The Biological and Clinical Relevance of G Protein-Coupled Receptors to the Outcomes of Hematopoietic Stem Cell Transplantation: A Systematized Review
Abstract
:1. Introduction
1.1. Hematopoietic Stem Cell Transplantation (HSCT)
1.2. G Protein-Coupled Receptors (GPCRs)
1.3. HSCT and GPCR: Plerixafor and Beyond
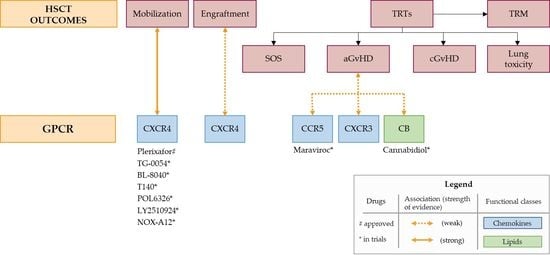
2. Results and Discussion
2.1. Mobilization
2.2. Engraftment
2.3. Sinusoidal Obstruction Syndrome (SOS)
2.4. Graft-Versus-Host Disease (GvHD)
2.4.1. Acute GvHD
2.4.2. Chronic GvHD
2.5. Lung Toxicity
2.6. Treatment-Related Mortality (TRM)
3. Methods
3.1. Systematized Search
3.2. Reporting of the Results
4. Conclusions and Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| aGvHD | acute graft-versus-host disease |
| cGvHD | chronic graft-versus-host disease |
| aPC | activated protein C |
| AT1R | angiotensin 1 receptor |
| AR | adrenergic receptor |
| BM | bone marrow |
| BMP | bone morphogenetic protein |
| CAR | chimeric antigen receptor |
| CB | cannabinoid receptor |
| DIC | disseminated intravascular coagulation |
| DKT | Dai-kenchu-to |
| ECS | endocannabinoid system |
| G-CSF | granulocyte colony-stimulating factor |
| GTEx | Genotype-Tissue expression |
| GF | graft failure |
| GPCR | G protein-coupled receptor |
| GRK | GPCR-related kinases |
| GvL | graft-versus-leukemia |
| HPA | Human Protein Atlas |
| HSC | hematopoietic stem cell |
| HSCT | hematopoietic stem cell transplantation |
| IPS | idiopathic pneumonia syndrome |
| MCT | Monocrotaline |
| MM | multiple myeloma |
| NHL | non-Hodgkin lymphoma |
| NRM | non-relapse mortality |
| P2Y2 | P2Y purinoreceptor 2 |
| PAF | platelet-activating factor |
| PAR | protease-activated receptor |
| PB | peripheral blood |
| PBSC | peripheral blood stem cell |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RANTES | Regulated on Activation, Normal T Cell Expressed and Secreted (CCL5) |
| 7TMR | seven trans-membrane spanning receptor |
| SCFA | short-chain fatty acid |
| SMO | Smoothened |
| SOS | sinusoidal obstruction syndrome |
| SPM | specialized pro-resolving mediators |
| THC | tetrahydrocannabinol |
| TM(E5) | thrombomodulin (fifth epidermal growth factor-like region) |
| Tregs | regulatory T cells |
| TRM | transplantation- or treatment-related mortality |
| TRTs | treatment-related toxicities |
| VOD | veno-occlusive disease (the term formerly used for SOS) |
Appendix A. Methodology
Appendix A1. Administrative Information
Appendix A2. Rationale and Objectives
Appendix A3. Eligibility/Exclusion Criteria
Appendix A3.1. Study Designs
Appendix A3.2. Interventions/Observations
- any association between the expression of a GPCR, a GPCR ligand, or a related protein (e.g. GPCR kinase, beta-arrestins) and one of the selected outcomes (see Appendix A8) of autologous and/or allogeneic HSCT;
- any intervention on a GPCR, a GPCR ligand, or a related protein to change one of the selected outcomes of autologous/allogeneic HSCT.
Appendix A4. Information Sources and Search Strategy
- Cochrane (www.cochranelibrary.com/), Prospero (www.crd.york.ac.uk/prospero/) and Epistomonikos (www.epistemonikos.org/): queried using Medical Subject Headings (MeSH®) descriptors (explode all trees) or free text terms, to probe for already existing systematic reviews.
- MEDLINE (Pubmed interface, 1966 onwards; www.ncbi.nlm.nih.gov/pubmed/) database: queried using either MeSH® or free text terms.
- EMBASE (Elsevier interface, 1947 onwards; www.embase.com/) database: queried using either Embase Subject Headings (Emtree ®) or free text terms.
| Key Concepts | G Protein-Coupled Receptor | Hematopoietic Stem Cell Transplantation |
|---|---|---|
| Free text terms | “G protein-coupled receptor”[Title/Abstract] OR “GPCR” [Title/Abstract] OR “G protein coupled receptor” [Title/Abstract] OR “chemokine” [Text Word] | “Hematopoietic stem cell transplantation” [Title/Abstract] OR “HSCT” [Title/Abstract] OR “haematopoietic stem cell therapy” [Title/Abstract] OR “haematopoietic stem cell transplantation” [Title/Abstract] OR “hematopoietic stem cell (hsc) transplantation” [Title/Abstract] OR “hematopoietic stem cell therapy” [Title/Abstract] OR “hsc therapy” [Title/Abstract] OR “hsc transplantation” [Title/Abstract] OR “Hematopoietic cell transplantation” [Title/Abstract] |
| MeSH terms | “Receptors, G-protein-coupled”[MeSH Terms] OR “beta-Arrestins”[Mesh Terms] OR “G-Protein-Coupled Receptor Kinases”[Mesh Terms] OR “Receptors, Thyrotropin”[Mesh Terms] OR “Receptors, Thyrotropin-Releasing Hormone”[Mesh Terms] | (“Hematopoietic Stem Cell Transplantation”[Mesh Terms] OR “Bone Marrow Transplantation”[Mesh Terms] OR “Hematopoietic Stem Cell Mobilization”[Mesh Terms] OR “Transplantation Conditioning”[Mesh Terms] OR “Cord Blood Stem Cell Transplantation”[Mesh Terms] |
| Others | “MSH receptor” [Supplementary Concept]NOT “editorial”[Publication Type] NOT “review”[Publication Type] AND “english”[Language] |
Appendix A5. Data Management
Appendix A6. Selection Process
Appendix A6.1. De-Duplications
Appendix A6.2. Screening
Appendix A7. Data Collection Process
- General information: Article ID (1st author’s name, 2nd author’s name if ambiguity), year of publication, PDF retrievability;
- Study types (if applicable): animal, human, observational, interventional, prospective/retrospective, gene manipulation;
- Intervention (if applicable): drug used, drug type, mode of action, polymorphism;
- Outcome, effect of GPCR, and direction of the effect: mobilization, engraftment, VOD, acute GvHD, chronic GvHD, lung toxicity, treatment-related mortality.
Appendix A8. Outcomes and Measurement
- -
- Stem cell mobilization in donors (allogeneic) or hosts (autologous), as measured using circulating CD34+ (HSC) and/or nucleated blood cells harvested through leukapheresis.
- -
- Engraftment (neutrophil and/or platelet recovery, lab diagnosis). Better engraftment was measured by shorter recovery times or lower rates of graft failure.
- -
- Hepatic sinusoidal obstruction syndrome (SOS), formerly known as hepatic veno-occlusive disease (VOD) or liver inflammation (clinical diagnosis).
- -
- Acute graft-versus-host disease (aGvHD) (clinical diagnosis).
- -
- Chronic graft-versus-host disease (cGvHD) (clinical diagnosis).
- -
- Treatment-related mortality.
Appendix A9. Risk of Bias
Appendix A10. Data Synthesis
Appendix A11. Meta-Biases and Cumulative Evidence
References
- Thomas, E.D.; Lochte, H.L., Jr.; Lu, W.C.; Ferrebee, J.W. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N. Engl. J. Med. 1957, 257, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Gratwohl, A. Hematopoietic Stem Cell TransplantationA Global Perspective. JAMA 2010, 303, 1617–1624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gratwohl, A.; Pasquini, M.C.; Aljurf, M.; Atsuta, Y.; Baldomero, H.; Foeken, L.; Gratwohl, M.; Bouzas, L.F.; Confer, D.; Frauendorfer, K.; et al. One million haemopoietic stem-cell transplants: A retrospective observational study. Lancet Haematol. 2015, 2, e91–e100. [Google Scholar] [CrossRef]
- Korbling, M.; Freireich, E.J. Twenty-five years of peripheral blood stem cell transplantation. Blood 2011, 117, 6411–6416. [Google Scholar] [CrossRef] [PubMed]
- Niederwieser, D.; Baldomero, H.; Szer, J.; Gratwohl, M.; Aljurf, M.; Atsuta, Y.; Bouzas, L.F.; Confer, D.; Greinix, H.; Horowitz, M.; et al. Hematopoietic stem cell transplantation activity worldwide in 2012 and a SWOT analysis of the Worldwide Network for Blood and Marrow Transplantation Group including the global survey. Bone Marrow Transplant. 2016, 51, 778–785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cottler-Fox, M.H. Stem Cell Mobilization. Hematology (Amsterdam Netherlands) 2003, 2003, 419–437. [Google Scholar] [CrossRef] [Green Version]
- Bender, J.G.; To, L.B.; Williams, S.; Schwartzberg, L.S. Defining a therapeutic dose of peripheral blood stem cells. J. Hematother. 1992, 1, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Blazar, B.R.; Murphy, W.J.; Abedi, M. Advances in graft-versus-host disease biology and therapy. Nat. Rev. Immunol. 2012, 12, 443–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apostolova, P.; Zeiser, R. The role of danger signals and ectonucleotidases in acute graft-versus-host disease. Hum. Immunol. 2016, 77, 1037–1047. [Google Scholar] [CrossRef]
- Pándy-Szekeres, G.; Munk, C.; Tsonkov, T.M.; Mordalski, S.; Harpsøe, K.; Hauser, A.S.; Bojarski, A.J.; Gloriam, D.E. GPCRdb in 2018: Adding GPCR structure models and ligands. Nucleic Acids Res. 2018, 46, D440–D446. [Google Scholar] [CrossRef]
- Gurevich, V.; Gurevich, E. Molecular Mechanisms of GPCR Signaling: A Structural Perspective. Int. J. Mol. Sci. 2017, 18, 2519. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, R. The G-Protein-Coupled Receptors in the Human Genome Form Five Main Families. Phylogenetic Analysis, Paralogon Groups, and Fingerprints. Mol. Pharmacol. 2003, 63, 1256–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weis, W.I.; Kobilka, B.K. The Molecular Basis of G Protein–Coupled Receptor Activation. Annu. Rev. Biochem. 2018, 87, 897–919. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Rajagopal, S. The β-Arrestins: Multifunctional Regulators of G Protein-coupled Receptors. J. Biol. Chem. 2016, 291, 8969–8977. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Shikano, S. Differential phosphorylation signals control endocytosis of GPR15. Mol. Biol. Cell 2017, 28, 2267–2281. [Google Scholar] [CrossRef]
- Nogués, L.; Reglero, C.; Rivas, V.; Neves, M.; Penela, P.; Mayor, F. G-Protein–Coupled Receptor Kinase 2 as a Potential Modulator of the Hallmarks of Cancer. Mol. Pharmacol. 2017, 91, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Hanyaloglu, A.C. Advances in Membrane Trafficking and Endosomal Signaling of G Protein-Coupled Receptors. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 339, pp. 93–131. [Google Scholar]
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schiöth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 16, 829–842. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, Y. Recent Trends and Applications of Molecular Modeling in GPCR–Ligand Recognition and Structure-Based Drug Design. Int. J. Mol. Sci. 2018, 19, 2105. [Google Scholar] [CrossRef]
- Shimada, I.; Ueda, T.; Kofuku, Y.; Eddy, M.T.; Wüthrich, K. GPCR drug discovery: Integrating solution NMR data with crystal and cryo-EM structures. Nat. Rev. Drug Discov. 2018, 18, 59–82. [Google Scholar] [CrossRef]
- Smith, J.S.; Lefkowitz, R.J.; Rajagopal, S. Biased signalling: From simple switches to allosteric microprocessors. Nat. Rev. Drug Discov. 2018, 17, 243–260. [Google Scholar] [CrossRef]
- Wisler, J.W.; Rockman, H.A.; Lefkowitz, R.J. Biased G Protein–Coupled Receptor Signaling: Changing the Paradigm of Drug Discovery. Circulation 2018, 137, 2315–2317. [Google Scholar] [CrossRef] [PubMed]
- Bar-Shavit, R.; Maoz, M.; Kancharla, A.; Nag, J.K.; Agranovich, D.; Grisaru-Granovsky, S.; Uziely, B. G Protein-Coupled Receptors in Cancer. Int. J. Mol. Sci. 2016, 17, 1320. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gareri, C.; Rockman, H.A. G-Protein-Coupled Receptors in Heart Disease. Circ. Res. 2018, 123, 716–735. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Todd, N.; Thathiah, A. The role of GPCRs in neurodegenerative diseases: Avenues for therapeutic intervention. Curr. Opin. Pharmacol. 2017, 32, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.D.; Hendy, G.N.; Percy, M.E.; Bichet, D.G.; Cole, D.E. G protein-coupled receptor mutations and human genetic disease. Methods Mol. Biol. (Clifton N.J.) 2014, 1175, 153–187. [Google Scholar] [CrossRef]
- Umamaheswaran, S.; Dasari, S.K.; Yang, P.; Lutgendorf, S.K.; Sood, A.K. Stress, inflammation, and eicosanoids: An emerging perspective. Cancer Metastasis Rev. 2018, 37, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Witkamp, R.; Meijerink, J. The endocannabinoid system: An emerging key player in inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 130–138. [Google Scholar] [CrossRef]
- Albi, E.; Alessenko, A.; Grösch, S. Sphingolipids in Inflammation. Mediat. Inflamm. 2018, 2018, 7464702. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Charo, I.F.; Ransohoff, R.M. The Many Roles of Chemokines and Chemokine Receptors in Inflammation. N. Engl. J. Med. 2006, 354, 610–621. [Google Scholar] [CrossRef]
