The Effects of Rebamipide 2% Ophthalmic Solution Application on Murine Subbasal Corneal Nerves After Environmental Dry Eye Stress
Abstract
:1. Introduction
2. Results
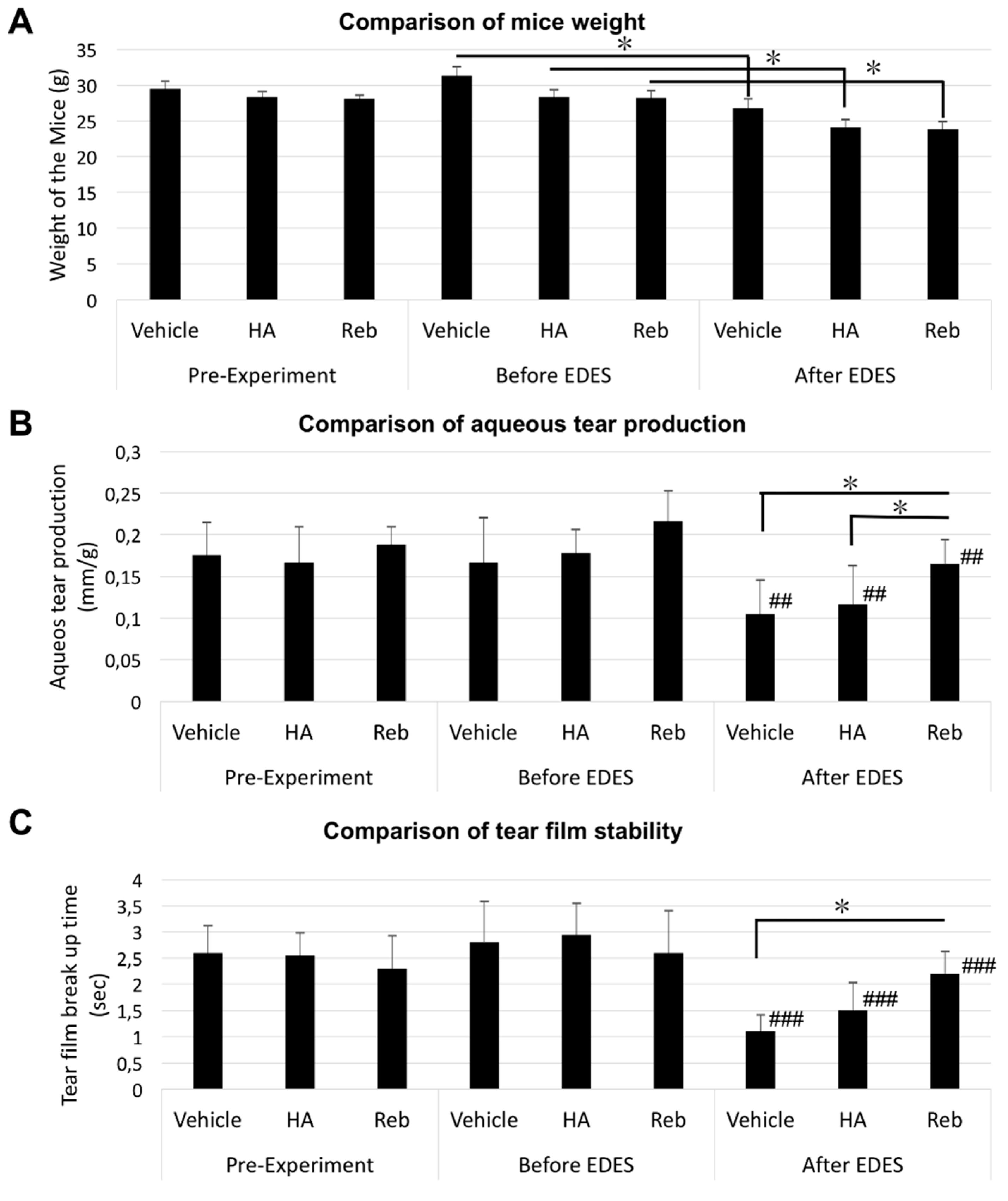
2.1. Aqueous Tear Secretion Quantity and Changes in Tear Film Stability
2.2. Changes in Vital Staining Score
2.3. Corneal Sensitivity Alterations
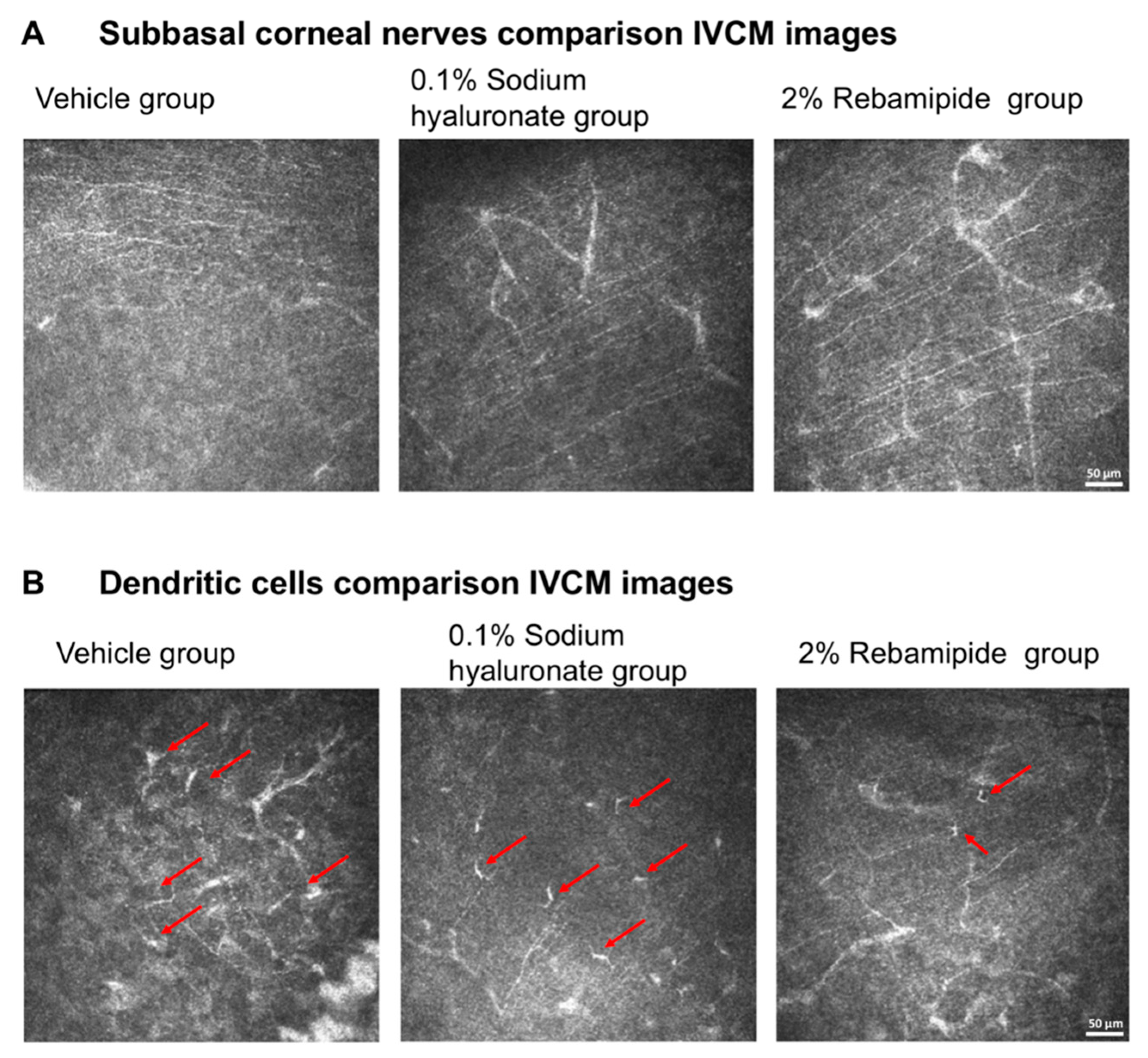
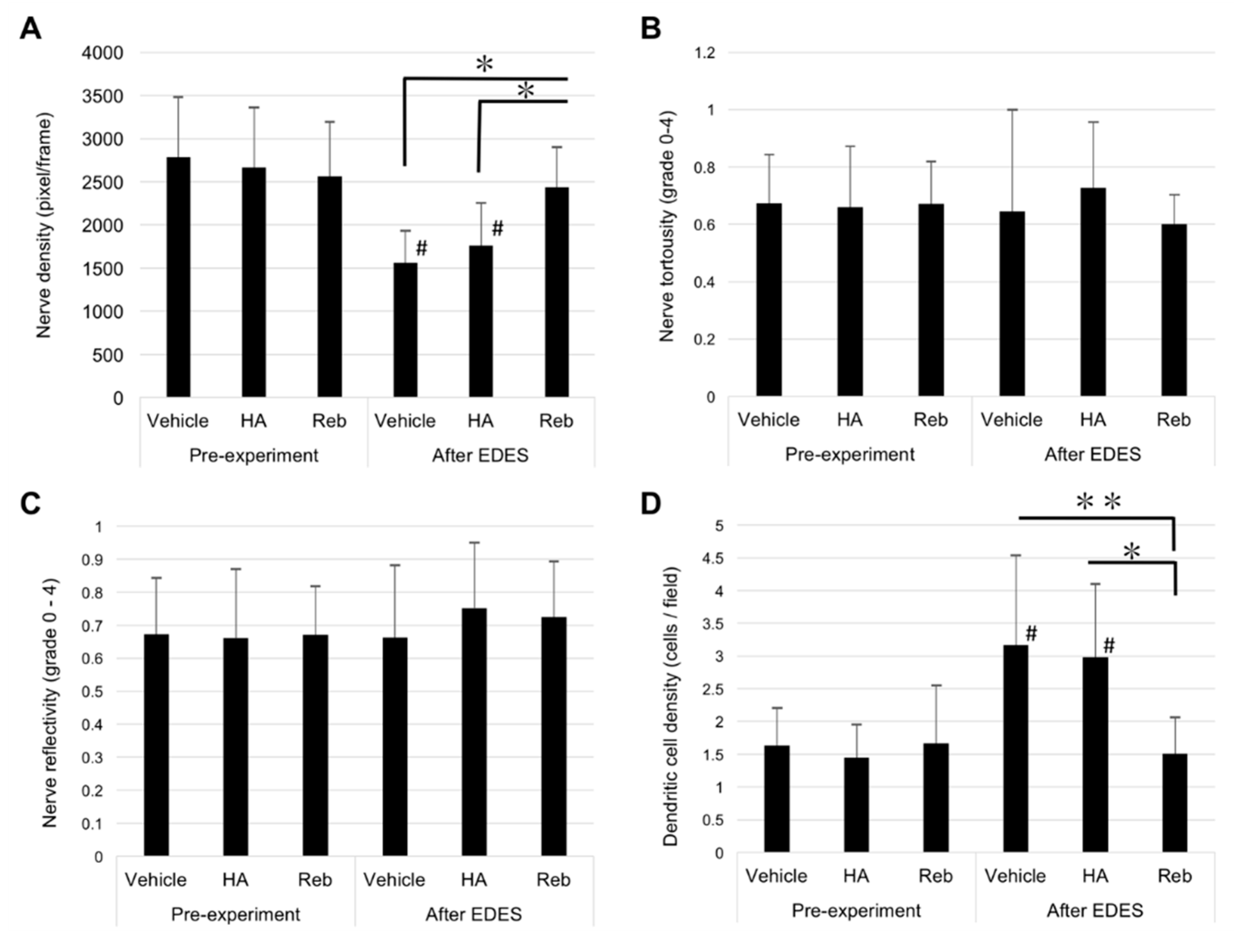
2.4. In Vivo Confocal Microscopy Findings
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Procedure
4.2. Application of Environmental Dry Eye Stress
4.3. Aqueous Tear Secretion Quantity and Tear Film Stability Assessment
4.4. Ocular Surface Vital Staining Assessment
4.5. Corneal Sensitivity Assessment
4.6. In Vivo Laser Scanning Confocal Microscopic Examination and Image Analysis
- (1)
- The NFD was assessed by measuring the total length of CSN fibers within a frame (160,000 µm2), as defined in our previous study [3]. After semi-automatically marking the CSN in each frame, the NFD was automatically measured by the NeuronJ plug-in for ImageJ software (National Institutes of Health, Bethesda, MD, USA). Four different representative images for each cornea were analyzed in pixels. The mean CSN for each cornea was calculated by averaging these total values. The data were determined as density (µm/mm2) ± standard error of the mean (SEM).
- (2)
- Nerve tortuosity was evaluated at the subbasal layer according to previously published grading scales [3]. The grading scales consisted of a series of images derived from our previous work and other studies of corneal innervation using confocal microscopy. Briefly, Oliveira-Soto and Efron (2001) classified human corneal nerve tortuosity into five grades [52]. Similarly, in our previous study, we classified mice corneal nerve tortuosity into five grades, ranging from 0 to 5, according to the subbasal nerve curve magnitude [3].
