The Effect of the ortho Nitro Group in the Solvolysis of Benzyl and Benzoyl Halides
Abstract
:1. Introduction



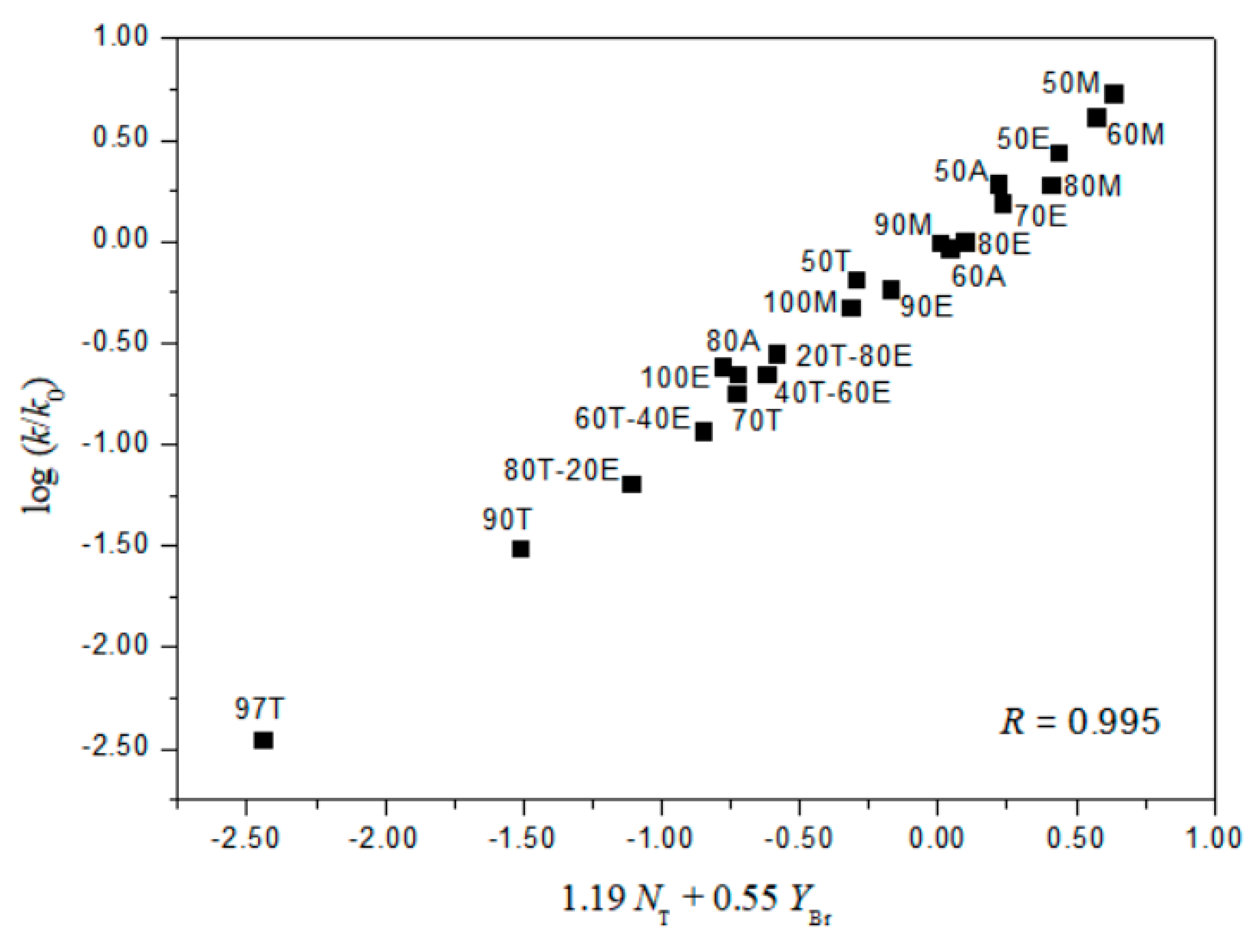
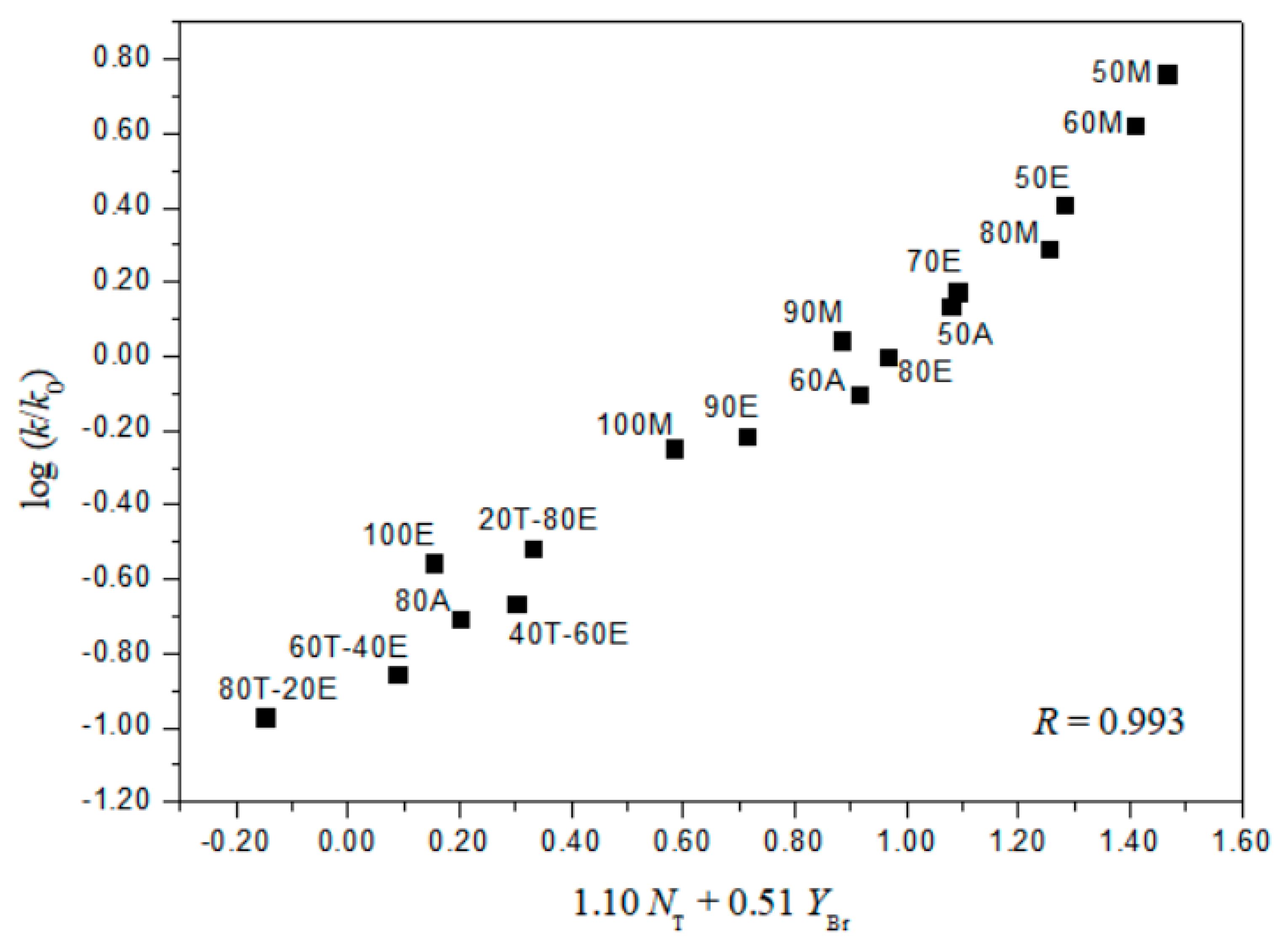
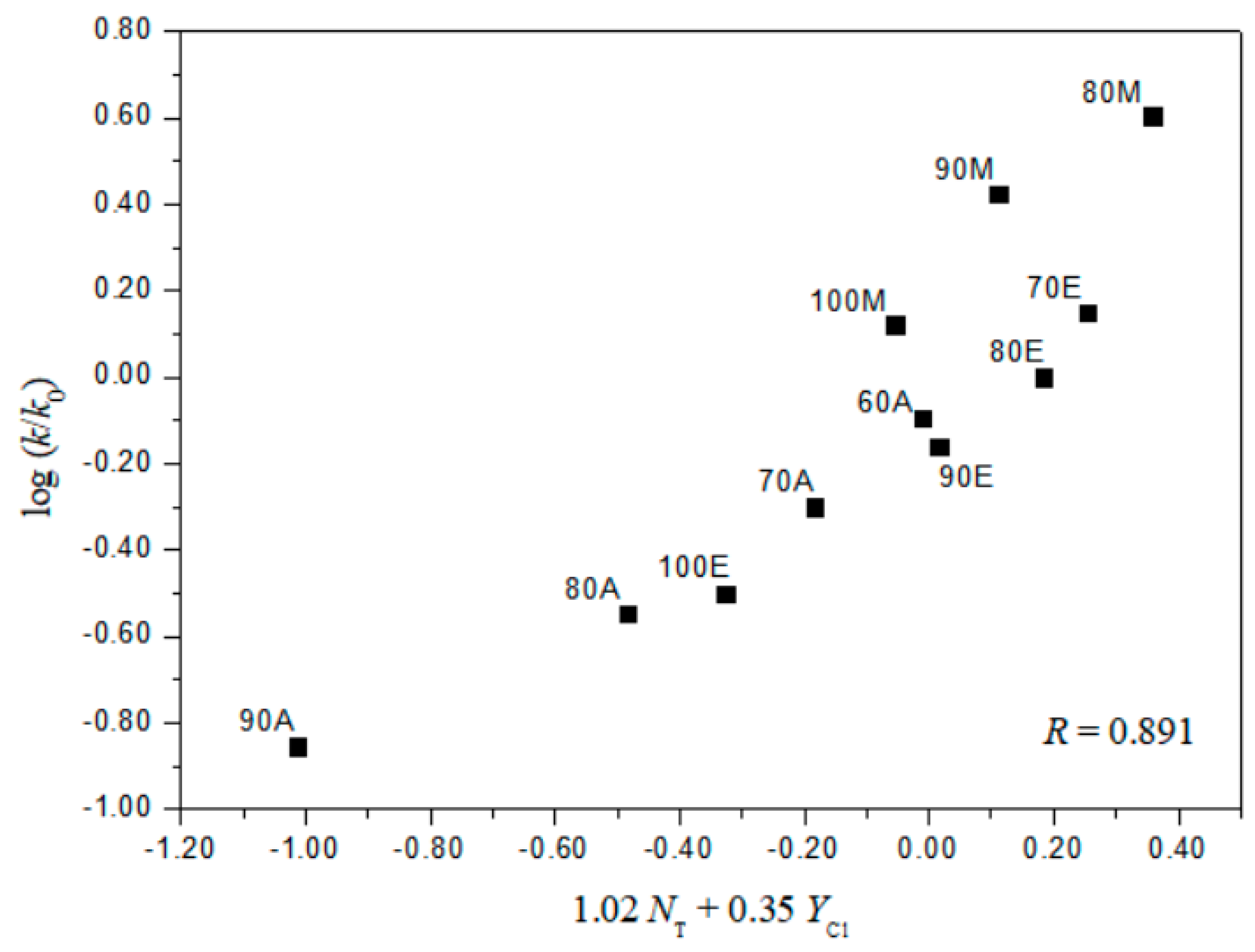
2. Results and Discussion
3. Experimental Section
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Singh, A.; Andrews, L.J.; Keefer, R.M. Intramolecular Nucleophilic Participation. The Effect of Certain ortho Substituents on Solvolysis Rates of Benzyl and Benzhydryl Bromides. J. Am. Chem. Soc. 1962, 84, 1179–1185. [Google Scholar] [CrossRef]
- Kim, S.; Friedrich, S.S.; Andrews, L.J.; Keefer, R.M. Intramolecular nucleophilic participation. VIII. Acetolysis of o- and p-nitro- and o- and p-carbophenoxybenzhydryl bromides. J. Am. Chem. Soc. 1970, 92, 5452–5456. [Google Scholar] [CrossRef]
- Kyong, J.B.; Hoshik Won, H.; Lee, Y.H.; Kevill, D.N. Correlation of the Rates of Solvolyses of Carbomethoxybenzyl Bromides Using the Grunwald-Winstein Equation. Bull. Korean Chem. Soc. 2005, 26, 661–664. [Google Scholar] [Green Version]
- Kevill, D.N.; D’Souza, M.J. Correlation of the rates of solvolysis of benzoyl chloride and derivatives using extended forms of the Grunwald–Winstein equation. J. Phys. Org. Chem 2002, 15, 881–888. [Google Scholar] [CrossRef]
- Grunwald, E.; Winstein, S. The Correlation of Solvolysis Rates. J. Am. Chem. Soc. 1948, 70, 846–854. [Google Scholar] [CrossRef]
- Winstein, S.; Grunwald, E.; Jones, H.W. The Correlation of Solvolysis Rates and the Classification of Solvolysis Reactions into Mechanistic Categories. J. Am. Chem. Soc. 1951, 73, 2700–2707. [Google Scholar] [CrossRef]
