Selective Separation of Acetic and Hexanoic Acids across Polymer Inclusion Membrane with Ionic Liquids as Carrier
Abstract
:1. Introduction
2. Results and Discussion
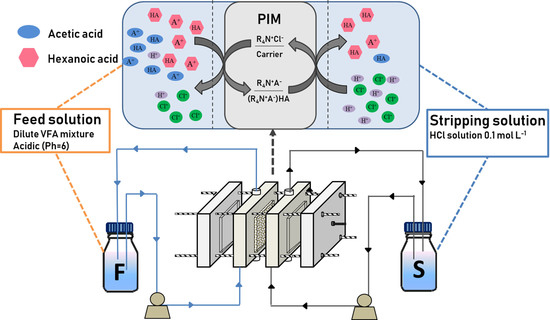
2.1. Transport Mechanism
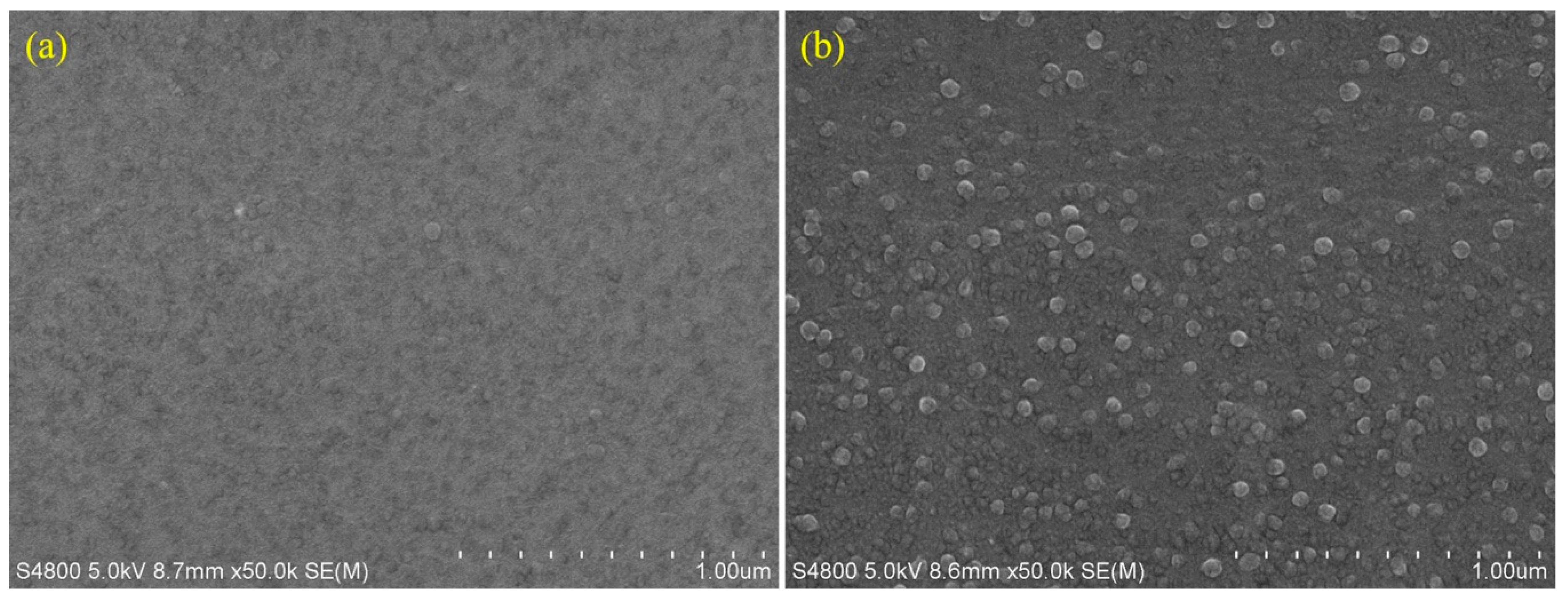
2.2. SEM Analysis
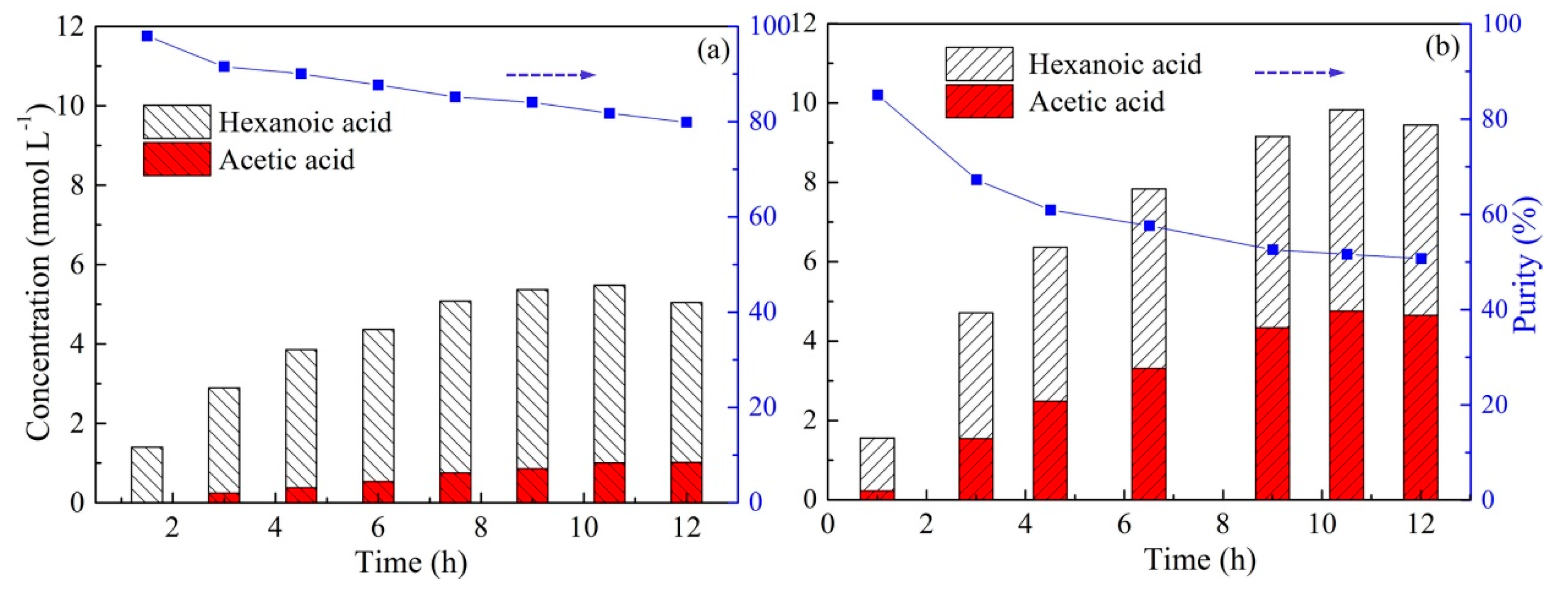
2.3. Effect of Carrier Type and Transport Kinetics across the PIM
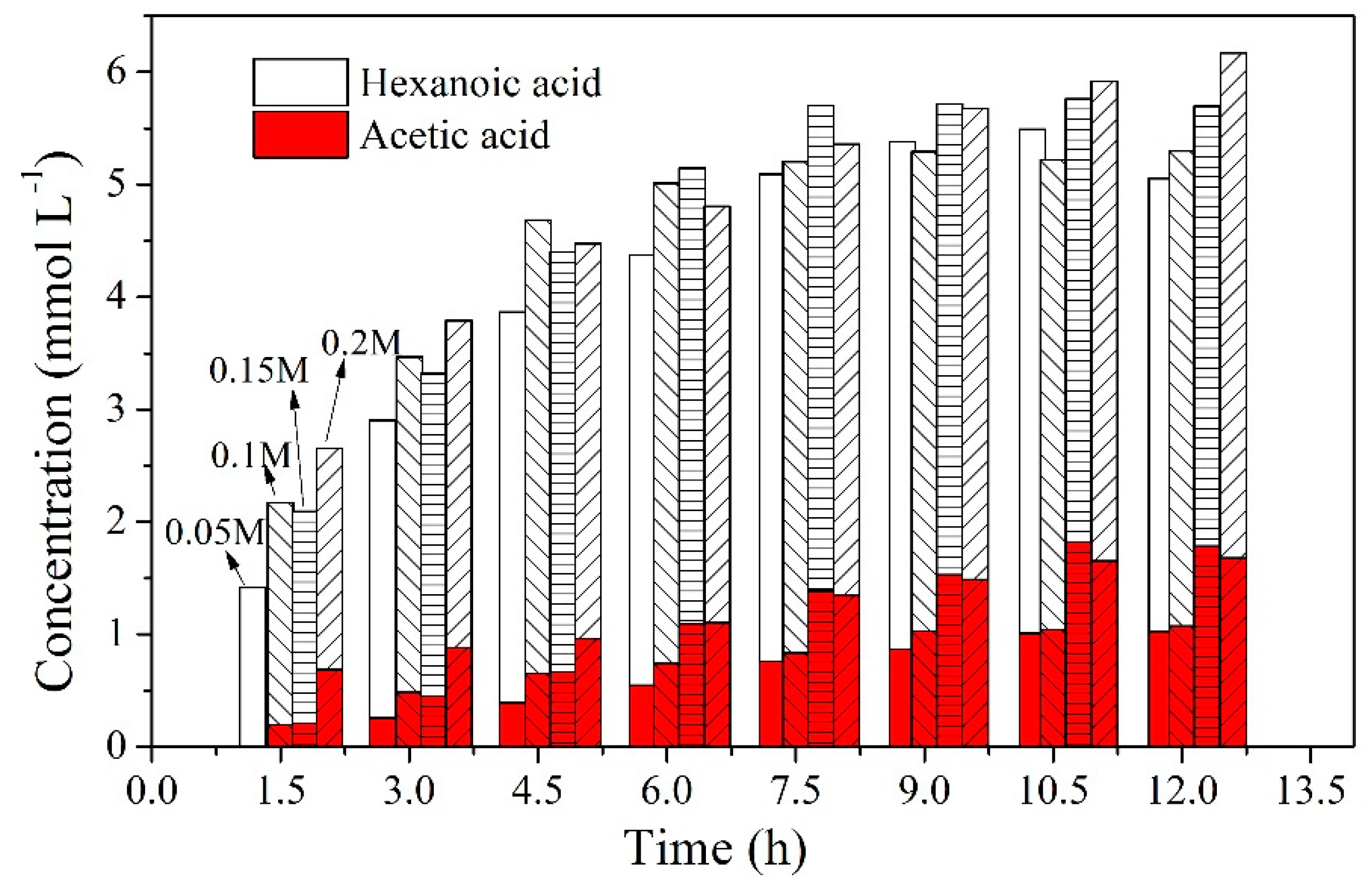
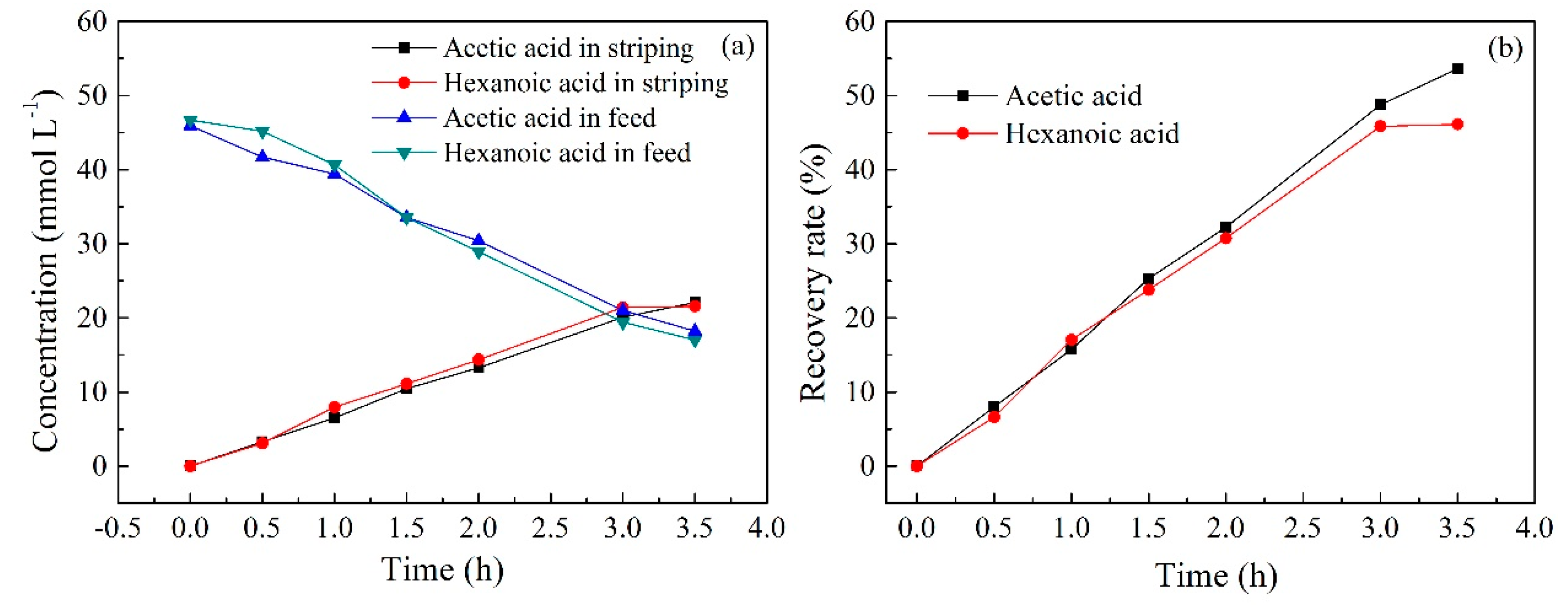
2.4. Effect of Carrier Content
2.5. Effect of Initial pH in Feed Solution
2.6. Effect of Initial Concentration in Feed Solution
2.7. Effect of Stripping Solution Type
2.8. Effect of Acid Concentration in the Stripping Solution
2.9. Adsorption Experiment
2.10. Transport Experiment during Electrodialysis
3. Materials and Methods
3.1. Materials
3.2. Membrane Preparation
3.3. Transport Experiments
3.4. Adsorption Capacity Test
3.5. Data Processing
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koller, M.; Marsalek, L.; Dias, M.M.D.S.; Braunegg, G. Producing microbial polyhydroxyalkanoate (PHA) biopolyesters in a sustainable manner. New Biotechnol. 2017, 37, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Chua, A.S.M.; Yeoh, H.K.; Ngoh, G.C. A review of the production and applications of waste-derived volatile fatty acids. Chem. Eng. J. 2014, 235, 83–99. [Google Scholar] [CrossRef]
- Cai, M.M.; Chua, H.; Zhao, Q.L.; Shirley, S.N.; Ren, J. Optimal production of polyhydroxyalkanoates (PHA) in activated sludge fed by volatile fatty acids (VFAs) generated from alkaline excess sludge fermentation. Bioresour. Technol. 2009, 100, 1399–1405. [Google Scholar]
- Choi, J.D.R.; Chang, H.N.; Han, J.I. Performance of microbial fuel cell with volatile fatty acids from food wastes. Biotechnol. Lett. 2011, 33, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.O.; Chen, Y.G.; Liu, C.C. Waste activated sludge alkaline fermentation liquid as carbon source for biological nutrients removal in anaerobic followed by alternating aerobic-anoxic sequencing batch reactors. Chin. J. Chem. Eng. 2010, 18, 478–485. [Google Scholar] [CrossRef]
