiovascular Diseases. Int. J. Mol. Sci. 2018, 19, 540. [Google Scholar] [CrossRef] [PubMed]
- Kiemer, A.K.; Weber, N.C.; Vollmar, A.M. Induction of IkappaB: atrial natriuretic peptide as a regulator of the NF-kappaB pathway. Biochem. Biophys. Res. Commun. 2002, 295, 1068–1076. [Google Scholar] [CrossRef]
- Kiemer, A.K.; Lehner, M.D.; Hartung, T.; Vollmar, A.M. Inhibition of cyclooxygenase-2 by natriuretic peptides. Endocrinology 2002, 143, 846–852. [Google Scholar] [CrossRef]
- Kiemer, A.K.; Vollmar, A.M. Autocrine regulation of inducible nitric-oxide synthase in macrophages by atrial natriuretic peptide. J. Biol. Chem. 1998, 273, 13444–13451. [Google Scholar] [CrossRef]
- Mtairag, E.M.; Houard, X.; Rais, S.; Pasquier, C.; Oudghiri, M.; Jacob, M.P.; Meilhac, O.; Michel, J.B. Pharmacological potentiation of natriuretic peptide limits polymorphonuclear neutrophil-vascular cell interactions. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1824–1831. [Google Scholar] [CrossRef]
- Kuhn, M. Endothelial actions of atrial and B-type natriuretic peptides. Br. J. Pharmacol. 2012, 166, 522–531. [Google Scholar] [CrossRef]
- Tamura, N.; Ogawa, Y.; Chusho, H.; Nakamura, K.; Nakao, K.; Suda, M.; Kasahara, M.; Hashimoto, R.; Katsuura, G.; Mukoyama, M.; et al. Cardiac fibrosis in mice lacking brain natriuretic peptide. Proc. Natl. Acad. Sci. USA 2000, 97, 4239–4244. [Google Scholar] [CrossRef] [Green Version]
- Kapoun, A.M.; Liang, F.; O’Young, G.; Damm, D.L.; Quon, D.; White, R.T.; Munson, K.; Lam, A.; Schreiner, G.F.; Protter, A.A. B-type natriuretic peptide exerts broad functional opposition to transforming growth factor-beta in primary human cardiac fibroblasts: fibrosis, myofibroblast conversion, proliferation, and inflammation. Circ. Res. 2004, 94, 453–461. [Google Scholar] [CrossRef]
- Soeki, T.; Kishimoto, I.; Okumura, H.; Tokudome, T.; Horio, T.; Mori, K.; Kangawa, K. C-type natriuretic peptide, a novel antifibrotic and antihypertrophic agent, prevents cardiac remodeling after myocardial infarction. J. Am. Coll. Cardiol. 2005, 45, 608–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; de Waard, M.C.; Sterner-Kock, A.; Stepan, H.; Schultheiss, H.P.; Duncker, D.J.; Walther, T. Cardiomyocyte-restricted over-expression of C-type natriuretic peptide prevents cardiac hypertrophy induced by myocardial infarction in mice. Eur. J. Heart Fail. 2007, 9, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Langenickel, T.H.; Buttgereit, J.; Pagel-Langenickel, I.; Lindner, M.; Monti, J.; Beuerlein, K.; Al-Saadi, N.; Plehm, R.; Popova, E.; Tank, J.; et al. Cardiac hypertrophy in transgenic rats expressing a dominant-negative mutant of the natriuretic peptide receptor B. Proc. Natl. Acad. Sci. USA 2006, 103, 4735–4740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izumiya, Y.; Araki, S.; Usuku, H.; Rokutanda, T.; Hanatani, S.; Ogawa, H. Chronic C-Type Natriuretic Peptide Infusion Attenuates Angiotensin II-Induced Myocardial Superoxide Production and Cardiac Remodeling. Int. J. Vasc. Med. 2012, 2012, 246058. [Google Scholar] [CrossRef] [PubMed]
- Nakao, K.; Kuwahara, K.; Nishikimi, T.; Nakagawa, Y.; Kinoshita, H.; Minami, T.; Kuwabara, Y.; Yamada, C.; Yamada, Y.; Tokudome, T.; et al. Endothelium-Derived C-Type Natriuretic Peptide Contributes to Blood Pressure Regulation by Maintaining Endothelial Integrity. Hypertension 2017, 69, 286–296. [Google Scholar] [CrossRef]
- Moyes, A.J.; Khambata, R.S.; Villar, I.; Bubb, K.J.; Baliga, R.S.; Lumsden, N.G.; Xiao, F.; Gane, P.J.; Rebstock, A.S.; Worthington, R.J.; et al. Endothelial C-type natriuretic peptide maintains vascular homeostasis. J. Clin. Investig. 2014, 124, 4039–4051. [Google Scholar] [CrossRef]
- Spiranec, K.; Chen, W.; Werner, F.; Nikolaev, V.O.; Naruke, T.; Koch, F.; Werner, A.; Eder-Negrin, P.; Dieguez-Hurtado, R.; Adams, R.H.; et al. Endothelial C-Type Natriuretic Peptide Acts on Pericytes to Regulate Microcirculatory Flow and Blood Pressure. Circulation 2018, 138, 494–508. [Google Scholar] [CrossRef]
- Honing, M.L.; Smits, P.; Morrison, P.J.; Burnett, J.C., Jr.; Rabelink, T.J. C-type natriuretic peptide-induced vasodilation is dependent on hyperpolarization in human forearm resistance vessels. Hypertension 2001, 37, 1179–1183. [Google Scholar] [CrossRef]