- Dixon, R.A.F.; Kobilka, B.K.; Strader, D.J.; Benovic, J.L.; Dohlman, H.G.; Frielle, T.; Bolanowski, M.A.; Bennett, C.D.; Rands, E.; Diehl, R.E.; et al. Cloning of the gene and cDNA for mammalian β-adrenergic receptor and homology with rhodopsin. Nature 1986, 321, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.; Cosentino, M. Adrenergic modulation of immune cells: An update. Amino Acids 2013, 45, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Kolmus, K.; Tavernier, J.; Gerlo, S. β2-Adrenergic receptors in immunity and inflammation: Stressing NF-κB. Brain Behav. Immun. 2015, 45, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Scanzano, A.; Cosentino, M. Adrenergic regulation of innate immunity: A review. Front. Pharmacol. 2015, 6, 171. [Google Scholar] [CrossRef] [PubMed]
- Bendall, L. Extracellular molecules in hematopoietic stem cell mobilisation. Int. J. Hematol. 2017, 105, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, T. CXCL12/SDF-1 and CXCR4. Front. Immunol. 2015, 6, 301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DiPersio, J.F.; Micallef, I.N.; Stiff, P.J.; Bolwell, B.J.; Maziarz, R.T.; Jacobsen, E.; Nademanee, A.; McCarty, J.; Bridger, G.; Calandra, G.; et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 4767–4773. [Google Scholar] [CrossRef]
- Brave, M.; Farrell, A.; Ching Lin, S.; Ocheltree, T.; Pope Miksinski, S.; Lee, S.L.; Saber, H.; Fourie, J.; Tornoe, C.; Booth, B.; et al. FDA review summary: Mozobil in combination with granulocyte colony-stimulating factor to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation. Oncology 2010, 78, 282–288. [Google Scholar] [CrossRef] [PubMed]
- DiPersio, J.F.; Stadtmauer, E.A.; Nademanee, A.; Micallef, I.N.; Stiff, P.J.; Kaufman, J.L.; Maziarz, R.T.; Hosing, C.; Fruehauf, S.; Horwitz, M.; et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood 2009, 113, 5720–5726. [Google Scholar] [CrossRef]
- Hartmann, T.; Hübel, K.; Monsef, I.; Engert, A.; Skoetz, N. Additional plerixafor to granulocyte colony-stimulating factors for haematopoietic stem cell mobilisation for autologous transplantation in people with malignant lymphoma or multiple myeloma. Cochrane Database Syst. Rev. 2015, CD010615. [Google Scholar] [CrossRef]
- Hopman, R.K.; DiPersio, J.F. Advances in stem cell mobilization. Blood Rev. 2014, 28, 31–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendall, L.J.; Bradstock, K.F. G-CSF: From granulopoietic stimulant to bone marrow stem cell mobilizing agent. Cytokine Growth Factor Rev. 2014, 25, 355–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben Nasr, M.; Reguaya, Z.; Berraies, L.; Maamar, M.; Ladeb, S.; Ben Othmen, T.; Mellouli, F.; Bejaoui, M.; Domenech, J.; Jenhani, F. Association of stromal cell-derived factor-1-3′A polymorphism to higher mobilization of hematopoietic stem cells CD34+ in Tunisian population. Transplant. Proc. 2011, 43, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Benboubker, L.; Watier, H.; Carion, A.; Georget, M.T.; Desbois, I.; Colombat, P.; Bardos, P.; Binet, C.; Domenech, J. Association between the SDF1-3′A allele and high levels of CD34(+) progenitor cells mobilized into peripheral blood in humans. Br. J. Haematol. 2001, 113, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Bogunia-Kubik, K.; Gieryng, A.; Dlubek, D.; Lange, A. The CXCL12-3′A allele is associated with a higher mobilization yield of CD34 progenitors to the peripheral blood of healthy donors for allogeneic transplantation. Bone Marrow Transplant. 2009, 44, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Soriano, A.; Martinez, C.; Garcia, F.; Plana, M.; Palou, E.; Lejeune, M.; Arostegui, J.I.; De Lazzari, E.; Rodriguez, C.; Barrasa, A.; et al. Plasma stromal cell-derived factor (SDF)-1 levels, SDF1-3′A genotype, and expression of CXCR4 on T lymphocytes: Their impact on resistance to human immunodeficiency virus type 1 infection and its progression. J. Infect. Dis. 2002, 186, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Pantin, J.; Purev, E.; Tian, X.; Cook, L.; Donohue-Jerussi, T.; Cho, E.; Reger, R.; Hsieh, M.; Khuu, H.; Calandra, G.; et al. Effect of high-dose plerixafor on CD34(+) cell mobilization in healthy stem cell donors: Results of a randomized crossover trial. Haematologica 2017, 102, 600–609. [Google Scholar] [CrossRef]
- Flomenberg, N.; Comenzo, R.L.; Badel, K.; Calandra, G. Plerixafor (Mozobil) alone to mobilize hematopoietic stem cells from multiple myeloma patients for autologous transplantation. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2010, 16, 695–700. [Google Scholar] [CrossRef]
- Cheng, J.; Schmitt, M.; Wuchter, P.; Buss, E.C.; Witzens-Harig, M.; Neben, K.; Hundemer, M.; Hillengass, J.; Alexi, R.; Goldschmidt, H.; et al. Plerixafor is effective given either preemptively or as a rescue strategy in poor stem cell mobilizing patients with multiple myeloma. Transfusion 2015, 55, 275–283. [Google Scholar] [CrossRef]
- Fadini, G.P.; Fiala, M.; Cappellari, R.; Danna, M.; Park, S.; Poncina, N.; Menegazzo, L.; Albiero, M.; DiPersio, J.; Stockerl-Goldstein, K.; et al. Diabetes Limits Stem Cell Mobilization Following G-CSF but Not Plerixafor. Diabetes 2015, 64, 2969–2977. [Google Scholar] [CrossRef] [Green Version]
- Patel, B.; Pearson, H.; Zacharoulis, S. Mobilisation of haematopoietic stem cells in paediatric patients, prior to autologous transplantation following administration of plerixafor and G-CSF. Pediatr. Blood Cancer 2015, 62, 1477–1480. [Google Scholar] [CrossRef] [PubMed]
- Teusink, A.; Pinkard, S.; Davies, S.; Mueller, M.; Jodele, S. Plerixafor is safe and efficacious for mobilization of peripheral blood stem cells in pediatric patients. Transfusion 2016, 56, 1402–1405. [Google Scholar] [CrossRef]
- Bitan, M.; Eshel, R.; Sadot, E.; Friedman, S.; Pinhasov, A.; Levin, D.; Dvir, R.; Manisterski, M.; Berger-Achituv, S.; Rosenfeld-Keidar, H.; et al. Combined plerixafor and granulocyte colony-stimulating factor for harvesting high-dose hematopoietic stem cells: Possible niche for plerixafor use in pediatric patients. Pediatr. Transplant. 2016, 20, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Douglas, K.W.; Parker, A.N.; Hayden, P.J.; Rahemtulla, A.; D’Addio, A.; Lemoli, R.M.; Rao, K.; Maris, M.; Pagliuca, A.; Uberti, J.; et al. Plerixafor for PBSC mobilisation in myeloma patients with advanced renal failure: Safety and efficacy data in a series of 21 patients from Europe and the USA. Bone Marrow Transplant. 2012, 47, 18–23. [Google Scholar] [CrossRef] [PubMed]
- MacFarland, R.; Hard, M.L.; Scarborough, R.; Badel, K.; Calandra, G. A pharmacokinetic study of plerixafor in subjects with varying degrees of renal impairment. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2010, 16, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Liles, W.C.; Broxmeyer, H.E.; Rodger, E.; Wood, B.; Hubel, K.; Cooper, S.; Hangoc, G.; Bridger, G.J.; Henson, G.W.; Calandra, G.; et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood 2003, 102, 2728–2730. [Google Scholar] [CrossRef] [PubMed]
- Devine, S.M.; Vij, R.; Rettig, M.; Todt, L.; McGlauchlen, K.; Fisher, N.; Devine, H.; Link, D.C.; Calandra, G.; Bridger, G.; et al. Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. Blood 2008, 112, 990–998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeder, M.A.; Rettig, M.P.; Lopez, S.; Christ, S.; Fiala, M.; Eades, W.; Mir, F.A.; Shao, J.; McFarland, K.; Trinkaus, K.; et al. Mobilization of allogeneic peripheral blood stem cell donors with intravenous plerixafor mobilizes a unique graft. Blood 2017, 129, 2680–2692. [Google Scholar] [CrossRef] [Green Version]
- Chung, D.T.; Chang, L.W.; Huang, Y.H.; Tsai, C.Y.; Hsu, C.H.; King, C.H.R.; Yuan, J.H.; Yen, C.F.; Chen, Y.M.; Lu, Y.C.; et al. TG-0054, a novel and potent stem cell mobilizer, displays excellent PK/PD and safety profile in phase I trial. Blood 2009, 114, 866. [Google Scholar]
- Schuster, M.W.; Hagog, N.; Jalilizeinali, B.; Funkhauser, S.; Yohannan, M.S.; Sadler, J.; Wood, S.; Carey, S.; Kelleher, K.; Tsai, C.E.; et al. Rapid mobilization of CD34+ progenitor cells with TG0054-03, a novel CXC chemokine receptor 4 (CXCR4) antagonoist. Blood 2013, 122, 905. [Google Scholar]
- Setia, G.; Hagog, N.; Jalilizeinali, B.; Funkhouser, S.; Pierzchanowski, L.; Lan, F.; Gabig, T.G.; Kiner-Strachan, B.; Kelleher, K.; Hsu, M.C.; et al. A phase ii, open-label pilot study to evaluate the hematopoietic stem cell mobilization of TG-0054 combined with G-CSF in 12 patients with multiple myeloma, non-hodgkin lymphoma or hodgkin lymphoma—An interim analysis. Blood 2015, 126, 515. [Google Scholar]
- Abraham, M.; Pereg, Y.; Bulvik, B.; Klein, S.; Mishalian, I.; Wald, H.; Eizenberg, O.; Beider, K.; Nagler, A.; Golan, R.; et al. Single Dose of the CXCR4 Antagonist BL-8040 Induces Rapid Mobilization for the Collection of Human CD34(+) Cells in Healthy Volunteers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 6790–6801. [Google Scholar] [CrossRef] [PubMed]
- Peled, A.; Abraham, M.; Avivi, I.; Rowe, J.M.; Beider, K.; Wald, H.; Tiomkin, L.; Ribakovsky, L.; Riback, Y.; Ramati, Y.; et al. The high-affinity CXCR4 antagonist BKT140 is safe and induces a robust mobilization of human CD34+ cells in patients with multiple myeloma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Karpova, D.; Brauninger, S.; Wiercinska, E.; Kramer, A.; Stock, B.; Graff, J.; Martin, H.; Wach, A.; Escot, C.; Douglas, G.; et al. Mobilization of hematopoietic stem cells with the novel CXCR4 antagonist POL6326 (balixafortide) in healthy volunteers-results of a dose escalation trial. J. Transl. Med. 2017, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.B.; Van Horn, R.D.; Yin, T.; Brown, R.M.; Roell, W.C.; Obungu, V.H.; Ruegg, C.; Wroblewski, V.J.; Raddad, E.; Stille, J.R. Distinct mobilization of leukocytes and hematopoietic stem cells by CXCR4 peptide antagonist LY2510924 and monoclonal antibody LY2624587. Oncotarget 2017, 8, 94619–94634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vater, A.; Sahlmann, J.; Kroger, N.; Zollner, S.; Lioznov, M.; Maasch, C.; Buchner, K.; Vossmeyer, D.; Schwoebel, F.; Purschke, W.G.; et al. Hematopoietic stem and progenitor cell mobilization in mice and humans by a first-in-class mirror-image oligonucleotide inhibitor of CXCL12. Clin. Pharmacol. Ther. 2013, 94, 150–157. [Google Scholar] [CrossRef]
- Costa, L.J.; Alexander, E.T.; Hogan, K.R.; Schaub, C.; Fouts, T.V.; Stuart, R.K. Development and validation of a decision-making algorithm to guide the use of plerixafor for autologous hematopoietic stem cell mobilization. Bone Marrow Transplant. 2011, 46, 64–69. [Google Scholar] [CrossRef]
- Dabusti, M.; Lanza, F.; Campioni, D.; Castagnari, B.; Tieghi, A.; Moretti, S.; Punturieri, M.; De Angeli, C.; Spanedda, R.; Ferrazzi, E.; et al. CXCR-4 expression on bone marrow CD34+ cells prior to mobilization can predict mobilization adequacy in patients with hematologic malignancies. J. Hematother. Stem Cell Res. 2003, 12, 425–434. [Google Scholar] [CrossRef]
- Cecyn, K.Z.; Schimieguel, D.M.; Kimura, E.Y.; Yamamoto, M.; Oliveira, J.S. Plasma levels of FL and SDF-1 and expression of FLT-3 and CXCR4 on CD34+ cells assessed pre and post hematopoietic stem cell mobilization in patients with hematologic malignancies and in healthy donors. Transfus. Apher. Sci. Off. J. World Apher. Assoc. Off. J. Eur. Soc. Haemapher. 2009, 40, 159–167. [Google Scholar] [CrossRef]
- Dlubek, D.; Drabczak-Skrzypek, D.; Lange, A. Low CXCR4 membrane expression on CD34(+) cells characterizes cells mobilized to blood. Bone Marrow Transplant. 2006, 37, 19–23. [Google Scholar] [CrossRef]
- Watanabe, T.; Kawano, Y.; Kanamaru, S.; Onishi, T.; Kaneko, S.; Wakata, Y.; Nakagawa, R.; Makimoto, A.; Kuroda, Y.; Takaue, Y.; et al. Endogenous interleukin-8 (IL-8) surge in granulocyte colony-stimulating factor-induced peripheral blood stem cell mobilization. Blood 1999, 93, 1157–1163. [Google Scholar] [PubMed]