- Pratt, S.; Liew, D.; Batstone, D.J.; Werker, A.G.; Morgan-Sagastume, F.; Lant, P.A. Inhibition by fatty acids during fermentation of pre-treated waste activated sludge. J. Biotechnol. 2012, 159, 38–43. [Google Scholar] [CrossRef]
- Alkaya, E.; Kaptan, S.; Ozkan, L.; Uludag-Demirer, S.; Demirer, G.N. Recovery of acids from anaerobic acidification broth by liquid-liquid extraction. Chemosphere 2009, 77, 1137–1142. [Google Scholar] [CrossRef]
- IJmker, H.M.; Gramblicka, M.; Kersten, S.R.A.; van der Ham, A.G.J.; Schuur, B. Acetic acid extraction from aqueous solutions using fatty acids. Sep. Purif. Technol. 2014, 125, 256–263. [Google Scholar] [CrossRef]
- Van den Bruinhorst, A.; Raes, S.; Maesara, S.A.; Kroon, M.C.; Esteves, A.C.C.; Meuldijk, J. Hydrophobic eutectic mixtures as volatile fatty acid extractants. Sep. Purif. Technol. 2019, 216, 147–157. [Google Scholar] [CrossRef]
- Kouki, N.; Tayeb, R.; Zarrougui, R.; Dhahbi, M. Transport of salicylic acid through supported liquid membrane based on ionic liquids. Sep. Purif. Technol. 2010, 76, 8–14. [Google Scholar] [CrossRef]
- Li, Q.; Xing, J.M.; Li, W.L.; Liu, Q.F.; Su, Z.G. Separation of Succinic Acid from Fermentation Broth Using Weak Alkaline Anion Exchange Adsorbents. Ind. Eng. Chem. Res. 2009, 48, 3595–3599. [Google Scholar] [CrossRef]
- Dey, P.; Linnanen, L.; Pal, P. Separation of lactic acid from fermentation broth by cross flow nanofiltration: Membrane characterization and transport modelling. Desalination 2012, 288, 47–57. [Google Scholar] [CrossRef]
- Li, Q.; Wang, D.; Wu, Y.; Li, W.L.; Zhang, Y.J.; Xing, J.M.; Su, Z.G. One step recovery of succinic acid from fermentation broths by crystallization. Sep. Purif. Technol. 2010, 72, 294–300. [Google Scholar] [CrossRef]
- Jones, R.J.; Massanet-Nicolau, J.; Guwy, A.; Premier, G.C.; Dinsdale, R.M.; Reilly, M. Removal and recovery of inhibitory volatile fatty acids from mixed acid fermentations by conventional electrodialysis. Bioresour. Technol. 2015, 189, 279–284. [Google Scholar] [CrossRef]