- Hutchinson, H.G.; Trindade, P.T.; Cunanan, D.B.; Wu, C.F.; Pratt, R.E. Mechanisms of natriuretic-peptide-induced growth inhibition of vascular smooth muscle cells. Cardiovasc. Res. 1997, 35, 158–167. [Google Scholar] [CrossRef]
- Furuya, M.; Miyazaki, T.; Honbou, N.; Kawashima, K.; Ohno, T.; Tanaka, S.; Kangawa, K.; Matsuo, H. C-type natriuretic peptide inhibits intimal thickening after vascular injury. Ann. N. Y. Acad. Sci. 1995, 748, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.Y.; Haruno, A.; Asada, Y.; Nishida, T.; Saito, Y.; Matsuda, T.; Ueno, H. Local expression of C-type natriuretic peptide suppresses inflammation, eliminates shear stress-induced thrombosis, and prevents neointima formation through enhanced nitric oxide production in rabbit injured carotid arteries. Circ. Res. 2002, 91, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Cody, R.J.; Atlas, S.A.; Laragh, J.H.; Kubo, S.H.; Covit, A.B.; Ryman, K.S.; Shaknovich, A.; Pondolfino, K.; Clark, M.; Camargo, M.J.; et al. Atrial natriuretic factor in normal subjects and heart failure patients. Plasma levels and renal, hormonal, and hemodynamic responses to peptide infusion. J. Clin. Investig. 1986, 78, 1362–1374. [Google Scholar] [CrossRef] [PubMed]
- Scriven, T.A.; Burnett, J.C., Jr. Effects of synthetic atrial natriuretic peptide on renal function and renin release in acute experimental heart failure. Circulation 1985, 72, 892–897. [Google Scholar] [CrossRef] [PubMed]
- Wambach, G.; Schittenhelm, U.; Bonner, G.; Kaufmann, W. Renal and adrenal resistance against atrial natriuretic peptide in congestive heart failure: effect of angiotensin I-converting-enzyme inhibition. Cardiology 1989, 76, 418–427. [Google Scholar] [CrossRef] [PubMed]
- Riegger, G.A.; Elsner, D.; Kromer, E.P.; Daffner, C.; Forssmann, W.G.; Muders, F.; Pascher, E.W.; Kochsiek, K. Atrial natriuretic peptide in congestive heart failure in the dog: plasma levels, cyclic guanosine monophosphate, ultrastructure of atrial myoendocrine cells, and hemodynamic, hormonal, and renal effects. Circulation 1988, 77, 398–406. [Google Scholar] [CrossRef]
- Clerico, A.; Recchia, F.A.; Passino, C.; Emdin, M. Cardiac endocrine function is an essential component of the homeostatic regulation network: physiological and clinical implications. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H17–H29. [Google Scholar] [CrossRef] [Green Version]
- Charloux, A.; Piquard, F.; Doutreleau, S.; Brandenberger, G.; Geny, B. Mechanisms of renal hyporesponsiveness to ANP in heart failure. Eur. J. Clin. Investig. 2003, 33, 769–778. [Google Scholar] [CrossRef]
- Goetze, J.P.; Kastrup, J.; Rehfeld, J.F. The paradox of increased natriuretic hormones in congestive heart failure patients: does the endocrine heart also fail in heart failure? Eur. Heart J. 2003, 24, 1471–1472. [Google Scholar] [CrossRef]
- Rubattu, S.; Calvieri, C.; Pagliaro, B.; Volpe, M. Atrial natriuretic peptide and regulation of vascular function in hypertension and heart failure: implications for novel therapeutic strategies. J. Hypertens. 2013, 31, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Colucci, W.S.; Elkayam, U.; Horton, D.P.; Abraham, W.T.; Bourge, R.C.; Johnson, A.D.; Wagoner, L.E.; Givertz, M.M.; Liang, C.S.; Neibaur, M.; et al. Intravenous nesiritide, a natriuretic peptide, in the treatment of decompensated congestive heart failure. Nesiritide Study Group. N. Engl. J. Med. 2000, 343, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Publication Committee for the VMAC Investigators (Vasodilatation in the Management of Acute CHF). Intravenous nesiritide vs nitroglycerin for treatment of decompensated congestive heart failure: a randomized controlled trial. JAMA 2002, 287, 1531–1540. [Google Scholar] [CrossRef]
- O’Connor, C.M.; Starling, R.C.; Hernandez, A.F.; Armstrong, P.W.; Dickstein, K.; Hasselblad, V.; Heizer, G.M.; Komajda, M.; Massie, B.M.; McMurray, J.J.; et al. Effect of nesiritide in patients with acute decompensated heart failure. N. Engl. J. Med. 2011, 365, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Sackner-Bernstein, J.D.; Skopicki, H.A.; Aaronson, K.D. Risk of worsening renal function with nesiritide in patients with acutely decompensated heart failure. Circulation 2005, 111, 1487–1491. [Google Scholar] [CrossRef] [PubMed]