- (3)
- The grading of nerve reflectivity was again performed according to our previous study [3]. In brief, we classified mice corneal nerve reflectivity into five grades from 0 to 5. Similarly, only nerves longer than 50% of the width frame underwent reflectivity assessment.
- (4)
- The images were also examined for the density of DCs. The measurement of density of epithelial DCs was performed at the CSN plexus area and basal epithelial layer. Four representative images were used to evaluate the density of epithelial DCs.
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Uchino, M.; Yokoi, N.; Uchino, Y.; Dogru, M.; Kawashima, M.; Komuro, A.; Sonomura, Y.; Kato, H.; Kinoshita, S.; Schaumberg, D.A.; et al. Prevalence of dry eye disease and its risk factors in visual display terminal users: The Osaka study. Am. J. Ophthalmol. 2013, 156, 759–766. [Google Scholar] [CrossRef]
- Nakamura, S.; Kinoshita, S.; Yokoi, N.; Ogawa, Y.; Shibuya, M.; Nakashima, H.; Hisamura, R.; Imada, T.; Imagawa, T.; Uehara, M.; et al. Lacrimal hypofunction as a new mechanism of dry eye in visual display terminal users. PLoS ONE 2010, 5, e11119. [Google Scholar] [CrossRef] [PubMed]
- Simsek, C.; Kojima, T.; Dogru, M.; Tsubota, K. Alterations of Murine Subbasal Corneal Nerves After Environmental Dry Eye Stress. Invest. Ophthalmol. Vis. Sci. 2018, 59, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Marfurt, C.F.; Cox, J.; Deek, S.; Dvorscak, L. Anatomy of the human corneal innervation. Exp. Eye Res. 2010, 90, 478–492. [Google Scholar] [CrossRef] [PubMed]
- Muller, L.J.; Marfurt, C.F.; Kruse, F.; Tervo, T.M. Corneal nerves: structure, contents and function. Exp. Eye Res. 2003, 76, 521–542. [Google Scholar] [CrossRef]
- Muller, L.J.; Pels, L.; Vrensen, G.F. Ultrastructural organization of human corneal nerves. Invest. Ophthalmol. Vis. Sci. 1996, 37, 476–488. [Google Scholar] [PubMed]
- Craig, J.P.; Nelson, J.D.; Azar, D.T.; Belmonte, C.; Bron, A.J.; Chauhan, S.K.; de Paiva, C.S.; Gomes, J.A.P.; Hammitt, K.M.; Jones, L.; et al. TFOS DEWS II Report Executive Summary. Ocul. Surf. 2017, 15, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Benitez del Castillo, J.M.; Wasfy, M.A.; Fernandez, C.; Garcia-Sanchez, J. An in vivo confocal masked study on corneal epithelium and subbasal nerves in patients with dry eye. Invest. Ophthalmol. Vis. Sci. 2004, 45, 3030–3035. [Google Scholar] [CrossRef]
- Davis, E.A.; Dohlman, C.H. Neurotrophic keratitis. Int. Ophthalmol. Clin. 2001, 41, 1–11. [Google Scholar] [CrossRef]
- Bonini, S.; Rama, P.; Olzi, D.; Lambiase, A. Neurotrophic keratitis. Eye 2003, 17, 989–995. [Google Scholar] [CrossRef] [Green Version]
- Tsubota, K.; Yokoi, N.; Shimazaki, J.; Watanabe, H.; Dogru, M.; Yamada, M.; Kinoshita, S.; Kim, H.M.; Tchah, H.W.; Hyon, J.Y.; et al. New Perspectives on Dry Eye Definition and Diagnosis: A Consensus Report by the Asia Dry Eye Society. Ocul. Surf. 2017, 15, 65–76. [Google Scholar] [CrossRef]
- Uchino, Y.; Uchino, M.; Yokoi, N.; Dogru, M.; Kawashima, M.; Okada, N.; Inaba, T.; Tamaki, S.; Komuro, A.; Sonomura, Y.; et al. Alteration of tear mucin 5AC in office workers using visual display terminals: The Osaka Study. JAMA Ophthalmol. 2014, 132, 985–992. [Google Scholar] [CrossRef]
- Miri, A.; Alomar, T.; Nubile, M.; Al-Aqaba, M.; Lanzini, M.; Fares, U.; Said, D.G.; Lowe, J.; Dua, H.S. In vivo confocal microscopic findings in patients with limbal stem cell deficiency. Br. J. Ophthalmol. 2012, 96, 523–529. [Google Scholar] [CrossRef]