- Torri, C.; Samori, C.; Ajao, V.; Baraldi, S.; Galletti, P.; Tagliavini, E. Pertraction of volatile fatty acids through biodiesel-based liquid membranes. Chem. Eng. J. 2019, 366, 254–263. [Google Scholar] [CrossRef]
- Nuchnoi, P.; Yano, T.; Nishio, N.; Nagai, S. Extraction of volatile fatty-acids from diluted aqueous-solution using a supported liquid membrane. J. Ferment. Bioeng. 1987, 65, 301–310. [Google Scholar] [CrossRef]
- Kaya, A.; Onac, C.; Alpoguz, H.K.; Yilmaz, A.; Atar, N. Removal of Cr(VI) through calixarene based polymer inclusion membrane from chrome plating bath water. Chem. Eng. J. 2016, 283, 141–149. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, Y.; Liu, R.; She, Z.L.; Tan, M.; Mao, D.B.; Fu, R.Q.; Zhang, Y. Polymer inclusion membrane (PIM) containing ionic liquid as a proton blocker to improve waste acid recovery efficiency in electrodialysis process. J. Membr. Sci. 2019, 581, 18–27. [Google Scholar] [CrossRef]
- Garcia, A.; Alvarez, A.; Matamoros, V.; Salvadó, V.; Fontàs, C. Development of polymer inclusion membranes for the extraction of antibiotics from environmental waters. Procedia Eng. 2012, 44, 804–806. [Google Scholar] [CrossRef]
- Garcia-Rodríguez, A.; Fontàs, C.; Matamoros, V.; Almeida, M.I.G.S.; Kolev, S.D. Development of a polymer inclusion membrane-based passive sampler for monitoring of sulfamethoxazole in natural waters. Minimizing the effect of the flow pattern of the aquatic system. Microchem. J. 2016, 124, 175–180. [Google Scholar] [CrossRef]
- Ershad, M.; Almeida, M.I.G.S.; Spassov, T.G.; Cattrall, R.W.; Kolev, S.D. Polymer inclusion membranes (PIMs) containing purified dinonylnaphthalene sulfonic acid (DNNS): Performance and selectivity. Sep. Purif. Technol. 2018, 195, 446–452. [Google Scholar] [CrossRef]
- Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Recent trends in extraction and transport of metal ions using polymer inclusion membranes (PIMs). J. Membr. Sci. 2012, 415, 9–23. [Google Scholar] [CrossRef]