- Kozuka, T.; Ishimaru, F.; Fujii, K.; Masuda, K.; Kaneda, K.; Imai, T.; Fujii, N.; Ishikura, H.; Hongo, S.; Watanabe, T.; et al. Plasma stromal cell-derived factor-1 during granulocyte colony-stimulating factor-induced peripheral blood stem cell mobilization. Bone Marrow Transplant. 2003, 31, 651–654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devine, S.M.; Flomenberg, N.; Vesole, D.H.; Liesveld, J.; Weisdorf, D.; Badel, K.; Calandra, G.; DiPersio, J.F. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin’s lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004, 22, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Gazitt, Y.; Freytes, C.O.; Akay, C.; Badel, K.; Calandra, G. Improved mobilization of peripheral blood CD34+ cells and dendritic cells by AMD3100 plus granulocyte-colony-stimulating factor in non-Hodgkin’s lymphoma patients. Stem Cells Dev. 2007, 16, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Stiff, P.; Micallef, I.; McCarthy, P.; Magalhaes-Silverman, M.; Weisdorf, D.; Territo, M.; Badel, K.; Calandra, G. Treatment with plerixafor in non-Hodgkin’s lymphoma and multiple myeloma patients to increase the number of peripheral blood stem cells when given a mobilizing regimen of G-CSF: Implications for the heavily pretreated patient. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2009, 15, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Modak, S.; Cheung, I.Y.; Kushner, B.H.; Kramer, K.; Reich, L.; Cheung, N.K. Plerixafor plus granulocyte-colony stimulating factor for autologous hematopoietic stem cell mobilization in patients with metastatic neuroblastoma. Pediatr. Blood Cancer 2012, 58, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Teusink, A.; Pinkard, S.L.; Davies, S.M.; Mueller, M.; Jodele, S. Safety and efficacy of plerixafor for mobilization of peripheral blood stem cells in pediatric patients. Biol. Blood Marrow Transplant. 2014, 20, S294–S295. [Google Scholar] [CrossRef]
- Bekadja, M.A.; Bouhass, R.; Osmani, S.; Brahimi, M.; Talhi, S.; Yafour, N.; Arabi, A. Plerixafor in the treatment of progenitor cell mobilization failure: First experience in Algeria. Hematol. Oncol. Stem Cell Ther. 2015, 8, 93–94. [Google Scholar] [CrossRef] [Green Version]
- Danylesko, I.; Sareli, R.; Varda-Bloom, N.; Yerushalmi, R.; Shem-Tov, N.; Shimoni, A.; Nagler, A. Plerixafor (Mozobil): A Stem Cell-Mobilizing Agent for Transplantation in Lymphoma Patients Predicted to Be Poor Mobilizers—A Pilot Study. Acta Haematol. 2016, 135, 29–36. [Google Scholar] [CrossRef]
- López-Parra, M.; López Villar, O.; Bastida Bermejo, J.M.; López-Godino, O.; Cabrero, M.; Ramos Sevillano, M.I.; Oreja Martín, B.; López Cadenas, F.; Dávila Vals, J.; Nieto, M.J.; et al. Preemptive use of plerixafor on day 5 of G-CSF treatment. Experience of University Hospital of Salamanca. Bone Marrow Transplant. 2016, 51, S334–S335. [Google Scholar] [CrossRef]
- Andritsos, L.A.; Huang, Y.; Fan, T.; Huff, K.; Drea, E.; McBride, A. A retrospective evaluation of the impact of pre-emptive plerixafor administration on collection efficiency in patients with myeloma undergoing stem cell mobilization. Blood 2016, 128, 5740. [Google Scholar]
- Basak, G.W.; Knopinska-Posluszny, W.; Matuszak, M.; Kisiel, E.; Hawrylecka, D.; Szmigielska-Kaplon, A.; Urbaniak-Kujda, D.; Dybko, J.; Zielinska, P.; Dabrowska-Iwanicka, A.; et al. Hematopoietic stem cell mobilization with the reversible CXCR4 receptor inhibitor plerixafor (AMD3100)-Polish compassionate use experience. Ann. Hematol. 2011, 90, 557–568. [Google Scholar] [CrossRef] [PubMed]
- Boulad, F.; Shore, T.B.; Van Besien, K.; Guarneri, D.; Greenberg, J.; Minniti, C.; Fedus, S.W.; Perna, F.; Wang, X.; Riviere, I.; et al. Safety and efficacy trial of escalation of plerixafor for mobilization of CD34+ hematopoietic progenitor cells (HPCS) for globin gene transfer in patients with sickle cell disease. Blood 2017, 130, 3531. [Google Scholar]
- Haen, S.; Schober-Melms, I.; Schumm, M.; Henes, J.; Möhle, R.; Bethge, W.; Kanz, L.; Vogel, W. Addition of plerixafor overcomes poor mobilization in autologous and allogeneic stem cell grafts and leads to efficient and sustained engraftment. Blood 2017, 130, 5457. [Google Scholar]
- Ogunniyi, A.; Rodriguez, M.; Devlin, S.; Adel, N.; Landau, H.; Chung, D.J.; Lendvai, N.; Lesokhin, A.; Koehne, G.; Mailankody, S.; et al. Upfront use of plerixafor and granulocyte-colony stimulating factor (GCSF) for stem cell mobilization in patients with multiple myeloma: Efficacy and analysis of risk factors associated with poor stem cell collection efficiency. Leuk. Lymphoma 2017, 58, 1123–1129. [Google Scholar] [CrossRef] [PubMed]
- Lanza, F.; Lemoli, R.M.; Olivieri, A.; Laszlo, D.; Martino, M.; Specchia, G.; Pavone, V.; Imola, M.; Pasini, A.; Milone, G.; et al. Factors affecting successful mobilization with plerixafor: An Italian prospective survey in 215 patients with multiple myeloma and lymphoma. Transfusion 2014, 54, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Haen, S.P.; Schober-Melms, I.; Bethge, W.A.; Möhle, R.; Schumm, M.; Kanz, L.; Vogel, W. Plerixafor significantly increases the hematopoietic stem cell yield in documented poor mobilizers in the autologous and allogeneic setting. Onkologie 2013, 36. [Google Scholar] [CrossRef]
- Fink, G.; Kleber, M.; Scherer, E.; Gross, B.; Müller-Schmah, C.; Duyster, J.; Wäsch, R.; Engelhardt, M. Plerixafor (AMD3100) for stem cell mobilization: Once dosing in poor mobilizers: Efficacious and economically feasible? Onkologie 2013, 36, 236–237. [Google Scholar] [CrossRef]
- Khaled, Y.; Solh, M.; Lamontagne, D.; Batista, A.; Sullivan, J.; Chan-Fong, S.; Fondaw, M.; Reddy, V. Successful stem cell mobilization and engraftment in heavily pretreated multiple myeloma patients with prior high dose melphalan and autologous stem cell transplantation. Biol. Blood Marrow Transplant. 2012, 18, S300. [Google Scholar] [CrossRef]
- Abusin, G.A.; Abu-Arja, R.F.; Gingrich, R.D.; Silverman, M.D.; Zamba, G.K.; Schlueter, A.J. An algorithm for utilizing peripheral blood CD34 count as a predictor of the need for plerixafor in autologous stem cell mobilization--cost-effectiveness analysis. J. Clin. Apher. 2013, 28, 293–300. [Google Scholar] [CrossRef]
- Sunu, C.; Onder Savas, O.; Ozet, G.; Dagdas, S.; Ceran, F.; Falay, M.; Koyuncu, N. Stem cell mobilization with plerixafor: A single center experience. Transfus. Apher. Sci. 2012, 47, S53. [Google Scholar] [CrossRef]
- Kasparu, H.; Kolb, A.; Böhm, A.; Hauser, H.; Weltermann, A. Overcoming poor stem cell mobilization and long term recovery after autologous transplantation with plerixafor primed stem cells. Eur. Surg.-Acta Chir. Austriaca 2012, 44. [Google Scholar]
- Worel, N.; Rosskopf, K.; Neumeister, P.; Kasparu, H.; Nachbaur, D.; Russ, G.; Namberger, K.; Witt, V.; Schloegl, E.; Zojer, N.; et al. Plerixafor and granulocyte-colony-stimulating factor (G-CSF) in patients with lymphoma and multiple myeloma previously failing mobilization with G-CSF with or without chemotherapy for autologous hematopoietic stem cell mobilization: The Austrian experience on a named patient program. Transfusion 2011, 51, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Selleslag, D.; Dierickx, D.; Breems, D.A.; Huynh, P.; Van De Velde, A.; Meers, S.; Brouwer, E.; Mertens, A. Plerixafor in poor stem cell mobilizers: The Belgian Compassionate Use Program. Acta Clin. Belg. 2011, 66, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Basak, G.W.; Jaksic, O.; Koristek, Z.; Mikala, G.; Basic-Kinda, S.; Mayer, J.; Masszi, T.; Giebel, S.; Labar, B.; Wiktor-Jedrzejczak, W. Haematopoietic stem cell mobilization with plerixafor and G-CSF in patients with multiple myeloma transplanted with autologous stem cells. Eur. J. Haematol. 2011, 86, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Nademanee, A.P.; DiPersio, J.F.; Maziarz, R.T.; Stadtmauer, E.A.; Micallef, I.N.; Stiff, P.J.; Hsu, F.J.; Bridger, G.; Bolwell, B.J. Plerixafor plus granulocyte colony-stimulating factor versus placebo plus granulocyte colony-stimulating factor for mobilization of CD34(+) hematopoietic stem cells in patients with multiple myeloma and low peripheral blood CD34(+) cell count: Results of a subset analysis of a randomized trial. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2012, 18, 1564–1572. [Google Scholar] [CrossRef]
- Jantunen, E.; Kuittinen, T.; Mahlamaki, E.; Pyorala, M.; Mantymaa, P.; Nousiainen, T. Efficacy of pre-emptively used plerixafor in patients mobilizing poorly after chemomobilization: A single centre experience. Eur. J. Haematol. 2011, 86, 299–304. [Google Scholar] [CrossRef]
- Jing, D.; Alakel, N.; Bornhauser, M.; Ehninger, G.; Ordemann, R. SDF-1/CXCR4 blockade to mobilize hematopoietic progenitor cells from the placenta. Bone Marrow Transplant. 2010, 45, 1661–1662. [Google Scholar] [CrossRef] [Green Version]
- Horwitz, M.; Khan, T.; Long, G.; Gasparetto, C.; Sullivan, K.; Chute, J.; Rizzieri, D.; Drago, S.; Chao, N. Plerixafor given “just in time” for peripheral blood stem cell mobilization of patients with suboptimal response to G-CSF. Biol. Blood Marrow Transplant. 2010, 16, S208. [Google Scholar] [CrossRef]
- Flomenberg, N.; Devine, S.M.; Dipersio, J.F.; Liesveld, J.L.; McCarty, J.M.; Rowley, S.D.; Vesole, D.H.; Badel, K.; Calandra, G. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood 2005, 106, 1867–1874. [Google Scholar] [CrossRef]
- Shastri, A.; Budhathoki, A.; Barta, S.K.; Kornblum, N.; Derman, O.; Battini, R.; Raghupathy, R.; Verma, A.K.; Frenette, P.S.; Braunschweig, I.; et al. Stimulation of adrenergic activity by desipramine enhances hematopoietic stem and progenitor cell mobilization along with G-CSF in multiple myeloma: A pilot study. Am. J. Hematol. 2017, 92, 1047–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nevo, N.; Gur-Cohen, S.; Kollet, O.; Avemaria, F.; Avci, S.; Itkin, T.; Chakrabarty, S.; Zuckerman, T.; Brenner, B.; Nadir, Y.; et al. Inverse PAR1 activity of hematopoietic stem cells and BM stromal cells mediates G-CSF-induced mobilization by regulation of nitric oxide generation. Blood 2016, 128, 3370. [Google Scholar]
- Shin, S.; Kim, J.; Kim-Wanner, S.Z.; Bonig, H.; Cho, S.R.; Kim, S.; Choi, J.R.; Lee, K.A. A novel association between relaxin receptor polymorphism and hematopoietic stem cell yield after mobilization. PLoS ONE 2017, 12, e0179986. [Google Scholar] [CrossRef] [PubMed]
- Olsson, R.; Remberger, M.; Schaffer, M.; Berggren, D.M.; Svahn, B.M.; Mattsson, J.; Ringden, O. Graft failure in the modern era of allogeneic hematopoietic SCT. Bone Marrow Transplant. 2013, 48, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Bach, C.; Steffen, M.; Roesler, W.; Winkler, J.; Mackensen, A.; Stachel, K.D.; Metzler, M.; Spriewald, B.M. Systematic comparison of donor chimerism in peripheral blood and bone marrow after hematopoietic stem cell transplantation. Blood Cancer J. 2017, 7, e566. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.I. Engraftment and Chimerism. Hematop. Stem Cell Transplant. Pediatr. Hematol./Oncol. 2017, 177–186. [Google Scholar] [CrossRef]
- Ozdemir, Z.N.; Civriz Bozdag, S. Graft failure after allogeneic hematopoietic stem cell transplantation. Transfus. Apher. Sci. Off. J. World Apher. Assoc. Off. J. Eur. Soc. Haemapher. 2018, 57, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Purton, L.E.; Scadden, D.T. Limiting factors in murine hematopoietic stem cell assays. Cell Stem Cell 2007, 1, 263–270. [Google Scholar] [CrossRef]
- Girbl, T.; Lunzer, V.; Greil, R.; Namberger, K.; Hartmann, T.N. The CXCR4 and adhesion molecule expression of CD34+ hematopoietic cells mobilized by “on-demand” addition of plerixafor to granulocyte-colony-stimulating factor. Transfusion 2014, 54, 2325–2335. [Google Scholar] [CrossRef]
- Varmavuo, V.; Rimpilainen, J.; Kuitunen, H.; Nihtinen, A.; Vasala, K.; Mikkola, M.; Kutila, A.; Lehtonen, P.; Kuittinen, T.; Mantymaa, P.; et al. Engraftment and outcome after autologous stem cell transplantation in plerixafor-mobilized non-Hodgkin’s lymphoma patients. Transfusion 2014, 54, 1243–1250. [Google Scholar] [CrossRef]
- Bonig, H.; Chudziak, D.; Priestley, G.; Papayannopoulou, T. Insights into the biology of mobilized hematopoietic stem/progenitor cells through innovative treatment schedules of the CXCR4 antagonist AMD3100. Exp. Hematol. 2009, 37, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.A.; Bonde, J.; Craft, T.P.; Wirthlin, L.; Hohm, S.; Lahey, R.; Todt, L.M.; Dipersio, J.F.; Devine, S.M.; Nolta, J.A. Human progenitor cells rapidly mobilized by AMD3100 repopulate NOD/SCID mice with increased frequency in comparison to cells from the same donor mobilized by granulocyte colony stimulating factor. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2007, 13, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Lidonnici, M.R.; Aprile, A.; Frittoli, M.; Mandelli, G.; Paleari, Y.; Spinelli, A.; Gentner, B.; Zambelli, M.; Parisi, C.; Bellio, L.; et al. Human CD34+ cells from different sources disclose a specific stemness signature. Blood 2016, 128, 4709. [Google Scholar]