- Itoh, S.; Itoh, K.; Shinohara, H. Regulation of human corneal epithelial mucins by rebamipide. Curr. Eye Res. 2014, 39, 133–141. [Google Scholar] [CrossRef]
- Kinoshita, S.; Awamura, S.; Nakamichi, N.; Suzuki, H.; Oshiden, K.; Yokoi, N.; Rebamipide Ophthalmic Suspension Long-term Study Group. A multicenter, open-label, 52-week study of 2% rebamipide (OPC-12759) ophthalmic suspension in patients with dry eye. Am. J. Ophthalmol. 2014, 157, 576–583.e1. [Google Scholar] [CrossRef]
- Kinoshita, S.; Awamura, S.; Oshiden, K.; Nakamichi, N.; Suzuki, H.; Yokoi, N.; Rebamipide Ophthalmic Suspension Phase II Study Group. Rebamipide (OPC-12759) in the treatment of dry eye: A randomized, double-masked, multicenter, placebo-controlled phase II study. Ophthalmology 2012, 119, 2471–2478. [Google Scholar] [CrossRef]
- Simsek, C.; Dogru, M.; Shinzawa, M.; Den, S.; Kojima, T.; Iseda, H.; Suzuki, M.; Shibasaki, Y.; Yoshida, N.; Shimazaki, J. The Efficacy of 2% Topical Rebamipide on Conjunctival Squamous Metaplasia and Goblet Cell Density in Dry Eye Disease. J. Ocul. Pharmacol. Ther. 2019, 35, 350–358. [Google Scholar] [CrossRef]
- Cruzat, A.; Pavan-Langston, D.; Hamrah, P. In vivo confocal microscopy of corneal nerves: analysis and clinical correlation. Semin Ophthalmol. 2010, 25, 171–177. [Google Scholar] [CrossRef]
- Patel, D.V.; McGhee, C.N. In vivo confocal microscopy of human corneal nerves in health, in ocular and systemic disease, and following corneal surgery: A review. Br. J. Ophthalmol. 2009, 93, 853–860. [Google Scholar] [CrossRef]
- Itakura, H.; Kashima, T.; Itakura, M.; Akiyama, H.; Kishi, S. Topical rebamipide improves lid wiper epitheliopathy. Clin Ophthalmol. 2013, 7, 2137–2141. [Google Scholar] [CrossRef] [Green Version]
- Ohguchi, T.; Kojima, T.; Ibrahim, O.M.; Nagata, T.; Shimizu, T.; Shirasawa, T.; Kawakita, T.; Satake, Y.; Tsubota, K.; Shimazaki, J.; et al. The effects of 2% rebamipide ophthalmic solution on the tear functions and ocular surface of the superoxide dismutase-1 (sod1) knockout mice. Invest. Ophthalmol. Vis. Sci. 2013, 54, 7793–7802. [Google Scholar] [CrossRef]
- Kojima, T.; Simsek, C.; Igarashi, A.; Aoki, K.; Higa, K.; Shimizu, T.; Dogru, M.; Tsubota, K.; Shimazaki, J. The Role of 2% Rebamipide Eye Drops Related to Conjunctival Differentiation in Superoxide Dismutase-1 (Sod1) Knockout Mice. Invest. Ophthalmol. Vis. Sci. 2018, 59, 1675–1681. [Google Scholar] [CrossRef] [Green Version]
- Condon, P.I.; McEwen, C.G.; Wright, M.; Mackintosh, G.; Prescott, R.J.; McDonald, C. Double blind, randomised, placebo controlled, crossover, multicentre study to determine the efficacy of a 0.1% (w/v) sodium hyaluronate solution (Fermavisc) in the treatment of dry eye syndrome. Br. J. Ophthalmol. 1999, 83, 1121–1124. [Google Scholar] [CrossRef]
- Aragona, P.; Di Stefano, G.; Ferreri, F.; Spinella, R.; Stilo, A. Sodium hyaluronate eye drops of different osmolarity for the treatment of dry eye in Sjogren’s syndrome patients. Br. J. Ophthalmol. 2002, 86, 879–884. [Google Scholar] [CrossRef]
- Kinoshita, S.; Oshiden, K.; Awamura, S.; Suzuki, H.; Nakamichi, N.; Yokoi, N.; Rebamipide Ophthalmic Suspension Phase 3 Study Group. A randomized, multicenter phase 3 study comparing 2% rebamipide (OPC-12759) with 0.1% sodium hyaluronate in the treatment of dry eye. Ophthalmology 2013, 120, 1158–1165. [Google Scholar] [CrossRef]