- Long, D.N.; Mornane, P.; Potter, I.D.; Perera, J.M.; Cattrall, R.W.; Kolev, S.D. Extraction and transport of metal ions and small organic compounds using polymer inclusion membranes (PIMs). J. Membr. Sci. 2006, 281, 7–41. [Google Scholar]
- O’Rourke, M.; Cattrall, R.W.; Kolev, S.D.; Potter, I.D. The Extraction and Transport of Organic Molecules Using Polymer Inclusion Membranes. Solvent Extr. Res. Dev. 2009, 16, 1–12. [Google Scholar]
- Baczyńska, M.; Słomka, Ż.; Rzelewska, M.; Waszak, M.; Nowicki, M.; Regel-Rosocka, M. Characterization of polymer inclusion membranes (PIM) containing phosphonium ionic liquids and their application for separation of Zn(II) from Fe(III). J. Chem. Technol. Biotechnol. 2018, 93, 1767–1777. [Google Scholar] [CrossRef]
- O’Bryan, Y.; Cattrall, R.W.; Truong, Y.B.; Kyratzis, I.L.; Kolev, S.D. The use of poly(vinylidenefluoride-co-hexafluoropropylene) for the preparation of polymer inclusion membranes. Application to the extraction of thiocyanate. J. Membr. Sci. 2016, 510, 481–488. [Google Scholar] [CrossRef]
- Zaheri, P.; Ghassabzadeh, H. Preparation of polymer inclusion membrane including mixture of D2EHPA and Cyanex272 for the extraction of Eu from nitrate media. Chem. Pap. 2017, 71, 1–9. [Google Scholar] [CrossRef]
- Rynkowska, E.; Fatyeyeva, K.; Kujawski, W. Application of polymer-based membranes containing ionic liquids in membrane separation processes: A critical review. Rev. Chem. Eng. 2017, 34, 341–363. [Google Scholar] [CrossRef]
- Baczyńska, M.; Waszak, M.; Nowicki, M.; Prządka, D.; Borysiak, S.; Regel-Rosocka, M. Characterization of polymer inclusion membranes (PIMs) containing phosphonium ionic liquids as Zn(II) carriers. Ind. Eng. Chem. Res. 2018, 57, 5070–5082. [Google Scholar] [CrossRef]
- Darvishi, R.; Sabet, J.K.; Esfahany, M.N. Preparation and characterization of a novel calcium-conducting polymer inclusion membrane: Part, I. Korean J. Chem. Eng. 2018, 35, 1–13. [Google Scholar] [CrossRef]
- Tonova, K.; Svinyarov, I.; Bogdanov, M.G. Hydrophobic 3-alkyl-1-methylimidazolium saccharinates as extractants for l-lactic acid recovery. Sep. Purif. Technol. 2014, 125, 239–246. [Google Scholar] [CrossRef]