- Arai, Y.; Choi, U.; Corsino, C.I.; Koontz, S.M.; Tajima, M.; Sweeney, C.L.; Black, M.A.; Feldman, S.A.; Dinauer, M.C.; Malech, H.L. Enhanced expression of CXCR4 facilitates C-Kit-targeted CAR-T cell trafficking to bone marrow and enables donor stem cell engraftment. Biol. Blood Marrow Transplant. 2018, 24, S311. [Google Scholar] [CrossRef]
- Arai, Y.; Corsino, C.I.; Koontz, S.M.; Tajima, M.; Black, M.A.; Sweeney, C.L.; Choi, U.; Feldman, S.A.; Dinauer, M.C.; Malech, H.L. Enhanced expression of CXCR4 facilitates the trafficking of anti-C-kit chimeric antigen receptor (CAR) T cells to bone marrow and achieves effective donor stem cell engraftment. Blood 2017, 130, 4446. [Google Scholar]
- Chen, J.; Larochelle, A.; Fricker, S.; Bridger, G.; Dunbar, C.E.; Abkowitz, J.L. Mobilization as a preparative regimen for hematopoietic stem cell transplantation. Blood 2006, 107, 3764–3771. [Google Scholar] [CrossRef]
- Jiang, Y.; Ulyanova, T.; Papayannopoulou, T. Is the post-transplantation treatment with AMD beneficial? Blood Cells Mol. Dis. 2012, 49, 29–31. [Google Scholar] [CrossRef] [Green Version]
- Green, M.M.; Chao, N.; Chhabra, S.; Corbet, K.; Gasparetto, C.; Horwitz, A.; Li, Z.; Venkata, J.K.; Long, G.; Mims, A.; et al. Plerixafor (a CXCR4 antagonist) following myeloablative allogeneic hematopoietic stem cell transplantation enhances hematopoietic recovery. J. Hematol. Oncol. 2016, 9, 71. [Google Scholar] [CrossRef]
- Lai, C.Y.; Otsu, M.; Okabe, M.; Suzuki, S.; Yamazaki, S.; Onodera, M.; Ema, H.; Kakuta, S.; Iwakura, Y.; Nakauchi, H. Evidence for the involvement of CXCR4 signaling in in vivo self-renewal of transplanted hematopoietic stem cells. Blood 2011, 118. [Google Scholar]
- Lai, C.Y.; Suzuki, S.; Okabe, M.; Yamazaki, S.; Otsu, M.; Nakauchi, H. Role of cxcr4 signaling in hematopoietic stem cell repopulation. J. Gene Med. 2012, 14, 682–683. [Google Scholar] [CrossRef]
- Asfour, I.; Afify, H.; Elkourashy, S.; Ayoub, M.; Kamal, G.; Gamal, M.; Elgohary, G. CXCR4 (CD184) expression on stem cell harvest and CD34(+) cells post-transplant. Hematol./Oncol. Stem Cell Ther. 2017, 10, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.; Jackson, J.; Baulch-Brown, C. Enumeration of bone marrow ‘homing’ haemopoietic stem cells from G-CSF-mobilised normal donors and influence on engraftment following allogeneic transplantation. Bone Marrow Transplant. 2001, 28, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Hicks, C.; Isaacs, A.; Wong, R.; Chong, B.H. CXCR4 expression on transplanted peripheral blood CD34+ cells: Relationship to engraftment after autologous transplantation in a cohort of multiple myeloma patients. Ann. Hematol. 2011, 90, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Gieryng, A.; Bogunia-Kubik, K.; Lange, A. CXCL12 gene polymorphism and hematologic recovery after transplantation of peripheral blood progenitor cells. Transplant. Proc. 2010, 42, 3280–3283. [Google Scholar] [CrossRef] [PubMed]
- Hoggatt, J.; Singh, P.; Sampath, J.; Pelus, L.M. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood 2009, 113, 5444–5455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelus, L.M.; Hoggatt, J.; Singh, P. Pulse exposure of haematopoietic grafts to prostaglandin E2 in vitro facilitates engraftment and recovery. Cell Prolif. 2011, 44, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Speth, J.M.; Hoggatt, J.; Pelus, L.M. Enhanced homing of hspcs after treatment with prostaglandin E2 (PGE2) may be an effect of transcriptional regulation of CXC chemokine receptor 4 (CXCR4) through hypoxia inducible factor 1α (HIF1α). Blood 2011, 118, 918. [Google Scholar]
- Speth, J.M.; Hoggatt, J.; Singh, P.; Pelus, L.M. Pharmacologic increase in HIF1alpha enhances hematopoietic stem and progenitor homing and engraftment. Blood 2014, 123, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Melacarne, A.; Yadak, R.; Schouteden, S.; Notelaers, T.; Pistoni, M.; Maes, C.; Verfaillie, C.M. SMAD signaling regulates CXCL12 expression in the bone marrow niche, affecting homing and mobilization of hematopoietic progenitors. Stem Cells (Dayton Ohio) 2014, 32, 3012–3022. [Google Scholar] [CrossRef]
- Blaser, B.W.; Moore, J.L.; Hagedorn, E.J.; Li, B.; Riquelme, R.; Lichtig, A.; Yang, S.; Zhou, Y.; Tamplin, O.J.; Binder, V.; et al. CXCR1 remodels the vascular niche to promote hematopoietic stem and progenitor cell engraftment. J. Exp. Med. 2017, 214, 1011–1027. [Google Scholar] [CrossRef]
- Merli, P.; Caruana, I.; De Vito, R.; Del Bufalo, F.; Strocchio, L.; Weber, G.A.; Pitisci, A.; Cirillo, V.; Galaverna, F.; Algeri, M.; et al. Role of IFN-gamma in immune-mediated graft failure after allogeneic hematopoietic stem cell transplantation. HemaSphere 2018, 2, 676–677. [Google Scholar] [CrossRef]
- Merli, P.; Caruana, I.; De Vito, R.; Strocchio, L.; Weber, G.; Del Bufalo, F.; Buatois, V.; Montanari, P.; Cefalo, M.G.; Pitisci, A.; et al. Role of IFNgamma in immune-mediated graft failure occurring after allogeneic hematopoietic stem cell transplantation. Haematologica 2019. [Google Scholar] [CrossRef] [PubMed]
- Hale, S.J.; Hale, A.B.; Zhang, Y.; Sweeney, D.; Fisher, N.; van der Garde, M.; Grabowska, R.; Pepperell, E.; Channon, K.; Martin-Rendon, E.; et al. CXCR2 modulates bone marrow vascular repair and haematopoietic recovery post-transplant. Br. J. Haematol. 2015, 169, 552–564. [Google Scholar] [CrossRef] [PubMed]
- de Wynter, E.A.; Heyworth, C.M.; Mukaida, N.; Matsushima, K.; Testa, N.G. NOD/SCID repopulating cells but not LTC-IC are enriched in human CD34+ cells expressing the CCR1 chemokine receptor. Leukemia 2001, 15, 1092–1101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jorda, M.A.; Jiang, S.; Zagozdzon, R.; Parmar, K.; Mauch, P.; Fu, Y.; Makriyannis, A.; Zvonok, A.M.; Tenen, D.G.; Avraham, S.; et al. The peripheral cannabinoid receptor regulates human and mouse hematopoiesis, bone marrow recovery, and hematopoietic stem and progenitor cell mobilization. Haematologica 2009, 94, 208. [Google Scholar]
- Schulte, G. International Union of Basic and Clinical Pharmacology. LXXX. The class Frizzled receptors. Pharmacol. Rev. 2010, 62, 632–667. [Google Scholar] [CrossRef]
- Abidin, B.M.; Owusu Kwarteng, E.; Heinonen, K.M. Frizzled-6 Regulates Hematopoietic Stem/Progenitor Cell Survival and Self-Renewal. J. Immunol. (Baltimore Md. 1950) 2015, 195, 2168–2176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zong, W.N.; Chen, X.M.; Yang, Y.Q.; Cao, J.L.; Zou, H.Y.Y.; Sun, H.W.; Hou, M.H.; Huang, H.J.; Zheng, H.J.; Qin, X.Y.; et al. Plasma auto-antibodies to angiotensin II receptors are correlated with blood pressure and inflammatory factors in hypertension patients. Eur. Heart J. Suppl. 2015, 17, B65–B70. [Google Scholar] [CrossRef] [Green Version]
- Dragun, D.; Philippe, A.; Catar, R. Role of non-HLA antibodies in organ transplantation. Curr. Opin. Organ Transplant. 2012, 17, 440–445. [Google Scholar] [CrossRef]
- Taniguchi, M.; Gendzekhadze, K.; Lee, J.H.; Senitzer, D. Anti-angiotensin II type-1 receptor antibodies in failed chimerism after hematopoietic stem cell transplantation. Hum. Immunol. 2017, 78, 89. [Google Scholar] [CrossRef]
- Richter, R.; Rüster, B.; Bistrian, R.; Forssmann, W.G.; Seifried, E.; Henschler, R. Beta-chemokine CCL15 affects the adhesion and migration of hematopoietic progenitor cells. Transfus. Med. Hemother. 2015, 42, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Hoggatt, J.; Singh, P.; Tate, T.A.; Chou, B.K.; Datari, S.R.; Fukuda, S.; Liu, L.; Kharchenko, P.V.; Schajnovitz, A.; Baryawno, N.; et al. Rapid Mobilization Reveals a Highly Engraftable Hematopoietic Stem Cell. Cell 2018, 172, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Foguenne, J.; Di Stefano, I.; Giet, O.; Beguin, Y.; Gothot, A. Ex vivo expansion of hematopoietic progenitor cells is associated with downregulation of alpha4 integrin-and CXCR4-mediated engraftment in NOD/SCID beta2-microglobulin-null mice. Haematologica 2009, 94, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Peled, A.; Petit, I.; Kollet, O.; Magid, M.; Ponomaryov, T.; Byk, T.; Nagler, A.; Ben-Hur, H.; Many, A.; Shultz, L.; et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science (New York N.Y.) 1999, 283, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Huang, X.; Cooper, S.; Broxmeyer, H.E. Glucocorticoid hormone-induced chromatin remodeling enhances human hematopoietic stem cell homing and engraftment. Nat. Med. 2017, 23, 424–428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brenner, S.; Whiting-Theobald, N.; Kawai, T.; Linton, G.F.; Rudikoff, A.G.; Choi, U.; Ryser, M.F.; Murphy, P.M.; Sechler, J.M.; Malech, H.L. CXCR4-transgene expression significantly improves marrow engraftment of cultured hematopoietic stem cells. Stem Cells (Dayton Ohio) 2004, 22, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Lozano, M.L.; Niño, E.A.; Cerezo, J.J.; Castilla-Llorente, C.; Anton, A.I.; Padilla, J.; Heras, I.; Garcia, V. CXCR4 allelic variations influence hematological recovery following autologous stem cell transplantation. Blood 2011, 118, 4098. [Google Scholar]
- Abraham, M.; Biyder, K.; Begin, M.; Wald, H.; Weiss, I.D.; Galun, E.; Nagler, A.; Peled, A. Enhanced unique pattern of hematopoietic cell mobilization induced by the CXCR4 antagonist 4F-benzoyl-TN14003. Stem Cells (Dayton Ohio) 2007, 25, 2158–2166. [Google Scholar] [CrossRef]
- Wiesmann, A.; Spangrude, G.J. Marrow engraftment of hematopoietic stem and progenitor cells is independent of Galphai-coupled chemokine receptors. Exp. Hematol. 1999, 27, 946–955. [Google Scholar] [CrossRef]
- Kazemi, Z.; Bergmayr, C.; Prchal-Murphy, M.; Javaheri, T.; Themanns, M.; Pham, H.T.; Strohmaier, W.; Sexl, V.; Freissmuth, M.; Zebedin-Brandl, E. Repurposing Treprostinil for Enhancing Hematopoietic Progenitor Cell Transplantation. Mol. Pharmacol. 2016, 89, 630–644. [Google Scholar] [CrossRef] [Green Version]
- Bergmayr, C.; Balasz, C.; Kazemi, Z.; Hussain, F.; Bauer, T.; Strobl, H.; Selzer, P.; Strohmaier, W.; Freissmuth, M.; Zebedin-Brandl, E. Pharmacological stimulation of murine and human hematopoetic stem cells. BMC Pharmacol. Toxicol. 2012, 13. [Google Scholar] [CrossRef]
- Ogle, M.E.; Olingy, C.E.; Awojoodu, A.O.; Das, A.; Ortiz, R.A.; Cheung, H.Y.; Botchwey, E.A. Sphingosine-1-Phosphate Receptor-3 Supports Hematopoietic Stem and Progenitor Cell Residence Within the Bone Marrow Niche. Stem Cells (Dayton Ohio) 2017, 35, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.S.; Cunningham, C.; Adams, G.B. Pharmacologic modulation of the calcium-sensing receptor enhances hematopoietic stem cell lodgment in the adult bone marrow. Blood 2011, 117, 1167–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferdous, M.; Ganuza, M.; Holmfeldt, P.; Hall, T.; Walker, M.; McKinney-Freeman, S. The G protein-coupled receptor associated sorting proteins, Gprasp2 and Armcx1 are putative negative regulators of HSC engraftment and repopulation. Blood 2015, 126, 2386. [Google Scholar]
- Cutler, C.; Kim, H.T.; Ayanian, S.; Bradwin, G.; Revta, C.; Aldridge, J.; Ho, V.; Alyea, E.; Koreth, J.; Armand, P.; et al. Prediction of veno-occlusive disease using biomarkers of endothelial injury. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2010, 16, 1180–1185. [Google Scholar] [CrossRef] [PubMed]
- Carreras, E.; Diaz-Ricart, M. The role of the endothelium in the short-term complications of hematopoietic SCT. Bone Marrow Transplant. 2011, 46, 1495–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corbacioglu, S.; Cesaro, S.; Faraci, M.; Valteau-Couanet, D.; Gruhn, B.; Rovelli, A.; Boelens, J.J.; Hewitt, A.; Schrum, J.; Schulz, A.S.; et al. Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell transplantation: An open-label, phase 3, randomised controlled trial. Lancet 2012, 379, 1301–1309. [Google Scholar] [CrossRef]