- Kevill, D.N.; Anderson, S.W. An improved scale of solvent nucleophilicity based on the solvolysis of the S-methyldibenzothiophenium ion. J. Org. Chem. 1991, 56, 1845–1850. [Google Scholar] [CrossRef]
- Kevill, D.N.; D’Souza, M.J. Additional YCl Values and the Correlation of the Specific Rates of Solvolysis of tert-Butyl Chloride in Terms of NT and YCl Scales. J. Chem. Res. Synop. 1993, 174–175. [Google Scholar]
- Schadt, F.L.; Bentley, T.W.; Schleyer, P.v.R. The SN2-SN1 spectrum. 2. Quantitative treatments of nucleophilic solvent assistance. A scale of solvent nucleophilicities. J. Am. Chem. Soc. 1976, 98, 7667–7674. [Google Scholar] [CrossRef]
- Bentley, T.W.; Carter, G.E. The SN2-SN1 spectrum. 4. The SN2 (intermediate) mechanism for solvolyses of tert-butyl chloride: A revised Y scale of solvent ionizing power based on solvolyses of 1-adamantyl chloride. J. Am. Chem. Soc. 1982, 104, 5741–5747. [Google Scholar] [CrossRef]
- Bentley, T.W.; Llewellyn, G. YX Scales of Solvent Ionizing Power. Prog. Phys. Org. Chem. 1990, 17, 121–158. [Google Scholar]
- Bentley, T.W.; Carter, G.E.; Harris, H.C. Competing SN2 and carbonyl addition pathways for solvolyses of benzoyl chloride in aqueous media. J. Chem. Soc. Perkin Trans. 2 1985, 983–990. [Google Scholar] [CrossRef]
- Bentley, T.W.; Harris, H.C. Solvolyses of para-substituted benzoyl chlorides in trifluoroethanol and in highly aqueous media. J. Chem. Soc. Perkin Trans. 2 1986, 619–624. [Google Scholar] [CrossRef]
- Bentley, T.W.; Harris, H.C. Separation of mass law and solvent effects in kinetics of solvolyses of p-nitrobenzoyl chloride in aqueous binary mixtures. J. Org. Chem. 1988, 53, 724–728. [Google Scholar] [CrossRef]