- Reyhanitash, E.; Zaalberg, B.; Kersten, S.R.A.; Schuur, B. Extraction of volatile fatty acids from fermented wastewater. Sep. Purif. Technol. 2016, 161, 61–68. [Google Scholar] [CrossRef]
- Lopez, A.M.; Hestekin, J.A. Improved organic acid purification through wafer enhanced electrodeionization utilizing ionic liquids. J. Membr. Sci. 2015, 493, 200–205. [Google Scholar] [CrossRef]
- Yang, S.T.; White, S.A.; Hsu, S.T. Extraction of carboxylic-acids with tertiary and quaternary amines-effect of pH. Ind. Eng. Chem. Res. 1991, 30, 1335–1342. [Google Scholar] [CrossRef]
- Kagaya, S.; Ryokan, Y.; Cattrall, R.W.; Kolev, S.D. Stability studies of poly (vinyl chloride)-based polymer inclusion membranes containing Aliquat 336 as a carrier. Sep. Purif. Technol. 2012, 101, 69–75. [Google Scholar] [CrossRef]
- Yıldız, Y.; Manzak, A.; Tutkun, O. Selective extraction of cobalt ions through polymer inclusion membrane containing Aliquat 336 as a carrier. Desalin. Water Treat. 2016, 57, 4616–4623. [Google Scholar] [CrossRef]
- Litaiem, Y.; Dhahbi, M. Measurements and correlations of viscosity, conductivity and density of an hydrophobic ionic liquid (Aliquat 336) mixtures with a non-associated dipolar aprotic solvent (DMC). J. Mol. Liq. 2012, 169, 54–62. [Google Scholar] [CrossRef]
- Yaftian, M.R.; Almeida, M.I.G.S.; Cattrall, R.W.; Kolev, S.D. Selective extraction of vanadium(V) from sulfate solutions into a polymer inclusion membrane composed of poly(vinylidenefluoride-co-hexafluoropropylene) and Cyphos® IL 101. J. Membr. Sci. 2018, 545, 57–65. [Google Scholar] [CrossRef]
- Matsumoto, M.; Murakami, Y.; Minamidate, Y.; Kondo, K. Separation of lactic acid through polymer inclusion membranes containing ionic liquids. Sep. Sci. Technol. 2012, 47, 354–359. [Google Scholar] [CrossRef]
- Gajewski, P.; Bogacki, M.B. Influence of alkyl chain length in 1-alkylimidazol on the citric acid transport rate across polymer inclusion membrane. Sep. Sci. Technol. 2012, 47, 1374–1382. [Google Scholar] [CrossRef]