- Carreras, E.; Diaz-Beya, M.; Rosinol, L.; Martinez, C.; Fernandez-Aviles, F.; Rovira, M. The incidence of veno-occlusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2011, 17, 1713–1720. [Google Scholar] [CrossRef]
- Corbacioglu, S.; Carreras, E.; Ansari, M.; Balduzzi, A.; Cesaro, S.; Dalle, J.H.; Dignan, F.; Gibson, B.; Guengoer, T.; Gruhn, B.; et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: A new classification from the European society for blood and marrow transplantation. Bone Marrow Transplant. 2018, 53, 138–145. [Google Scholar] [CrossRef]
- Mohty, M.; Malard, F.; Abecassis, M.; Aerts, E.; Alaskar, A.S.; Aljurf, M.; Arat, M.; Bader, P.; Baron, F.; Bazarbachi, A.; et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: A new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2016, 51, 906–912. [Google Scholar] [CrossRef]
- Ikezoe, T.; Togitani, K.; Komatsu, N.; Isaka, M.; Yokoyama, A. Successful treatment of sinusoidal obstructive syndrome after hematopoietic stem cell transplantation with recombinant human soluble thrombomodulin. Bone Marrow Transplant. 2010, 45, 783–785. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S. The Preventative Effects of Recombinant Thrombomodulin on Transplantation-Associated Coagulopathy after Allogeneic Hematopoietic Stem Cell Transplantation. J. Stem Cell Res. Ther. 2014, 04. [Google Scholar] [CrossRef]
- Ikezoe, T.; Yang, J.; Nishioka, C.; Pan, B.; Xu, K.; Furihata, M.; Nakamura, K.; Yurimoto, H.; Sakai, Y.; Honda, G.; et al. The fifth epidermal growth factor-like region of thrombomodulin exerts cytoprotective function and prevents SOS in a murine model. Bone Marrow Transplant. 2017, 52, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pan, B.; Honda, G.; Wang, X.; Hashimoto, Y.; Ohkawara, H.; Xu, K.; Zeng, L.; Ikezoe, T. Cytoprotective and pro-angiogenic functions of thrombomodulin are preserved in the C loop of the fifth epidermal growth factor-like domain. Haematologica 2018, 103, 1730–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, B.; Wang, X.; Nishioka, C.; Honda, G.; Yokoyama, A.; Zeng, L.; Xu, K.; Ikezoe, T. G-protein coupled receptor 15 mediates angiogenesis and cytoprotective function of thrombomodulin. Sci. Rep. 2017, 7, 692. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Wang, X.; Kojima, S.; Nishioka, C.; Yokoyama, A.; Honda, G.; Xu, K.; Ikezoe, T. The Fifth Epidermal Growth Factor-like Region of Thrombomodulin Alleviates Murine Graft-versus-Host Disease in a G-Protein Coupled Receptor 15 Dependent Manner. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2017, 23, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Carreras, E.; Iacobelli, M.; Nejadnik, B. The use of defibrotide in blood and marrow transplantation. Blood Adv. 2018, 2, 1495–1509. [Google Scholar] [CrossRef]
- Zhou, Q.; Chu, X.; Ruan, C. Defibrotide Stimulates Expression of Thrombomodulin in Human Endothelial Cells. Thromb. Haemost. 1994, 72, 507–510. [Google Scholar] [CrossRef]
- Narita, M.; Hatano, E.; Tamaki, N.; Yamanaka, K.; Yanagida, A.; Nagata, H.; Asechi, H.; Takada, Y.; Ikai, I.; Uemoto, S. Dai-kenchu-to attenuates rat sinusoidal obstruction syndrome by inhibiting the accumulation of neutrophils in the liver. J. Gastroenterol. Hepatol. 2009, 24, 1051–1057. [Google Scholar] [CrossRef]
- Markey, K.A.; MacDonald, K.P.A.; Hill, G.R. The biology of graft-versus-host disease: Experimental systems instructing clinical practice. Blood 2014, 124, 354–362. [Google Scholar] [CrossRef]
- Ferrara, J.L.M.; Levine, J.E.; Reddy, P.; Holler, E. Graft-versus-host disease. Lancet 2009, 373, 1550–1561. [Google Scholar] [CrossRef]
- Jagasia, M.; Arora, M.; Flowers, M.E.; Chao, N.J.; McCarthy, P.L.; Cutler, C.S.; Urbano-Ispizua, A.; Pavletic, S.Z.; Haagenson, M.D.; Zhang, M.J.; et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood 2012, 119, 296–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeiser, R.; Blazar, B.R. Acute Graft-versus-Host Disease—Biologic Process, Prevention, and Therapy. N. Engl. J. Med. 2017, 377, 2167–2179. [Google Scholar] [CrossRef] [PubMed]
- Bouazzaoui, A.; Spacenko, E.; Mueller, G.; Miklos, S.; Huber, E.; Holler, E.; Andreesen, R.; Hildebrandt, G.C. Chemokine and chemokine receptor expression analysis in target organs of acute graft-versus-host disease. Genes Immun. 2009, 10, 687–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, M.; Ota, A.; Hori, T.; Imai, S.; Sohma, H.; Suzuki, N.; Hatakeyama, N.; Inazawa, N.; Ito, Y.M.; Kimura, H.; et al. Early expression of plasma CCL8 closely correlates with survival rate of acute graft-vs.-host disease in mice. Exp. Hematol. 2011, 39, 1101–1112. [Google Scholar] [CrossRef]
- Terwey, T.H.; Kim, T.D.; Kochman, A.A.; Hubbard, V.M.; Lu, S.; Zakrzewski, J.L.; Ramirez-Montagut, T.; Eng, J.M.; Muriglan, S.J.; Heller, G.; et al. CCR2 is required for CD8-induced graft-versus-host disease. Blood 2005, 106, 3322–3330. [Google Scholar] [CrossRef] [Green Version]
- Miklos, S.; Mueller, G.; Chang, Y.; Bouazzaoui, A.; Spacenko, E.; Schubert, T.E.O.; Grainger, D.J.; Holler, E.; Andreesen, R.; Hildebrandt, G.C. Preventive usage of broad spectrum chemokine inhibitor NR58-3.14.3 reduces the severity of pulmonary and hepatic graft-versus-host disease. Int. J. Hematol. 2009, 89, 383–397. [Google Scholar] [CrossRef]
- Li, N.; Chen, Y.; He, W.; Yi, T.; Zhao, D.; Zhang, C.; Lin, C.L.; Todorov, I.; Kandeel, F.; Forman, S.; et al. Anti-CD3 preconditioning separates GVL from GVHD via modulating host dendritic cell and donor T-cell migration in recipients conditioned with TBI. Blood 2009, 113, 953–962. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.Y.; Kim, I.; Kim, J.H.; Lee, Y.G.; Kang, E.J.; Cho, H.J.; Lee, K.H.; Kim, H.J.; Park, E.H.; Lee, J.E.; et al. RANTES polymorphisms and the risk of graft-versus-host disease in human leukocyte antigen-matched sibling allogeneic hematopoietic stem cell transplantation. Acta Haematol. 2013, 129, 137–145. [Google Scholar] [CrossRef]
- Brissot, E.; Bossard, C.; Malard, F.; Braudeau, C.; Chevallier, P.; Guillaume, T.; Delaunay, J.; Josien, R.; Gregoire, M.; Gaugler, B.; et al. Involvement of the CX3CL1 (fractalkine)/CX3CR1 pathway in the pathogenesis of acute graft-versus-host disease. J. Leukoc. Biol. 2015, 97, 227–235. [Google Scholar] [CrossRef]
- Coghill, J.M.; West, M.L.; Cook, D.N.; Serody, J.S. The absence of cc-chemokine receptor 8 on donor regulatory t cells impairs their ability to prevent lethal acute GVHD. Blood 2010, 116. [Google Scholar]
- Vinci, P.; Recordati, C.; Bardelli, D.; Cappuzzello, C.; Fumagalli, V.; Dander, E.; Del Prete, A.; Sozzani, S.; Biondi, A.; D’Amico, G. The chemerin/ChemR23 axis plays a pivotal role in the pathogenesis of intestinal damage in a murine model of graft versus host disease. Blood 2015, 126, 3077. [Google Scholar]
- Bogunia-Kubik, K.; Mizia, S.; Polak, M.; Gronkowska, A.; Nowak, J.; Kyrcz-Krzemien, S.; Markiewicz, M.; Dzierzak-Mietla, M.; Koclega, A.; Sedzimirska, M.; et al. Beneficial effect of the CXCL12-3′A variant for patients undergoing hematopoietic stem cell transplantation from unrelated donors. Cytokine 2015, 76, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Maruyama, K.; Yamauchi, N.; Kobayashi, R.; Nagano, S.; Ishikawa, T.; Yoshii, Y.; Uoshima, N.; Hosoi, H. Effects of humanized anti-CC chemokine receptor 4 monoclonal antibody on regulatory T cells and GVHD around hematopoietic stem cell transplantation for adult T-cell leukemia lymphoma. Haematologica 2013, 98, 377. [Google Scholar]
- Sugio, T.; Kato, K.; Aoki, T.; Ota, T.; Saito, N.; Yoshida, S.; Kawano, I.; Henzan, H.; Takase, K.; Muta, T.; et al. Prior use of mogamulizumab to allogenic hematopoietic stem cell transplantation induces severe acute graft-versus-host disease. Blood 2015, 126, 1940. [Google Scholar]
- Khandelwal, P.; Davies, S.M.; Jordan, M.B.; Lake, K.E.; Litts, B.; Owsley, E.; Marsh, R.A. α4β7 Integrin Is Upregulated on CD8+ Effector Memory T-Cells in Children with Gut Gvhd Prior to Clinical Symptoms and Represents a Therapeutic Target in Pediatric Allogeneic HSCT Patients. Biol. Blood Marrow Transplant. 2019, 25, S265–S266. [Google Scholar] [CrossRef]
- Julg, B.; Goebel, F.D. Susceptibility to HIV/AIDS: An individual characteristic we can measure? Infection 2005, 33, 160–162. [Google Scholar] [CrossRef]
- Bogunia-Kubik, K.; Duda, D.; Suchnicki, K.; Lange, A. CCR5 deletion mutation and its association with the risk of developing acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Haematologica 2006, 91, 1628–1634. [Google Scholar] [PubMed]
- Ma, Q.; Gooley, T.A.; Storb, R.F. CCR5 expression on cells from HLA-matched unrelated marrow donors and graft-versus-host disease. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2010, 16, 132–133. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Hammer, S.G.; Braun, T.; Hanash, S.; Ferrara, J.L.M.; Greenson, J.K.; Wang, H.; Zhang, Q.; Vander Lugt, M.; Pongtornpipat, P.; et al. A novel CD4+CD146+CCR5+T-cell population is a biomarker of intestinal graft-versus-host disease. Bone Marrow Transplant. 2013, 48, S66. [Google Scholar] [CrossRef]
- Li, W.; Liu, L.; Gomez, A.; Zhang, J.; Ramadan, A.; Zhang, Q.; Choi, S.W.; Zhang, P.; Greenson, J.K.; Liu, C.; et al. Proteomics analysis reveals a Th17-prone cell population in presymptomatic graft-versus-host disease. JCI Insight 2016, 1, 86660. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, L.; Gomez, A.; Zhang, Q.; Zhang, J.; Ramadan, A.; Mumaw, C.L.; Greenson, J.K.; Hammer, S.; Lin, J.; et al. A novel Th17-Prone CD146+CCR5+ T-Cell population as an early marker of intestinal graft-versus-host disease. Blood 2014, 124, 3. [Google Scholar]
- Sartor, M.; Lau, J.; Gottlieb, D.; Bradstock, K. CCR5 expression on circulating blood DC post-allogeneic haematopoietic cell transplant is highly predictive for the development of clinically significant acute graft-versushost disease. Bone Marrow Transplant. 2010, 45, S125–S126. [Google Scholar] [CrossRef]
- Sartor, M.M.; Lau, J.; Gottlieb, D.J.; Bradstock, K.F. CCR5 expression on circulating blood dc post-allogeneic hemopoietic cell transplant is highly predictive for the development of clinically significant acute graft versus host disease. Blood 2009, 114. [Google Scholar] [CrossRef]
- Shahin, K.; Sartor, M.; Hart, D.N.; Bradstock, K.F. Alterations in chemokine receptor CCR5 expression on blood dendritic cells correlate with acute graft-versus-host disease. Transplantation 2013, 96, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Reshef, R.; Luger, S.M.; Hexner, E.O.; Loren, A.W.; Frey, N.V.; Nasta, S.D.; Goldstein, S.C.; Stadtmauer, E.A.; Smith, J.; Bailey, S.; et al. Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N. Engl. J. Med. 2012, 367, 135–145. [Google Scholar] [CrossRef]
- Huffman, A.P.; Richman, L.P.; Crisalli, L.; Ganetsky, A.; Porter, D.L.; Vonderheide, R.H.; Reshef, R. Pharmacodynamic Monitoring Predicts Outcomes of CCR5 Blockade as Graft-versus-Host Disease Prophylaxis. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2018, 24, 594–599. [Google Scholar] [CrossRef] [Green Version]
- Moy, R.H.; Huffman, A.P.; Richman, L.P.; Crisalli, L.; Hoxie, J.A.; Vonderheide, R.H.; Porter, D.L.; Reshef, R. Immunologic effects of CCR5 blockade in graft-versus-host disease prophylaxis. Blood 2015, 126, 920. [Google Scholar]
- Moy, R.H.; Huffman, A.P.; Richman, L.P.; Crisalli, L.; Wang, X.K.; Hoxie, J.A.; Mick, R.; Emerson, S.G.; Zhang, Y.; Vonderheide, R.H.; et al. Clinical and immunologic impact of CCR5 blockade in graft-versus-host disease prophylaxis. Blood 2017, 129, 906–916. [Google Scholar] [CrossRef]
- Reshef, R.; Ganetsky, A.; Acosta, E.P.; Blauser, R.; Crisalli, L.; McGraw, J.; Frey, N.V.; Hexner, E.O.; Hoxie, J.A.; Loren, A.W.; et al. Extended CCR5 Blockade for Graft-versus-Host Disease Prophylaxis Improves Outcomes of Reduced-Intensity Unrelated Donor Hematopoietic Cell Transplantation: A Phase II Clinical Trial. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2018, 25, 515–521. [Google Scholar] [CrossRef]
- Reshef, R.; Luger, S.M.; Loren, A.W.; Frey, N.V.; Goldstein, S.C.; Hexner, E.O.; Stadtmauer, E.A.; Smith, J.; Mick, R.; Heitjan, D.F.; et al. Prevention of graft-versus-host disease by inhibition of lymphocyte trafficking using a CCR5 antagonist. Blood 2010, 116. [Google Scholar]