- Song, B.D.; Jencks, W.P. Mechanism of Solvolysis of Substituted Benzoyl Halides. J. Am. Chem. Soc. 1989, 111, 8470–8479. [Google Scholar] [CrossRef]
- Kevill, D.N.; Kyong, J.B. Intramolecular nucleophilic assistance in the solvolyses of benzyl derivatives: Solvolyses of o-nitrobenzyl bromide and tosylate. J. Chem. Res. Synop. 2007, 210–215. [Google Scholar] [CrossRef]
- Austin, R.P.; Ridd, J.H. Oxidation–reduction reactions involving nitro groups in trifluoromethanesulfonic acid.Part 3.The reactions of 2-nitrobenzyl alcohol and related compounds. J. Chem. Soc. Perkin Trans. 2 1994, 1411–1414. [Google Scholar] [CrossRef]
- Bakke, J. Reactions of Nitrobenzyl Alcohol under Acidic Conditions. Possibilities of Intramolecular Nucleophilic Participation by the Nitro Group. Acta Chem. Scand. B 1974, 28, 645–649. [Google Scholar] [CrossRef]
- Chen, L.J.; Burka, L.T. Formation of o-Nitrosobenzaldehyde from Hydrolysis of o-Nitrobenzyl Tosylate. Evidence of Intramolecular Nucleophilic. Interaction. Tetrahedron Lett. 1998, 39, 5351–5354. [Google Scholar] [CrossRef]
- Fujio, M.; Susuki, T.; Goto, M.; Tsuji, Y.; Yatsugi, K.; Kim, S.H.; Ahmed, G.A.-W.; Tsuno, Y. Solvent Effects on the Solvolysis of p-Nitrobenzyl and 3,5-Bis(trifluoromethyl)benzyl p-Toluenesulfonates. Bull. Chem. Soc. Jpn. 1995, 68, 673–682. [Google Scholar] [CrossRef]
- Kevill, D.N.; D’Souza, M.J.; Ren, H. Correlation of the rates of solvolysis of arylmethyl p-toluenesulfonates: Application of the aromatic ring parameter and a discussion of similarity models. Can. J. Chem. 1998, 76, 751–757. [Google Scholar] [CrossRef]