- Pratiwi, A.I.; Sato, T.; Matsumoto, M.; Kondo, K. Permeation mechanism of succinic acid through polymer inclusion membranes with ionic liquid Aliquat 336. J. Chem. Eng. Jpn. 2014, 47, 314–318. [Google Scholar] [CrossRef]
- Manzak, A.; Kursun, C.; Yildiz, Y. Characterization of humic acid extracted from aqueous solutions with polymer inclusion membranes. J. Taiwan Inst. Chem. Eng. 2017, 81, 14–20. [Google Scholar] [CrossRef]
- Przewozna, M.; Gajewski, P.; Michalak, N.; Bogacki, M.B.; Skrzypczak, A. Determination of the Percolation Threshold for the Oxalic, Tartaric, and Lactic Acids Transport through polymer inclusion membranes with 1-alkylimidazoles as a Carrier. Sep. Sci. Technol. 2014, 49, 1745–1755. [Google Scholar] [CrossRef]
- Wang, D.; Hu, J.; Liu, D.; Chen, Q.; Jie, L. Selective transport and simultaneous separation of Cu(II), Zn(II) and Mg(II) using a dual polymer inclusion membrane system. J. Membr. Sci. 2017, 524, 205–213. [Google Scholar] [CrossRef]
- O’Bryan, Y.; Truong, Y.B.; Cattrall, R.W.; Kyratzis, I.L.; Kolev, S.D. A new generation of highly stable and permeable polymer inclusion membranes (PIMs) with their carrier immobilized in a crosslinked semi-interpenetrating polymer network. Application to the transport of thiocyanate. J. Membr. Sci. 2017, 529, 55–62. [Google Scholar] [CrossRef]
- Yoshida, W.; Baba, Y.; Kubota, F.; Kolev, S.D.; Goto, M. Selective transport of scandium(III) across polymer inclusion membranes with improved stability which contain an amic acid carrier. J. Membr. Sci. 2019, 572, 291–299. [Google Scholar] [CrossRef]
- Kaya, A.; Onac, C.; Alpoguz, H.K.; Agarwal, S.; Gupta, V.K.; Atar, N.; Yilmaz, A. Reduced graphene oxide based a novel polymer inclusion membrane: Transport studies of Cr(VI). J. Mol. Liq. 2016, 219, 1124–1130. [Google Scholar] [CrossRef]
- Meng, X.; Wang, C.; Ren, T.; Lei, W.; Wang, X. Electrodriven transport of chromium (VI) using 1-octanol/PVC in polymer inclusion membrane under low voltage. Chem. Eng. J. 2018, 346, 506–514. [Google Scholar] [CrossRef]