- Khandelwal, P.; Fukuda, T.; Teusink-Cross, A.; Kashuba, A.D.; Marsh, R.A.; Mehta, P.A.; Vinks, A.; Nelson, A.S.; Myers, K.C.; Krupski, M.C.; et al. Pediatric Phase II Study of Maraviroc for Acute Graft Versus Host Disease Prophylaxis. Biol. Blood Marrow Transplant. 2019, 25, S251–S252. [Google Scholar] [CrossRef]
- Wang, Z.; Murphy, W.J. Relationship between CCR5 and acute graft-versus-host disease in murine bone marrow transplantation. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2006, 14, 934–940. [Google Scholar] [PubMed]
- Welniak, L.A.; Wang, Z.; Sun, K.; Kuziel, W.; Anver, M.R.; Blazar, B.R.; Murphy, W.J. An absence of CCR5 on donor cells results in acceleration of acute graft-vs-host disease. Exp. Hematol. 2004, 32, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, C.A.; Burkett, S.B.; Panoskaltsis-Mortari, A.; Kirby, S.L.; Luster, A.D.; McKinnon, K.; Blazar, B.R.; Serody, J.S. Differential roles for CCR5 expression on donor T cells during graft-versus-host disease based on pretransplant conditioning. J. Immunol. (Baltimore Md. 1950) 2004, 173, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Burger, D.R.; Parker, Y.; Guinta, K.; Lindner, D. PRO 140 Monoclonal Antibody to CCR5 Prevents Acute Xenogeneic Graft-versus-Host Disease in NOD-scid IL-2Rynull Mice. Biol. Blood Marrow Transplant. 2018, 24, 260–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.; Ren, H.Y.; Shi, Y.J.; Liu, W. Prophylaxis of acute graft-versus-host disease by CCR5 blockade combined with cyclosporine A in a murine model. Inflamm. Res. 2015, 64, 137–144. [Google Scholar] [CrossRef]
- Tang, B.; Ren, H.; Liu, H.; Shi, Y.; Liu, W.; Dong, Y.; Yin, Y.; Miao, S. CCR5 blockade combined with cyclosporine A attenuates liver GVHD by impairing T cells function. Inflamm. Res. 2016, 65, 917–924. [Google Scholar] [CrossRef]
- Tang, B.; Liu, H.; Miao, S.; Dong, Y.; Liu, W.; Shi, Y.; Qin, C.; Wang, Z.; Zhao, M.; Ren, H. Combined CCR5 and CXCR3 blockade attenuates murine agvhd through alternating donor-derived t cell distribution and function. Blood 2017, 130, 5449. [Google Scholar]
- Hasegawa, H.; Inoue, A.; Kohno, M.; Lei, J.; Miyazaki, T.; Yoshie, O.; Nose, M.; Yasukawa, M. Therapeutic effect of CXCR3-expressing regulatory T cells on liver, lung and intestinal damages in a murine acute GVHD model. Gene Ther. 2008, 15, 171–182. [Google Scholar] [CrossRef]
- Duffner, U.; Lu, B.; Hildebrandt, G.C.; Teshima, T.; Williams, D.L.; Reddy, P.; Ordemann, R.; Clouthier, S.G.; Lowler, K.; Liu, C.; et al. Role of CXCR3-induced donor T-cell migration in acute GVHD. Exp. Hematol. 2003, 31, 897–902. [Google Scholar] [CrossRef]
- He, S.; Cao, Q.; Qiu, Y.; Mi, J.; Zhang, J.Z.; Jin, M.; Ge, H.; Emerson, S.G.; Zhang, Y.; Zhang, Y. A new approach to the blocking of alloreactive T cell-mediated graft-versus-host disease by in vivo administration of anti-CXCR3 neutralizing antibody. J. Immunol. (Baltimore Md. 1950) 2008, 181, 7581–7592. [Google Scholar] [CrossRef] [PubMed]
- Satake, A.; Inoue, T.; Kubo, S.; Taniguchi, Y.; Imado, T.; Fujioka, T.; Horiuchi, M.; Xu, Y.; Ikegame, K.; Yoshihara, S.; et al. Separation of antileukemic effects from graft-versus-host disease in MHC-haploidentical murine bone marrow transplantation: Participation of host immune cells. Int. J. Hematol. 2010, 91, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Ziga, E.D.; Ritchey, J.; Collins, L.; Prior, J.L.; Cooper, M.L.; Piwnica-Worms, D.; DiPersio, J.F. IFNgammaR signaling mediates alloreactive T-cell trafficking and GVHD. Blood 2012, 120, 4093–4103. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Cooper, M.L.; Alahmari, B.; Ritchey, J.; Collins, L.; Holt, M.; DiPersio, J.F. Pharmacologic blockade of JAK1/JAK2 reduces GvHD and preserves the graft-versus-leukemia effect. PLoS ONE 2014, 9, e109799. [Google Scholar] [CrossRef]
- Miao, S.; Tang, B.; Liu, H.; Wang, Z.; Shi, Y.; Dong, Y.; Liu, W.; Qin, C.; Ren, H. CXCR3 blockade combined with cyclosporine A alleviates acute graft-versus-host disease by inhibiting alloreactive donor T cell responses in a murine model. Mol. Immunol. 2018, 94, 82–90. [Google Scholar] [CrossRef]
- O’Boyle, G.; Barker, C.; Mavin, E.; Wang, X.; Fox, C.; Walden, H.; Willet, J.; Hine, D.; Palmer, J.; Lamb, C.; et al. A small molecule agonist of the chemokine receptor CXCR3 prevents experimental graft-versus-host disease. Immunology 2013, 140, 149. [Google Scholar] [CrossRef]
- Broen, K.; van der Waart, A.B.; Greupink-Draaisma, A.; Metzig, J.; Feuth, T.; Schaap, N.P.; Blijlevens, N.M.; van der Velden, W.J.; Dolstra, H. Polymorphisms in CCR6 are associated with chronic graft-versus-host disease and invasive fungal disease in matched-related hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2011, 17, 1443–1449. [Google Scholar] [CrossRef]
- de Jager, S.C.; Cante-Barrett, K.; Bot, I.; Husberg, C.; van Puijvelde, G.H.; van Santbrink, P.J.; Yndestad, A.; van den Oever, J.M.; Kuiper, J.; van Berkel, T.J.; et al. Impaired effector memory T-cell regulation facilitates graft versus host disease in CCR7-deficient bone marrow transplant chimeras. Transplantation 2009, 88, 631–639. [Google Scholar] [CrossRef]
- Xu, Y.J.; Chen, W.R.; Li, D.P.; Song, L.X.; Wu, J.Q.; Zhang, P.; Li, Z.Y.; Huang, Y.H. Suppression of lentivirus-mediated transgenic dendritic cells in graft-versus-host disease after allogeneic bone marrow transplantation in mice. Genet. Mol. Res. GMR 2015, 14, 11444–11455. [Google Scholar] [CrossRef]
- Wang, M.; Hu, J.; Qiu, Z.X.; Liu, W.; Wang, M.J.; Li, Y.; Sun, Y.H.; Zhu, S.N.; Ren, H.Y.; Dong, Y.J. Alterations of CCR5 and CCR7 expression on donor peripheral blood T cell subsets after mobilization with rhG-CSF correlate with acute graft-versus-host disease. Clin. Immunol. 2018, 191, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ren, H.Y.; Dong, Y.J.; Liang, Z.Y.; Qiu, Z.X.; Liu, W.; Wang, L.H. Association of chemokine receptor CCR5, CCR6 and CCR7 expressions on T lymphocyte subsets in recipients after Allo-HSCT with acute GvHD. Blood 2013, 122, 4596. [Google Scholar]
- Ren, H.Y.; Wang, M.; Ma, X.J.; Dong, Y.J.; Qiu, Z.X.; Liu, W. Differential regulation of chemokine receptor expressions on T lymphocyte subsets in healthy donors after mobilization with RHG-CSF and its correlation with acute GvHD. Blood 2013, 122. [Google Scholar]
- Goncalves, K.A.; Falahee, P.C.; Hyzy, S.L.; Li, S.; Boitano, A.E.; Morrow, D.M.; Cooke, M.P. Co-Administration of Mgta-145 and Plerixafor Rapidly Mobilizes High Numbers of Hematopoietic Stem Cells and Graft-Versus-Host Disease Inhibiting Monocytic Cells in Non-Human Primates. Biol. Blood Marrow Transplant. 2019, 25, S291–S292. [Google Scholar] [CrossRef] [Green Version]
- Cho, K.A.; Woo, S.Y.; Park, Y.S.; Park, M.H.; Ryu, K.H. Macrophage inflammatory protein-2 (MIP-2)/CXCR2 blockade attenuates acute graft-versus-host disease while preserving graft-versus-leukemia activity. Biochem. Biophys. Res. Commun. 2012, 426, 558–564. [Google Scholar] [CrossRef]
- Lazarus, H.M.; Sommers, S.R.; Arfons, L.M.; Fu, P.; Ataergin, S.A.; Kaye, N.M.; Liu, F.; Kindwall-Keller, T.L.; Cooper, B.W.; Laughlin, M.J.; et al. Spontaneous autologous graft-versus-host disease in plasma cell myeloma autograft recipients: Flow cytometric analysis of hematopoietic progenitor cell grafts. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2011, 17, 970–978. [Google Scholar] [CrossRef]
- Arbez, J.; Saas, P.; Lamarthee, B.; Malard, F.; Couturier, M.; Mohty, M.; Gaugler, B. Impact of donor hematopoietic cells mobilized with G-CSF and plerixafor on murine acute graft-versus-host-disease. Cytotherapy 2015, 17, 948–955. [Google Scholar] [CrossRef]
- Lundqvist, A.; Smith, A.L.; Takahashi, Y.; Wong, S.; Bahceci, E.; Cook, L.; Ramos, C.; Tawab, A.; McCoy, J.P., Jr.; Read, E.J.; et al. Differences in the phenotype, cytokine gene expression profiles, and in vivo alloreactivity of T cells mobilized with plerixafor compared with G-CSF. J. Immunol. (Baltimore Md. 1950) 2013, 191, 6241–6249. [Google Scholar] [CrossRef]
- Han, K.L.; Changpriroa, C.M.; Malech, H.L.; Kang, E.M. Activation of the adenosine A2A receptor promotes the development of donor-derived T regulatory cells in a graft-versus host disease mouse model. Blood 2009, 114. [Google Scholar]
- Lappas, C.M.; Liu, P.C.; Linden, J.; Kang, E.M.; Malech, H.L. Adenosine A2A receptor activation limits graft-versus-host disease after allogenic hematopoietic stem cell transplantation. J. Leukoc. Biol. 2010, 87, 345–354. [Google Scholar] [CrossRef]
- Leigh, N.D.; Kokolus, K.M.; Eng, J.W.L.; Qiu, J.; Chen, G.L.; McCarthy, P.L.; Cao, X.; Repasky, E.A. The degree of adrenergic stress signaling regulates the severity of graft versus host disease following allogeneic hematopoietic cell transplantation. Cancer Immunol. Res. 2015, 3, B43. [Google Scholar] [CrossRef]
- Leigh, N.D.; Kokolus, K.M.; O’Neill, R.E.; Du, W.; Eng, J.W.; Qiu, J.; Chen, G.L.; McCarthy, P.L.; Farrar, J.D.; Cao, X.; et al. Housing Temperature-Induced Stress Is Suppressing Murine Graft-versus-Host Disease through beta2-Adrenergic Receptor Signaling. J. Immunol. (Baltimore Md. 1950) 2015, 195, 5045–5054. [Google Scholar] [CrossRef] [PubMed]
- Klambt, V.; Wohlfeil, S.A.; Schwab, L.; Hulsdunker, J.; Ayata, K.; Apostolova, P.; Schmitt-Graeff, A.; Dierbach, H.; Prinz, G.; Follo, M.; et al. A Novel Function for P2Y2 in Myeloid Recipient-Derived Cells during Graft-versus-Host Disease. J. Immunol. (Baltimore Md. 1950) 2015, 195, 5795–5804. [Google Scholar] [CrossRef] [PubMed]
- Sido, J.M.; Nagarkatti, P.S.; Nagarkatti, M. Delta(9)-Tetrahydrocannabinol attenuates allogeneic host-versus-graft response and delays skin graft rejection through activation of cannabinoid receptor 1 and induction of myeloid-derived suppressor cells. J. Leukoc. Biol. 2015, 98, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Kemter, A.M.; Scheu, S.; Huser, N.; Ruland, C.; Schumak, B.; Findeiss, M.; Cheng, Z.; Assfalg, V.; Arolt, V.; Zimmer, A.; et al. The cannabinoid receptor 2 is involved in acute rejection of cardiac allografts. Life Sci. 2015, 138, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Hegde, V.L.; Nagarkatti, M.; Nagarkatti, P.S. Targeting cannabinoid receptors as a novel approach in the treatment of graft-versus-host disease: Evidence from an experimental murine model. J. Pharmacol. Exp. Ther. 2011, 338, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Yuan, V.; Belle, L.; Komorowski, R.; Hillard, C.; Drobyski, W.R. The type 2 cannabinoid receptor regulates the severity of acute and chronic graft versus host disease in mice. Biol. Blood Marrow Transplant. 2018, 24, S175. [Google Scholar] [CrossRef]
- Yeshurun, M.; Shpilberg, O.; Herscovici, C.; Shargian, L.; Dreyer, J.; Peck, A.; Israeli, M.; Levy-Assaraf, M.; Gruenewald, T.; Mechoulam, R.; et al. Cannabidiol for the Prevention of Graft-versus-Host-Disease after Allogeneic Hematopoietic Cell Transplantation: Results of a Phase II Study. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2015, 21, 1770–1775. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.; O’Sullivan, C.; Gergely, P. Sphingosine 1-Phosphate Signaling and Its Pharmacological Modulation in Allogeneic Hematopoietic Stem Cell Transplantation. Int. J. Mol. Sci. 2017, 18, E2027. [Google Scholar] [CrossRef]
- Cheng, Q.; Ma, S.; Lin, D.; Mei, Y.; Gong, H.; Lei, L.; Chen, Y.; Zhao, Y.; Hu, B.; Wu, Y.; et al. The S1P1 receptor-selective agonist CYM-5442 reduces the severity of acute GVHD by inhibiting macrophage recruitment. Cell. Mol. Immunol. 2015, 12, 681–691. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Bastian, D.; Kuril, S.; Dany, M.; Fairlie, D.; Atkinson, C.; Ogretmen, B.; Tomlinson, S.; Yu, X.Z. Complement promotes GVHD through suppressing autophagy in recipient dendritic cells after allogeneic hematopoietic cell transplantation in mice. Blood 2017, 130, 1904. [Google Scholar]
- Castor, M.G.; Rezende, B.M.; Resende, C.B.; Bernardes, P.T.; Cisalpino, D.; Vieira, A.T.; Souza, D.G.; Silva, T.A.; Teixeira, M.M.; Pinho, V. Platelet-activating factor receptor plays a role in the pathogenesis of graft-versus-host disease by regulating leukocyte recruitment, tissue injury, and lethality. J. Leukoc. Biol. 2012, 91, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Harkensee, C.; Oka, A.; Onizuka, M.; Middleton, P.G.; Inoko, H.; Nakaoka, H.; Gennery, A.R.; Ando, K.; Morishima, Y. Microsatellite scanning of the immunogenome associates MAPK14 and ELTD1 with graft-versus-host disease in hematopoietic stem cell transplantation. Immunogenetics 2013, 65, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Hayase, E.; Hashimoto, D.; Takahashi, S.; Ohigashi, H.; Tateno, T.; Ara, T.; Yokoyama, E.; Teshima, T. Gastric stem cells are targeted in upper gastrointestinal GVHD. Blood 2017, 130, 3173. [Google Scholar]