- Kevill, D.N. Advances in Quantitative Structure-Property Relationships; Charton, M., Ed.; JAI Press: Greenwich, CT, USA, 1996; Volume 1, pp. 81–115. [Google Scholar]
- Koo, I.S.; Bentley, T.W.; Kang, D.H.; Lee, I. Limitations of the transition state variation model. Part 2. Dual reaction channels for solvolyses of 2,4,6-trimethylbenzenesulphonyl chloride. J. Chem. Soc. Perkin Trans. 2 1991, 2, 175–179. [Google Scholar] [CrossRef]
- Kevill, D.N.; Kyong, J.B.; Weitl, F.L. Solvolysis–Decomposition of 1-Adamantyl Chloroformate: Evidence for Ion-Pair Return in 1-Adamantyl Chloride Solvolysis. J. Org. Chem. 1990, 55, 4304–4311. [Google Scholar] [CrossRef]
- D’Souza, M.J.; Reed, D.N.; Erdman, K.J.; Kyong, J.B.; Kevill, D.N. Grunwald-Winstein Analysis-Isopropyl Chloroformate Solvolysis Revisited. Int. J. Mol. Sci. 2009, 10, 862–879. [Google Scholar] [CrossRef]
- Lee, Y.; Park, K.-H.; Seong, M.H.; Kyong, J.B.; Kevill, D.N. Correlation of the rates of solvolysis of i-butyl fluoroformate and a consideration of leaving group effects. Int. J. Mol. Sci. 2011, 12, 7806–7817. [Google Scholar] [CrossRef]
- Kyong, J.B.; Park, B.-C.; Kim, C.-B.; Kevill, D.N. Rate and Product Studies with Benzyl and p-Nitrobenzyl Chloroformates Under Solvolytic Conditions. J. Org. Chem. 2000, 65, 8051–8058. [Google Scholar] [CrossRef]
- D’Souza, M.J.; Ryu, Z.H.; Park, B.-C.; Kevill, D.N. Correlation of the rates of solvolysis of acetyl chloride and α-substituted derivatives. Can. J. Chem. 2008, 86, 359–367. [Google Scholar] [CrossRef]
- McDonald, R.N.; Davis, G.E. Some Observations on the Conductometric Method for Determining Solvolytic Rate Constants. J. Org. Chem. 1973, 38, 138–144. [Google Scholar] [CrossRef]