- Sadyrbaeva, T.Z. Removal of chromium(VI) from aqueous solutions using a novel hybrid liquid membrane—Electrodialysis process. Chem. Eng. Process. 2016, 99, 183–191. [Google Scholar] [CrossRef]
- Aydin, S.; Yesil, H.; Tugtas, A.E. Recovery of mixed volatile fatty acids from anaerobically fermented organic wastes by vapor permeation membrane contactors. Bioresour. Technol. 2018, 250, 548–555. [Google Scholar] [CrossRef]
- Lee, S.C.; Hyun, K.S. Development of an emulsion liquid membrane system for separation of acetic acid from succinic acid. J. Membr. Sci. 2010, 350, 333–339. [Google Scholar] [CrossRef]
- Yordanov, B.; Boyadzhiev, L. Pertraction of citric acid by means of emulsion liquid membranes. J. Membr. Sci. 2004, 238, 191–197. [Google Scholar] [CrossRef]
- Schlosser, Š.; Marták, J.; Blahušiak, M. Specifc phenomena in carboxylic acids extraction by selected types of hydrophobic ionic liquids. Chem. Pap. 2018, 72, 567–584. [Google Scholar] [CrossRef]
- Rao, R.V.S.; Sivakumar, P.; Natarajan, R.; Rao, P.R.V. Effect of Aliquat 336 concentration on transportation of hydrochloric acid across supported liquid membrane. J. Radioanal. Nucl. Chem. 2002, 252, 95–98. [Google Scholar]
- Cai, C.Q.; Yang, F.; Zhao, Z.G.; Liao, Q.X.; Bai, R.X.; Guo, W.H.; Chen, P.; Zhang, Y.; Zhang, H. Promising transport and high-selective separation of Li(I) from Na(I) and K(I) by a functional polymer inclusion membrane (PIM) system. J. Membr. Sci. 2019, 579, 1–10. [Google Scholar] [CrossRef]
- Kebiche-Senhadji, O.; Tingry, S.; Seta, P.; Benamor, M. Selective extraction of Cr(VI) over metallic species by polymer inclusion membrane (PIM) using anion (Aliquat 336) as carrier. Desalination 2010, 258, 59–65. [Google Scholar] [CrossRef]
- Kertes, A.S.; King, C.J. Extraction chemistry of fermentation product carboxylic-acids. Biotechnol. Bioeng. 1986, 28, 269–282. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Sun, Y.; Tang, N.; Miao, C.L.; Wang, Y.T.; Tang, L.H.; Wang, S.X.; Yang, X.J. Simultaneous extraction and recovery of gold(I) from alkaline solutions using an environmentally benign polymer inclusion membrane with ionic liquid as the carrier. Sep. Purif. Technol. 2019, 222, 136–144. [Google Scholar] [CrossRef]