- Ranjan, S.; Goihl, A.; Kohli, S.; Gadi, I.; Pierau, M.; Shahzad, K.; Gupta, D.; Bock, F.; Wang, H.; Shaikh, H.; et al. Activated protein C protects from GvHD via PAR2/PAR3 signalling in regulatory T-cells. Nat. Commun. 2017, 8, 311. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Ishii, K.; Fujita, S.; Nakaya, A.; Satake, A.; Ito, T. Associations between acute GVHD-related biomarkers and endothelial cell activation after allogeneic hematopoietic stem cell transplantation. Transpl. Immunol. 2017, 43–44, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; van Esch, B.; Henricks, P.A.J.; Folkerts, G.; Garssen, J. The Anti-inflammatory Effects of Short Chain Fatty Acids on Lipopolysaccharide-or Tumor Necrosis Factor alpha-Stimulated Endothelial Cells via Activation of GPR41/43 and Inhibition of HDACs. Front. Pharmacol. 2018, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Kamp, M.E.; Shim, R.; Nicholls, A.J.; Oliveira, A.C.; Mason, L.J.; Binge, L.; Mackay, C.R.; Wong, C.H. G Protein-Coupled Receptor 43 Modulates Neutrophil Recruitment during Acute Inflammation. PLoS ONE 2016, 11, e0163750. [Google Scholar] [CrossRef] [PubMed]
- Luu, M.; Weigand, K.; Wedi, F.; Breidenbend, C.; Leister, H.; Pautz, S.; Adhikary, T.; Visekruna, A. Regulation of the effector function of CD8(+) T cells by gut microbiota-derived metabolite butyrate. Sci. Rep. 2018, 8, 14430. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Docampo, M.D.; Riwes, M.; Peltier, D.; Toubai, T.; Henig, I.; Wu, S.J.; Kim, S.; Taylor, A.; Brabbs, S.; et al. Microbial metabolite sensor GPR43 controls severity of experimental GVHD. Nat. Commun. 2018, 9, 3674. [Google Scholar] [CrossRef] [PubMed]
- Lamarthee, B.; Malard, F.; Gamonet, C.; Bossard, C.; Couturier, M.; Renauld, J.C.; Mohty, M.; Saas, P.; Gaugler, B. Donor interleukin-22 and host type I interferon signaling pathway participate in intestinal graft-versus-host disease via STAT1 activation and CXCL10. Mucosal Immunol. 2016, 9, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Qin, X.; Wang, N.; Jiang, E.; Han, M.; Ma, Y.; Liang, B.; Yang, K.; Yu, K.; Ye, H. B Lymphocyte Chemoattractant (CXCL13) Is an Indicator of Acute Gastrointestinal GVHD in Murine Model. Inflammation 2017, 40, 1678–1687. [Google Scholar] [CrossRef] [PubMed]
- McDermott, D.H.; Conway, S.E.; Wang, T.; Ricklefs, S.M.; Agovi, M.A.; Porcella, S.F.; Tran, H.T.; Milford, E.; Spellman, S.; Abdi, R. Donor and recipient chemokine receptor CCR5 genotype is associated with survival after bone marrow transplantation. Blood 2010, 115, 2311–2318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mapara, M.Y.; Leng, C.; Kim, Y.M.; Bronson, R.; Lokshin, A.; Luster, A.; Sykes, M. Expression of chemokines in GVHD target organs is influenced by conditioning and genetic factors and amplified by GVHR. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2006, 12, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, T.; Gu, L.; Huang, C.; Zhu, H.; Meng, W.; Xi, Y.; Li, S.; Liu, Y. Immunomodulatory effects of mesenchymal stem cells involved in favoring type 2 T cell subsets. Transpl. Immunol. 2009, 22, 55–61. [Google Scholar] [CrossRef]
- Lee, S.J. Classification systems for chronic graft-versus-host disease. Blood 2017, 129, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Cooke, K.R.; Luznik, L.; Sarantopoulos, S.; Hakim, F.T.; Jagasia, M.; Fowler, D.H.; van den Brink, M.R.M.; Hansen, J.A.; Parkman, R.; Miklos, D.B.; et al. The Biology of Chronic Graft-versus-Host Disease: A Task Force Report from the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2017, 23, 211–234. [Google Scholar] [CrossRef]
- Kroger, N.; Solano, C.; Wolschke, C.; Bandini, G.; Patriarca, F.; Pini, M.; Nagler, A.; Selleri, C.; Risitano, A.; Messina, G.; et al. Antilymphocyte Globulin for Prevention of Chronic Graft-versus-Host Disease. N. Engl. J. Med. 2016, 374, 43–53. [Google Scholar] [CrossRef]
- Kanakry, C.G.; Tsai, H.L.; Bolanos-Meade, J.; Smith, B.D.; Gojo, I.; Kanakry, J.A.; Kasamon, Y.L.; Gladstone, D.E.; Matsui, W.; Borrello, I.; et al. Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS. Blood 2014, 124, 3817–3827. [Google Scholar] [CrossRef] [Green Version]
- Arai, S.; Arora, M.; Wang, T.; Spellman, S.R.; He, W.; Couriel, D.R.; Urbano-Ispizua, A.; Cutler, C.S.; Bacigalupo, A.A.; Battiwalla, M.; et al. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: A report from the Center for International Blood and Marrow Transplant Research. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2015, 21, 266–274. [Google Scholar] [CrossRef]
- Baker, K.S.; Ness, K.K.; Steinberger, J.; Carter, A.; Francisco, L.; Burns, L.J.; Sklar, C.; Forman, S.; Weisdorf, D.; Gurney, J.G.; et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: A report from the bone marrow transplantation survivor study. Blood 2007, 109, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Sugerman, P.B.; Faber, S.B.; Willis, L.M.; Petrovic, A.; Murphy, G.F.; Pappo, J.; Silberstein, D.; van den Brink, M.R. Kinetics of gene expression in murine cutaneous graft-versus-host disease. Am. J. Pathol. 2004, 164, 2189–2202. [Google Scholar] [CrossRef]
- Martinu, T.; Kinnier, C.V.; Sun, J.; Kelly, F.L.; Nelson, M.E.; Garantziotis, S.; Foster, W.M.; Palmer, S.M. Allogeneic splenocyte transfer and lipopolysaccharide inhalations induce differential T cell expansion and lung injury: A novel model of pulmonary graft-versus-host disease. PLoS ONE 2014, 9, e97951. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, J.N.; Nakamura, S.; Toyoshima, T.; Moriyama, M.; Sasaki, M.; Kawamura, E.; Ohyama, Y.; Kumamaru, W.; Shirasuna, K. Possible involvement of cytokines, chemokines and chemokine receptors in the initiation and progression of chronic GVHD. Bone Marrow Transplant. 2013, 48, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Piccari, S.; Chirumbolo, G.; Nicolini, B.; Ulbar, F.; Tolomelli, G.; Bandini, G.; Bonifazi, F.; Stanzani, M.; Lemoli, R.M.; Baccarani, M.; et al. Increased serum concentration of the inflammatory chemokines IL-8, MIP1-alpha and IP-10 at 3 months after allogeneic hsct correlate with the development of chronic GVHD. Haematologica 2011, 96, 154–155. [Google Scholar]
- Abu Zaid, M.; Wu, J.; Wu, C.; Logan, B.R.; Yu, J.; Cutler, C.; Antin, J.H.; Paczesny, S.; Choi, S.W. Plasma biomarkers of risk for death in a multicenter phase 3 trial with uniform transplant characteristics post-allogeneic HCT. Blood 2017, 129, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Storer, B.E.; Kushekhar, K.; Abu Zaid, M.; Zhang, Q.; Gafken, P.R.; Ogata, Y.; Martin, P.J.; Flowers, M.E.; Hansen, J.A.; et al. Biomarker Panel for Chronic Graft-Versus-Host Disease. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2016, 34, 2583–2590. [Google Scholar] [CrossRef]
- Westekemper, H.; Meller, S.; Citak, S.; Schulte, C.; Steuhl, K.P.; Homey, B.; Meller, D. Differential chemokine expression in chronic GVHD of the conjunctiva. Bone Marrow Transplant. 2010, 45, 1340–1346. [Google Scholar] [CrossRef]
- Croudace, J.E.; Inman, C.F.; Abbotts, B.E.; Nagra, S.; Nunnick, J.; Mahendra, P.; Craddock, C.; Malladi, R.; Moss, P.A. Chemokine-mediated tissue recruitment of CXCR3+ CD4+ T cells plays a major role in the pathogenesis of chronic GVHD. Blood 2012, 120, 4246–4255. [Google Scholar] [CrossRef] [Green Version]
- Imanguli, M.M.; Swaim, W.D.; League, S.C.; Gress, R.E.; Pavletic, S.Z.; Hakim, F.T. Increased T-bet+ cytotoxic effectors and type I interferon-mediated processes in chronic graft-versus-host disease of the oral mucosa. Blood 2009, 113, 3620–3630. [Google Scholar] [CrossRef]
- Croudace, J.; Malladi, R.; Nagra, S.; Craddock, C.; Moss, P. CXCL10 contributes to the pathogenesis of chronic skin GVHD by recruiting CXCR3+ T cells. Haematologica 2011, 96, 190–191. [Google Scholar]
- Croudace, J.; Inman, C.F.; Abbotts, B.; Nagra, S.; Malladi, R.; Craddock, C.; Moss, P. CD4+ T cells, recruited via CXCL10 and CXCR3 interactions mediate chronic skin GVHD and distinguish acute from chronic disease post allogeneic stem cell transplantation. Immunology 2011, 135, 122. [Google Scholar] [CrossRef]
- Imanguli, M.M.; Cowen, E.W.; Rose, J.; Dhamala, S.; Swaim, W.; Lafond, S.; Yagi, B.; Gress, R.E.; Pavletic, S.Z.; Hakim, F.T. Comparative analysis of FoxP3+ regulatory T cells in the target tissues and blood in chronic graft versus host disease. Leukemia 2014, 28, 2016–2027. [Google Scholar] [CrossRef]
- Klimczak, A.; Lach, A.; Jaskula, E.; Lange, A. Epithelial expression of CCR6 chemokine receptor and the presence of IL-17 producing cells is associated with skin pathology in chronic GvHD. Bone Marrow Transplant. 2011, 46, S102. [Google Scholar] [CrossRef]
- Cromvik, J.; Johnsson, M.; Vaht, K.; Johansson, J.E.; Wenneras, C. Eosinophils in the blood of hematopoietic stem cell transplanted patients are activated and have different molecular marker profiles in acute and chronic graft-versus-host disease. Immun. Inflamm. Dis. 2014, 2, 99–113. [Google Scholar] [CrossRef]
- Inamoto, Y.; Murata, M.; Katsumi, A.; Kuwatsuka, Y.; Tsujimura, A.; Ishikawa, Y.; Sugimoto, K.; Onizuka, M.; Terakura, S.; Nishida, T.; et al. Donor single nucleotide polymorphism in the CCR9 gene affects the incidence of skin GVHD. Bone Marrow Transplant. 2010, 45, 363–369. [Google Scholar] [CrossRef] [PubMed]
- McDonald-Hyman, C.; Flynn, R.; Panoskaltsis-Mortari, A.; Peterson, N.; MacDonald, K.P.; Hill, G.R.; Luznik, L.; Serody, J.S.; Murphy, W.J.; Maillard, I.; et al. Therapeutic regulatory T-cell adoptive transfer ameliorates established murine chronic GVHD in a CXCR5-dependent manner. Blood 2016, 128, 1013–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konuma, T.; Kohara, C.; Watanabe, E.; Mizukami, M.; Nagai, E.; Oiwa-Monna, M.; Tanoue, S.; Isobe, M.; Jimbo, K.; Kato, S.; et al. Circulating monocyte subsets in human chronic graft-versus-host disease. Bone Marrow Transplant. 2018, 53, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Vasu, S.; Jaglowski, S.M.; Geyer, S.; Bingman, A.; Elder, P.; Yu, J.; Andritsos, L.A.; Blum, W.; Klisovic, R.B.; Penza, S.; et al. Differential distribution of activated innate and adaptive immune subsets in G-CSF mobilized hematopoietic stem cell allografts may influence incidence of acute (AGVHD) and chronic graft-versus-host disease (CGVHD). Blood 2012, 120, 4192. [Google Scholar]
- Martinu, T.; Gowdy, K.M.; Nugent, J.L.; Sun, J.; Kinnier, C.V.; Nelson, M.E.; Lyes, M.A.; Kelly, F.L.; Foster, W.M.; Gunn, M.D.; et al. Role of C-C motif ligand 2 and C-C motif receptor 2 in murine pulmonary graft-versus-host disease after lipopolysaccharide inhalations. Am. J. Respir. Cell Mol. Biol. 2014, 51, 810–821. [Google Scholar] [CrossRef]
- Kalra, A.; Dharmani-Khan, P.; Patel, S.; Faridi, R.M.; Lewis, V.; Daly, A.; Mansoor, A.; Shabani-Rad, M.T.; Storek, J.; Khan, F.M. Differential Expression of Immunity Related Genes Can Predict Moderate-Severe Chronic GvHD Early after Myeloablative Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, S237. [Google Scholar] [CrossRef]
- Chiron, A.; Bouaziz, J.D.; Carmagnat, M.; Peffault de Latour, R.; Lafaurie-Bergeron, A.; Robin, M.; Xhaard, A.; Toubert, A.; Charron, D.; Guigue, N.; et al. Anti-Angiotensin type 1 receptor antibodies in chronic graft-versus-host disease. Transplantation 2014, 98, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Zerr, P.; Palumbo-Zerr, K.; Distler, A.; Tomcik, M.; Vollath, S.; Munoz, L.E.; Beyer, C.; Dees, C.; Egberts, F.; Tinazzi, I.; et al. Inhibition of hedgehog signaling for the treatment of murine sclerodermatous chronic graft-versus-host disease. Blood 2012, 120, 2909–2917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carreras, E.; Dufour, C.; Mohty, M.; Kröger, N. The EBMT Handbook; Springer Nature Switzerland: Cham, Switzerland, 2019. [Google Scholar]