- Lee, Y.H.; Seong, M.H.; Lee, E.S.; Lee, Y.W.; Won, H.; Kyong, J.B.; Kevill, D.N. Rate and Product Studies with 2-Methyl-2-Chloroadamantane under Solvolytic Conditions. Bull. Korean Chem. Soc. 2010, 31, 1209–1214. [Google Scholar] [CrossRef] [Green Version]


| Solvent d | k/10−5 s−1 (1) a | k/10−5 s−1 (3) b | k/10−3 s−1 (2) c | k(1)/k(3)e | k(2)/k(4)f |
|---|---|---|---|---|---|
| 100% EtOH g | 0.934 ± 0.012 | 0.834 ± 0.011 | 1.29 | 1.1 | 0.096 |
| 90% EtOH | 2.06 ± 0.07 | 2.01 ± 0.08 | 2.84 | 1.0 | 0.079 |
| 80% EtOH | 3.36 ± 0.06 | 3.43 ± 0.08 | 4.09 | 0.98 | 0.087 |
| 70% EtOH | 5.01 ± 0.13 | 5.33 ± 0.42 | 5.80 | 0.94 | 0.10 |
| 50% EtOH | 8.58 ± 0.20 | 9.43 ± 0.13 | - | 0.91 | |
| 100% MeOH h | 1.90 ± 0.03 | 1.63 ± 0.08 | 5.41 | 1.2 | 0.13 |
| 90% MeOH | 3.71 ± 0.11 | 3.37 ± 0.11 | 10.9 | 1.1 | 0.12 |
| 80% MeOH | 6.54 ± 0.18 | 6.58 ± 0.17 | 16.5 | 0.99 | 0.13 |
| 60% MeOH | 14.1 ± 0.2 | 14.2 ± 0.4 | - | 0.99 | |
| 50% MeOH | 19.4 ± 0.2 | 18.5 ± 0.5 | - | 1.0 | |
| 90% Acetone | 0.572 | 0.099 | |||
| 80% Acetone | 0.659 ± 0.011 | 0.761 ± 0.021 | 1.16 | 0.87 | 0.082 |
| 70% Acetone | - | - | 2.05 | 0.098 | |
| 60% Acetone | 2.67 ± 0.09 | 3.18 ± 0.14 | 3.30 | 0.84 | 0.10 |
| 50% Acetone | 4.58 ± 0.06 | 5.64 ± 0.13 | - | 0.81 | |
| 100% TFE | - | - | 3.73 | 5870 | |
| 97% TFE | 0.645 ± 0.052 j,k | 0.0121 ± 0.0013 | 5.14 | 53 | 519 |
| 90% TFE | 0.939 ± 0.041 k | 0.107 ± 0.030 | 6.73 | 8.8 | 114 |
| 70% TFE | 1.64 ± 0.03 k | 0.612 ± 0.046 | 17.8 | 2.7 | 29 |
| 50% TFE | 3.66 ± 0.07 k | 2.25 ± 0.12 | 36.0 | 1.6 | 14 |