- Sackner-Bernstein, J.D.; Kowalski, M.; Fox, M.; Aaronson, K. Short-term risk of death after treatment with nesiritide for decompensated heart failure: a pooled analysis of randomized controlled trials. JAMA 2005, 293, 1900–1905. [Google Scholar] [CrossRef] [PubMed]
- McMurray, J.J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Committees, Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; McMurray, J.J.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Coordinators, Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015, 131, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Vodovar, N.; Seronde, M.F.; Laribi, S.; Gayat, E.; Lassus, J.; Januzzi, J.L., Jr.; Boukef, R.; Nouira, S.; Manivet, P.; Samuel, J.L.; et al. Elevated Plasma B-Type Natriuretic Peptide Concentrations Directly Inhibit Circulating Neprilysin Activity in Heart Failure. JACC Heart Fail. 2015, 3, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Januzzi, J.L.; Butler, J.; Fombu, E.; Maisel, A.; McCague, K.; Pina, I.L.; Prescott, M.F.; Riebman, J.B.; Solomon, S. Rationale and methods of the Prospective Study of Biomarkers, Symptom Improvement, and Ventricular Remodeling During Sacubitril/Valsartan Therapy for Heart Failure (PROVE-HF). Am. Heart J. 2018, 199, 130–136. [Google Scholar] [CrossRef]
- Ishii, M.; Kaikita, K.; Sato, K.; Sueta, D.; Fujisue, K.; Arima, Y.; Oimatsu, Y.; Mitsuse, T.; Onoue, Y.; Araki, S.; et al. Cardioprotective Effects of LCZ696 (Sacubitril/Valsartan) After Experimental Acute Myocardial Infarction. JACC Basic Transl. Sci. 2017, 2, 655–668. [Google Scholar] [CrossRef]
- Torrado, J.; Cain, C.; Mauro, A.G.; Romeo, F.; Ockaili, R.; Chau, V.Q.; Nestler, J.A.; Devarakonda, T.; Ghosh, S.; Das, A.; et al. Sacubitril/Valsartan Averts Adverse Post-Infarction Ventricular Remodeling and Preserves Systolic Function in Rabbits. J. Am. Coll. Cardiol. 2018, 72, 2342–2356. [Google Scholar] [CrossRef] [PubMed]
- Pfau, D.; Thorn, S.L.; Zhang, J.; Mikush, N.; Renaud, J.M.; Klein, R.; deKemp, R.A.; Wu, X.; Hu, X.; Sinusas, A.J.; et al. Angiotensin Receptor Neprilysin Inhibitor Attenuates Myocardial Remodeling and Improves Infarct Perfusion in Experimental Heart Failure. Sci. Rep. 2019, 9, 5791. [Google Scholar] [CrossRef] [PubMed]
- Rouleau, J.L.; Pfeffer, M.A.; Stewart, D.J.; Isaac, D.; Sestier, F.; Kerut, E.K.; Porter, C.B.; Proulx, G.; Qian, C.; Block, A.J. Comparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trial. Lancet 2000, 356, 615–620. [Google Scholar] [CrossRef]
- Kostis, J.B.; Packer, M.; Black, H.R.; Schmieder, R.; Henry, D.; Levy, E. Omapatrilat and enalapril in patients with hypertension: The Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am. J. Hypertens. 2004, 17, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Califf, R.M.; Konstam, M.A.; Krum, H.; McMurray, J.J.; Rouleau, J.L.; Swedberg, K. Comparison of omapatrilat and enalapril in patients with chronic heart failure: The Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation 2002, 106, 920–926. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forte, M.; Madonna, M.; Schiavon, S.; Valenti, V.; Versaci, F.; Biondi Zoccai, G.; Frati, G.; Sciarretta, S. Cardiovascular Pleiotropic Effects of Natriuretic Peptides. Int. J. Mol. Sci. 2019, 20, 3874. https://doi.org/10.3390/ijms20163874
Forte M, Madonna M, Schiavon S, Valenti V, Versaci F, Biondi Zoccai G, Frati G, Sciarretta S. Cardiovascular Pleiotropic Effects of Natriuretic Peptides. International Journal of Molecular Sciences. 2019; 20(16):3874. https://doi.org/10.3390/ijms20163874
Chicago/Turabian StyleForte, Maurizio, Michele Madonna, Sonia Schiavon, Valentina Valenti, Francesco Versaci, Giuseppe Biondi Zoccai, Giacomo Frati, and Sebastiano Sciarretta. 2019. "Cardiovascular Pleiotropic Effects of Natriuretic Peptides" International Journal of Molecular Sciences 20, no. 16: 3874. https://doi.org/10.3390/ijms20163874