| pH | P (µm s−1) | Purity (%) | |
|---|---|---|---|
| Acetic Acid | Hexanoic Acid | ||
| 2 | 0.42 | 5.63 | 76.2 |
| 4 | 0.42 | 6.67 | 77.4 |
| 5 | 0.42 | 6.25 | 77.1 |
| 6 | 0.83 | 5.63 | 73.5 |
| 8 | 0.63 | 5.00 | 75.9 |
| 9 | 0.42 | 5.63 | 76.5 |
| 10 | 0.42 | 5.63 | 77.9 |
| Membrane Number | Base Polymer (wt%) | Carrier (wt%) | Total Weight (g) | Thickness (µm) | |
|---|---|---|---|---|---|
| CTA | Aliquat 336 | Cyphos IL101 | |||
| 1 | 70 | 30 | 1.1 | 58.1 ± 5.1 | |
| 2 | 60 | 40 | 1.1 | 57.8 ± 6.2 | |
| 3 | 50 | 50 | 1.1 | 58.6 ± 5.6 | |
| 4 | 40 | 60 | 1.1 | 60.4 ± 5.2 | |
| 5 | 30 | 70 | 1.1 | 59.6 ± 8.7 | |
| 6 | 40 | 60 | 1.1 | 61.4 ± 4.6 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.-Y.; Zhang, N.; Li, Z.-Y.; Lang, Q.-L.; Yan, B.-H.; Liu, Y.; Zhang, Y. Selective Separation of Acetic and Hexanoic Acids across Polymer Inclusion Membrane with Ionic Liquids as Carrier. Int. J. Mol. Sci. 2019, 20, 3915. https://doi.org/10.3390/ijms20163915
Wang B-Y, Zhang N, Li Z-Y, Lang Q-L, Yan B-H, Liu Y, Zhang Y. Selective Separation of Acetic and Hexanoic Acids across Polymer Inclusion Membrane with Ionic Liquids as Carrier. International Journal of Molecular Sciences. 2019; 20(16):3915. https://doi.org/10.3390/ijms20163915
Chicago/Turabian StyleWang, Bao-Ying, Na Zhang, Zhen-Yu Li, Qiao-Lin Lang, Bing-Hua Yan, Yang Liu, and Yang Zhang. 2019. "Selective Separation of Acetic and Hexanoic Acids across Polymer Inclusion Membrane with Ionic Liquids as Carrier" International Journal of Molecular Sciences 20, no. 16: 3915. https://doi.org/10.3390/ijms20163915
APA StyleWang, B.-Y., Zhang, N., Li, Z.-Y., Lang, Q.-L., Yan, B.-H., Liu, Y., & Zhang, Y. (2019). Selective Separation of Acetic and Hexanoic Acids across Polymer Inclusion Membrane with Ionic Liquids as Carrier. International Journal of Molecular Sciences, 20(16), 3915. https://doi.org/10.3390/ijms20163915