| 80T–20E i | 0.360 ± 0.022 | 0.220 ± 0.012 | 1.53 | 1.6 | 8.68 |
| 60T–40E i | 0.470 ± 0.072 | 0.403 ± 0.075 | 0.702 | 1.2 | 0.74 |
| 40T–60E i | 0.727 ± 0.031 | 0.762 ± 0.034 | 0.667 | 0.95 | 0.20 |
| 20T–80E i | 1.02 ± 0.02 | 0.970 ± 0.023 | 0.914 | 1.1 | 0.10 |
| 97% HFIP | - | - | 38.3 | - | 21,600 |
| 90% HFIP | - | - | 28.9 | - | |
| 70% HFIP | - | - | 22.9 | - |
| k/10−6 s−1 | |||||
|---|---|---|---|---|---|
| Temp. (°C) | o-isomer (1) | p-isomer (3) | |||
| 80% EtOH a | 70%TFE b | 97% TFE b | 80% EtOH a | 70% TFE b | |
| 45.0 | 3.36 ± 0.05 | 1.64 ± 0.003 | 0.645e | 3.43 ± 0.08 | 0.612 ± 0.046 |
| 58.1 | 12.0 ± 1.0c | - | 2.08 ± 0.007 | 12.1 ± 0.5 | - |
| 62.5 | - | 9.08 ± 0.03 d | 5.43 ± 0.005 | 18.8 ± 1.0 | - |
| 68.0 | 29.8 ± 1.1 | 16.9 ± 0.08 | 6.95 ± 0.008 f | 30.7 ± 1.0 | 6.63 ± 0.28 |
| 73.0 | - | 27.5 ± 0.07 | 9.97 ± 0.004 | - | 8.83 ± 0.22 |
| 83.0 | - | - | - | - | 21.3 ± 1.5 |
| ΔH≠318.15 (kcal/mol) | 19.8 ± 0.1 | 21.3 ± 0.6 | 21.5 ± 0.5 | 19.9 ± 0.2 | 18.5 ± 0.5 |
| −ΔS≠318.15 (cal/mol·K) | 21.4 ± 0.2 | 18.1 ± 2.0 | 19.3 ± 1.6 | 21.0 ± 0.7 | 28.9 ± 1.7 |
| Solvent a (%) | Temp. (°C) | k/10−3b(s−1) | ΔH≠298.15c(kcal/mol) | −ΔS≠298.15c(cal/mol·K) |
|---|---|---|---|---|
| 100EtOH | 25.0 20.0 15.0 10.0 | 1.29 0.833 0.571 0.410 | 12.2 ± 0.7 | 30.8 ± 2.4 |
| 80EtOH | 25.0 20.0 15.0 10.0 | 4.09 2.61 1.72 1.11 | 14.0 ± 0.2 | 22.6 ± 0.8 |
| 100MeOH | 25.0 20.0 15.0 10.0 | 5.41 3.74 2.58 1.76 | 11.9 ± 0.08 | 28.9 ± 0.3 |
| 70TFE | 25.0 20.0 15.0 10.0 | 17.8 10.4 6.91 3.95 | 15.9 ± 0.6 | 13.1 ± 2.1 |
| 70HFIP | 25.0 20.0 15.0 10.0 | 22.9 14.4 8.99 5.47 | 15.4 ± 0.06 | 14.3 ± 0.2 |
| Substrate | n a | l b | m b | c c | r d | l/m e |