- Panoskaltsis-Mortari, A.; Griese, M.; Madtes, D.K.; Belperio, J.A.; Haddad, I.Y.; Folz, R.J.; Cooke, K.R.; American Thoracic Society Committee on Idiopathic Pneumonia, S. An Official American Thoracic Society research statement: Noninfectious lung injury after hematopoietic stem cell transplantation: Idiopathic pneumonia syndrome. Am. J. Respir. Crit. Care Med. 2011, 183, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Panoskaltsis-Mortari, A.; Taylor, P.A.; Yaeger, T.M.; Wangensteen, O.D.; Bitterman, P.B.; Ingbar, D.H.; Vallera, D.A.; Blazar, B.R. The critical early proinflammatory events associated with idiopathic pneumonia syndrome in irradiated murine allogeneic recipients are due to donor T cell infusion and potentiated by cyclophosphamide. J. Clin. Investig. 1997, 100, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Hackman, R.C.; Guthrie, K.A.; Sandmaier, B.M.; Boeckh, M.; Maris, M.B.; Maloney, D.G.; Deeg, H.J.; Martin, P.J.; Storb, R.F.; et al. Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventional conditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood 2003, 102, 2777–2785. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yanik, G.; Kitko, C. Management of noninfectious lung injury following hematopoietic cell transplantation. Curr. Opin. Oncol. 2013, 25, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, G.C.; Corrion, L.A.; Olkiewicz, K.M.; Lu, B.; Lowler, K.; Duffner, U.A.; Moore, B.B.; Kuziel, W.A.; Liu, C.; Cooke, K.R. Blockade of CXCR3 receptor:ligand interactions reduces leukocyte recruitment to the lung and the severity of experimental idiopathic pneumonia syndrome. J. Immunol. (Baltimore Md. 1950) 2004, 173, 2050–2059. [Google Scholar] [CrossRef]
- Hildebrandt, G.C.; Olkiewicz, K.M.; Choi, S.; Corrion, L.A.; Clouthier, S.G.; Liu, C.; Serody, J.S.; Cooke, K.R. Donor T-cell production of RANTES significantly contributes to the development of idiopathic pneumonia syndrome after allogeneic stem cell transplantation. Blood 2005, 105, 2249–2257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hildebrandt, G.C.; Duffner, U.A.; Olkiewicz, K.M.; Corrion, L.A.; Willmarth, N.E.; Williams, D.L.; Clouthier, S.G.; Hogaboam, C.M.; Reddy, P.R.; Moore, B.B.; et al. A critical role for CCR2/MCP-1 interactions in the development of idiopathic pneumonia syndrome after allogeneic bone marrow transplantation. Blood 2004, 103, 2417–2426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panoskaltsis-Mortari, A.; Hermanson, J.R.; Taras, E.; Wangensteen, O.D.; Charo, I.F.; Rollins, B.J.; Blazar, B.R. Post-BMT lung injury occurs independently of the expression of CCL2 or its receptor, CCR2, on host cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L284–L292. [Google Scholar] [CrossRef] [PubMed]
- Panoskaltsis-Mortari, A.; Hermanson, J.R.; Taras, E.; Wangensteen, O.D.; Serody, J.S.; Blazar, B.R. Acceleration of idiopathic pneumonia syndrome (IPS) in the absence of donor MIP-1 alpha (CCL3) after allogeneic BMT in mice. Blood 2003, 101, 3714–3721. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.; Pole, J.D.; Gibson, P.; Lee, M.; Hesser, T.; Chi, S.N.; Dvorak, C.C.; Fisher, B.; Hasle, H.; Kanerva, J.; et al. Classification of treatment-related mortality in children with cancer: A systematic assessment. Lancet Oncol. 2015, 16, e604–e610. [Google Scholar] [CrossRef]
- Ambruzova, Z.; Mrazek, F.; Raida, L.; Jindra, P.; Vidan-Jeras, B.; Faber, E.; Pretnar, J.; Indrak, K.; Petrek, M. Association of IL6 and CCL2 gene polymorphisms with the outcome of allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant. 2009, 44, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Takami, A.; Espinoza, J.L.; Matsuo, K.; Morishima, Y.; Onizuka, M.; Fukuda, T.; Kodera, Y.; Akiyama, H.; Miyamura, K.; et al. The recipient CXCL10 + 1642C>G variation predicts survival outcomes after HLA fully matched unrelated bone marrow transplantation. Clin. Immunol. (Orlando F.L.) 2013, 146, 104–111. [Google Scholar] [CrossRef] [PubMed]
- De Soria, V.G.G.; Portero, I.; Fernandez Arandojo, C.; Muñoz, C. GVHD-related mortality is associated with high levels of CCR7+ CD4 T lymphocytes in the graft. Bone Marrow Transplant. 2016, 51, S387. [Google Scholar] [CrossRef]
- Grant, M.J.; Booth, A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Inf. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef]
- Bramer, W.M.; Giustini, D.; de Jonge, G.B.; Holland, L.; Bekhuis, T. De-duplication of database search results for systematic reviews in EndNote. J. Med. Libr. Assoc. 2016, 104, 240–243. [Google Scholar] [CrossRef]
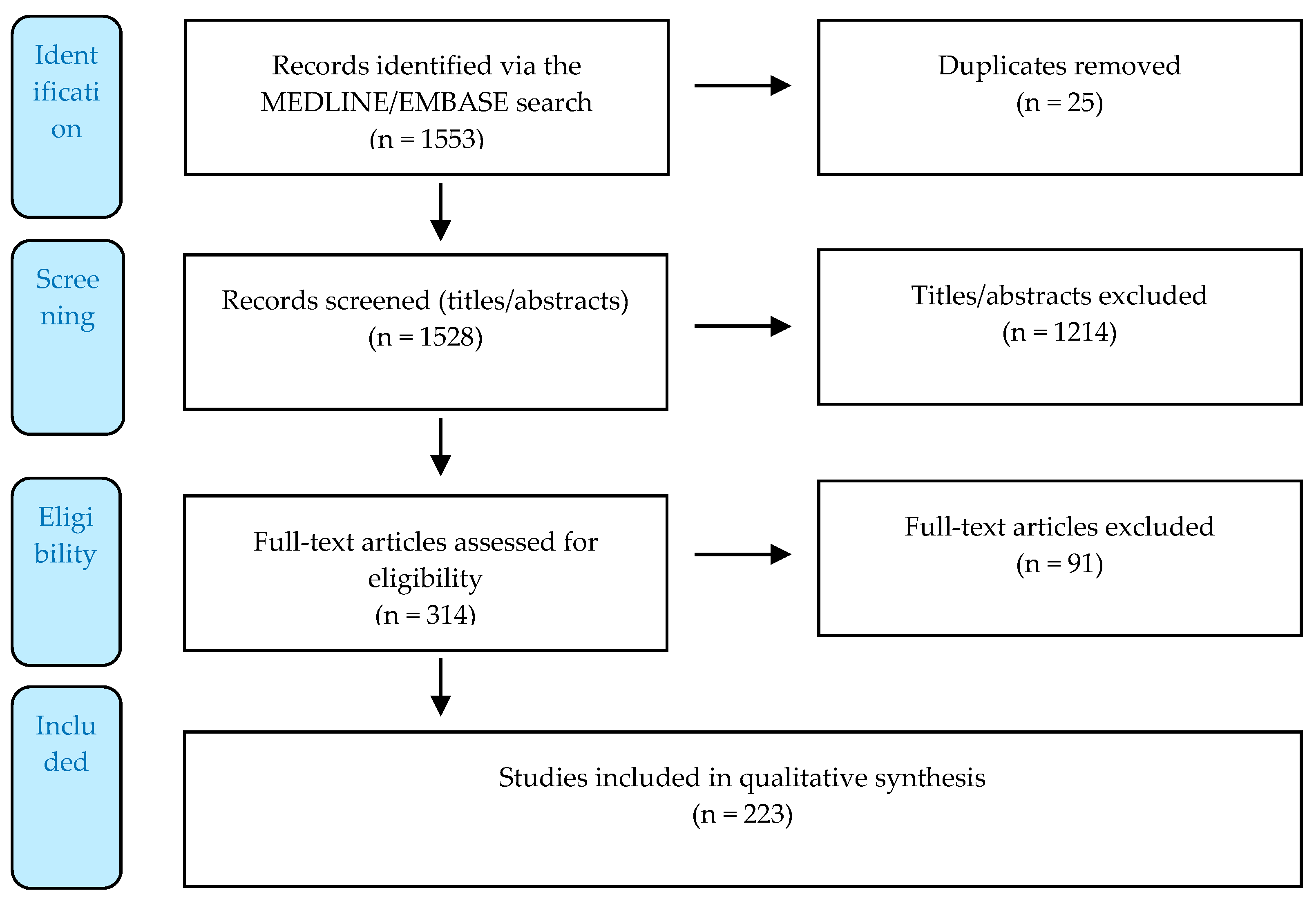

| Mobilization | |||
|---|---|---|---|
| Studies | Correlation with outcome | References | |
| C-X-C ligand 8 (CXCL8) | H | + | [72] |
| C-X-C ligand 12 (CXCL12) | H | 0 | [73] |
| H (rs1801157) | + | [44,45,46] | |
| C-X-C receptor 4 (CXCR4) | H | − | [49,50,51,52,53,54,57,58,60,61,62,63,64,65,66,67,69,70,71,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101] |
| Beta-3 adrenergic receptor (B3AR) | H | + | [102] |
| Protease-activated receptor 1 (PAR1) | H | + | [103] |
| Relaxin/insulin-like family peptide receptor 4 (RXFP4) | H | + | [104] |
| Engraftment | |||
|---|---|---|---|
| Studies | Correlation with Outcome | References | |
| C-C ligand 15 (CCL15) | A | + | [142] |
| C-X-C ligand 9 (CXCL9) | H | − | [132,133] |
| C-X-C ligand 12 (CXCL12) | H (rs1801157) | + | [125] |
| A | + | [130] | |
| C-C receptor 1 (CCR1) | A | + | [135] |
| C-X-C receptor 1 (CXCR1) | A | + | [131] |
| C-X-C receptor 2 (CXCR2) | A | + | [134,143] |
| C-X-C receptor 4 (CXCR4) | A, H | + | [120,121,144,145,146,147] (A), [122,123,124,148] (H) |
| 0 | [112,118] (A), [61,85,94,95,110,111] (H) | ||
| − | [113,114,115,116,117,149] (A), [119] (H) | ||
| Gai-coupled chemokine receptors (Pertussis toxin) | A | 0 | [150] |
| Angiotensin 1 receptor (AT1R) | H | − | [141] |
| Beta-3 adrenergic receptor (B3AR) | H | 0 | [102] |
| Cannabinoid receptors 1/2 (CB1/CB2) | A | 0 | [136] |
| Prostaglandin E2 (PGE2) | A | + | [126,127,128,129] |
| Prostaglandin I2 (PGI2) | A | + | [151,152] |
| Sphingosine-1-phosphate receptor 3 (S1PR3) | A | − | [153] |
| Calcium receptor (CaR) | A | + | [154] |
| Frizzled-6 (Fzd-6) | A | + | [138] |
| GPCR-associated sorting protein 2 (Gprasp2)/Armadillo repeat-containing X-linked protein 1 (Armcx1) | A | − | [155] |
| aGvHD | |||
|---|---|---|---|
| Studies | Correlation with Outcome | References | |
| C-C ligand 2 (CCL2) | A | + | [178] |
| C-C ligand 3 (CCL3) | A | + | [178] |
| C-C ligand 5 (CCL5; RANTES) | A | + | [178] |
| H | + | [180] | |
| H (haplotype) | + | [247] | |
| C-C ligand 8 (CCL8) | A | + | [176] |
| C-X-C ligand 10 (CXCL10) | A | + | [252] |
| C-X-C ligand 12 (CXCL 12) | H (rs1801157) | + | [184] |
| C-X-C ligand 13 (CXCL 13) | A | + | [253] |
| C-X3-C ligand 1 (CX3CL1) | A | + | [181] |
| C-C receptor 1 (CCR1) | A | + | [175] |
| C-C receptor 2 (CCR2) | A | + | [175,177] |
| C-C receptor 4 (CCR4) | H | − | [185,186] |
| C-C receptor 5 (CCR5) | A, H | + | [207,208,209,210](A), [187,189,190,191,192,193,194,195,196,197,198,199,200,201,202,254] (H) |
| H | 0 | [203] | |
| A | − | [204,205,206] | |
| C-C receptor 6 (CCR6) | A, H | +/− | [223] (A, −)/[219] (H, +) |
| C-C receptor 7 (CCR7) | H | + | [179,194,195] |
| A, H | − | [220,221] (A), [222,223,224] (H) | |
| C-C receptor 9 (CCR9) | H | + | [179,187] |
| C-C receptor 8 (CCR8) | A | − | [182] |
| Chemerin receptor 23 (Chem23R) | A | − | [183] |
| C-X-C receptor 2 (CXCR2) | A | +/− | [226](+)/[225] (−) |
| C-X-C receptor 3 (CXCR3) | A, H | + | [175,210,212,213,214,215,216,217](A), [200,218] (H) |
| A | 0 | [255] | |
| A | − | [211,256] | |
| C-X-C receptor 4 (CXCR4) | A, H | + | [225] (A), [227] (H) |
| A | − | [228,229] (A) | |
| Alpha-2 adrenergic receptor (A2AR) | A | − | [230,231] |
| Angiotensin 1 receptor (AT1R) | H | + | [141] |
| Beta-adrenergic receptor (BAR) | A | − | [232,233] |
| P2Y purinoreceptor 2 (P2Y2) | A | + | [234] |
| Cannabinoid receptor 1 and 2 (CB1/CB2) | A | − | [237] |
| Cannabinoid receptor 2 (CB2) | A | − | [238] |
| Sphingosine-1-Phosphate 1 (S1P1) | A | − | [241] |
| Complement 3/5 activator fragments receptors (C3aR/C5aR) | A | + | [242] |
| EGF, Latrophilin and Seven Transmembrane Domain-Containing Protein 1 (ELTD1) | H (microsatellite) | + | [244] |
| G Protein-Coupled Receptor 15 (GPR15) | A | − | [167] |
| G Protein-Coupled Receptor 43 (GPR43) | A | − | [251] |
| Leucine-rich repeat-containing G protein-coupled receptor 5 (LGR5) | A | − | [245] |
| Platelet-activating factor receptor (PAFR) | A | + | [243] |
| Protease-activated receptor 2/3 (PAR2/3) | A | − | [246] |
| cGvHD | |||
|---|---|---|---|
| Studies | Effect | References | |
| C-C ligand 2 (CCL2) | A | 0 | [281] |
| C-C ligand 3 (CCL3) | H | + | [266] |
| C-C ligand 5 (CCL5) | A | + | [264] |
| C-C ligand 6 (CCL6) | A | + | [263] |
| C-C ligand 7 (CCL7) | A | + | [263] |
| C-C ligand 8 (CCL8) | A | + | [263] |
| C-C ligand 9 (CCL9) | A | + | [263] |
| C-C ligand 11 (CCL11) | A | + | [263] |
| C-C ligand 19 (CCL19) | A | + | [263] |
| C-C ligand 22 (CCL22) | H | + | [265] |
| C-X-C ligand 2 (CXCL2) | A | + | [263] |
| C-X-C ligand 8 (CXCL8) | H | + | [266] |
| C-X-C ligand 9 (CXCL9) | A, H | + | [263] (A), [267,268,269,270,271] (H) |
| C-X-C ligand 10 (CXCL10) | A, H | + | [263] (A), [265,266,269,270,272,273] (H) |
| C-X-C ligand 11 (CXCL11) | H | + | [270] |
| C-X-C ligand 12 (CXCL12) | A | + | [263] |
| C-C receptor 1 (CCR1) | A | + | [263] |
| C-C receptor 2 (CCR2) | A | 0 | [281] |
| C-C receptor 3 (CCR3) | H | + | [276] |
| C-C receptor 4 (CCR4) | H | +/− | [265] (+)/[280] (−) |
| C-C receptor 5 (CCR5) | H | +/− | [265] (+)/[279] (−) |
| C-C receptor 6 (CCR6) | H | + | [275] |
| C-C receptor 7 (CCR7) | H | + | [282] |
| C-C receptor 9 (CCR9) | H | + | [277] |
| C-X-C receptor 3 (CXCR3) | H | + | [269,270,271,272,273,274] |
| C-X3-C receptor 1 (CX3CR1) | H | − | [279] |
| C-X-C receptor 5 (CXCR5) | A | − | [278] |
| Angiotensin 1 receptor (AT1R) | H | + | [283] |
| Cannabinoid receptor 2 (CB2) | A | − | [238] |
| Prostaglandin D2 receptor (PGD2R; CRTH2) | H | +/− | [276] (+)/[280] (−) |
| Smoothened (SMO) | A | + | [284] |
| Lung Toxicity | |||
|---|---|---|---|
| Studies | Effect | References | |
| C-C ligand 2 (CCL2) | A | +/0 | [178,292] (+)/[293](0) |
| C-C ligand 3 (CCL3) | A | +/− | [178] (+)/[294] (−) |
| C-C ligand 5 (CCL5) or Regulated on Activation, Normal T Cell Expressed and Secreted (RANTES) | A | + | [178,291] |
| C-X-C ligand 9 (CXCL9) | A | + | [290] |
| C-X-C ligand 10 (CXCL10) | A | + | [290] |
| C-C receptor 2 (CCR2) | A | 0 | [293] |
| C-X-C receptor 3 (CXCR3) | A | + | [290] |
| Treatment-Related Mortality | |||
|---|---|---|---|
| Studies | Effect | References | |
| C-C ligand 2 (CCL2) | H (rs1024610) | + | [296] |
| C-X-C ligand 9 (CXCL9) | H | + | [268] |
| C-X-C ligand 10 (CXCL10) | H (rs3921) | − | [297] |
| C-C receptor 5 (CCR5) | H | + | [191,198,254] |
| C-C receptor 7 (CCR7) | H | + | [298] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Golay, H.; Jurkovic Mlakar, S.; Mlakar, V.; Nava, T.; Ansari, M. The Biological and Clinical Relevance of G Protein-Coupled Receptors to the Outcomes of Hematopoietic Stem Cell Transplantation: A Systematized Review. Int. J. Mol. Sci. 2019, 20, 3889. https://doi.org/10.3390/ijms20163889
Golay H, Jurkovic Mlakar S, Mlakar V, Nava T, Ansari M. The Biological and Clinical Relevance of G Protein-Coupled Receptors to the Outcomes of Hematopoietic Stem Cell Transplantation: A Systematized Review. International Journal of Molecular Sciences. 2019; 20(16):3889. https://doi.org/10.3390/ijms20163889
Chicago/Turabian StyleGolay, Hadrien, Simona Jurkovic Mlakar, Vid Mlakar, Tiago Nava, and Marc Ansari. 2019. "The Biological and Clinical Relevance of G Protein-Coupled Receptors to the Outcomes of Hematopoietic Stem Cell Transplantation: A Systematized Review" International Journal of Molecular Sciences 20, no. 16: 3889. https://doi.org/10.3390/ijms20163889
APA StyleGolay, H., Jurkovic Mlakar, S., Mlakar, V., Nava, T., & Ansari, M. (2019). The Biological and Clinical Relevance of G Protein-Coupled Receptors to the Outcomes of Hematopoietic Stem Cell Transplantation: A Systematized Review. International Journal of Molecular Sciences, 20(16), 3889. https://doi.org/10.3390/ijms20163889