|---|---|---|---|---|---|---|
| 1 | 21 g | 0.66 ± 0.07 | 0.41 ± 0.05 | 0.02 ± 0.06 | 0.903 | - |
| 17 h | 1.10 ± 0.07 | 0.51 ± 0.02 | 0.08 ± 0.03 | 0.993 | 2.16 | |
| 04 i | 0.07 ± 0.05 ~0 | 1.23 ± 0.15 0.60 ± 0.08l | −3.58 ± 0.51 −2.22 ± 0.23 | 0.997 0.971 | 0.06 ~0 | |
| 2 | 23 g | 0.23 ± 0.11 | 0.35 ± 0.08 | −0.03 ± 0.11 | 0.779 | - |
| 11 j | 1.02 ± 0.26 | 0.35 ± 0.06 | 0.18 ± 0.08 | 0.891 | 2.94 | |
| 10 k | 0.32 ± 0.07 | 0.71 ± 0.07 | 0.92 ± 0.14 | 0.966 | 0.45 | |
| 3 | 21 g | 1.19 ± 0.03 | 0.55 ± 0.02 | 0.09 ± 0.02 | 0.995 | 2.16 |
| 4f | 34 g | 1.78 ± 0.08 | 0.54 ± 0.04 | 0.11 ± 0.37 | 0.969 | 3.30 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.-H.; Rhu, C.J.; Kyong, J.B.; Kevill, D.N. The Effect of the ortho Nitro Group in the Solvolysis of Benzyl and Benzoyl Halides. Int. J. Mol. Sci. 2019, 20, 4026. https://doi.org/10.3390/ijms20164026
Park K-H, Rhu CJ, Kyong JB, Kevill DN. The Effect of the ortho Nitro Group in the Solvolysis of Benzyl and Benzoyl Halides. International Journal of Molecular Sciences. 2019; 20(16):4026. https://doi.org/10.3390/ijms20164026
Chicago/Turabian StylePark, Kyoung-Ho, Chan Joo Rhu, Jin Burm Kyong, and Dennis N. Kevill. 2019. "The Effect of the ortho Nitro Group in the Solvolysis of Benzyl and Benzoyl Halides" International Journal of Molecular Sciences 20, no. 16: 4026. https://doi.org/10.3390/ijms20164026
APA StylePark, K.-H., Rhu, C. J., Kyong, J. B., & Kevill, D. N. (2019). The Effect of the ortho Nitro Group in the Solvolysis of Benzyl and Benzoyl Halides. International Journal of Molecular Sciences, 20(16), 4026. https://doi.org/10.3390/ijms20164026