- Urashima, H.; Takeji, Y.; Okamoto, T.; Fujisawa, S.; Shinohara, H. Rebamipide increases mucin-like substance contents and periodic acid Schiff reagent-positive cells density in normal rabbits. J. Ocul. Pharmacol. Ther. 2012, 28, 264–270. [Google Scholar] [CrossRef]
- Alhatem, A.; Cavalcanti, B.; Hamrah, P. In vivo confocal microscopy in dry eye disease and related conditions. Semin Ophthalmol. 2012, 27, 138–148. [Google Scholar] [CrossRef]
- Villani, E.; Baudouin, C.; Efron, N.; Hamrah, P.; Kojima, T.; Patel, S.V.; Pflugfelder, S.C.; Zhivov, A.; Dogru, M. In vivo confocal microscopy of the ocular surface: from bench to bedside. Curr. Eye Res. 2014, 39, 213–231. [Google Scholar] [CrossRef]
- Villani, E.; Magnani, F.; Viola, F.; Santaniello, A.; Scorza, R.; Nucci, P.; Ratiglia, R. In vivo confocal evaluation of the ocular surface morpho-functional unit in dry eye. Optom. Vis. Sci. 2013, 90, 576–586. [Google Scholar] [CrossRef]
- Benitez-Del-Castillo, J.M.; Acosta, M.C.; Wassfi, M.A.; Diaz-Valle, D.; Gegundez, J.A.; Fernandez, C.; Garcia-Sanchez, J. Relation between corneal innervation with confocal microscopy and corneal sensitivity with noncontact esthesiometry in patients with dry eye. Invest. Ophthalmol. Vis. Sci. 2007, 48, 173–181. [Google Scholar] [CrossRef]
- Labbe, A.; Alalwani, H.; Van Went, C.; Brasnu, E.; Georgescu, D.; Baudouin, C. The relationship between subbasal nerve morphology and corneal sensation in ocular surface disease. Invest. Ophthalmol. Vis. Sci. 2012, 53, 4926–4931. [Google Scholar] [CrossRef]
- Simsek, C.; Kojima, T.; Nagata, T.; Dogru, M.; Tsubota, K. Changes in Murine Subbasal Corneal Nerves After Scopolamine-Induced Dry Eye Stress Exposure. Invest. Ophthalmol. Vis. Sci. 2019, 60, 615–623. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.C.; Kainz, V.; Burstein, R.; Levy, D. Tumor necrosis factor-alpha induces sensitization of meningeal nociceptors mediated via local COX and p38 MAP kinase actions. Pain 2011, 152, 140–149. [Google Scholar] [CrossRef]
- Hosal, B.M.; Ornek, N.; Zilelioglu, G.; Elhan, A.H. Morphology of corneal nerves and corneal sensation in dry eye: a preliminary study. Eye 2005, 19, 1276–1279. [Google Scholar] [CrossRef]
- Tuominen, I.S.; Konttinen, Y.T.; Vesaluoma, M.H.; Moilanen, J.A.; Helinto, M.; Tervo, T.M. Corneal innervation and morphology in primary Sjogren’s syndrome. Invest. Ophthalmol. Vis. Sci. 2003, 44, 2545–2549. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, J.; Luo, L.; Xiao, Q.; Sun, M.; Liu, Z. Altered corneal nerves in aqueous tear deficiency viewed by in vivo confocal microscopy. Cornea 2005, 24, 818–824. [Google Scholar] [CrossRef]
- Villani, E.; Galimberti, D.; Viola, F.; Mapelli, C.; Del Papa, N.; Ratiglia, R. Corneal involvement in rheumatoid arthritis: An in vivo confocal study. Invest. Ophthalmol. Vis. Sci. 2008, 49, 560–564. [Google Scholar] [CrossRef]
- Villani, E.; Garoli, E.; Termine, V.; Pichi, F.; Ratiglia, R.; Nucci, P. Corneal Confocal Microscopy in Dry Eye Treated with Corticosteroids. Optom. Vis. Sci. 2015, 92, e290–e295. [Google Scholar] [CrossRef] [Green Version]
- Arakaki, R.; Eguchi, H.; Yamada, A.; Kudo, Y.; Iwasa, A.; Enkhmaa, T.; Hotta, F.; Mitamura-Aizawa, S.; Mitamura, Y.; Hayashi, Y.; et al. Anti-inflammatory effects of rebamipide eyedrop administration on ocular lesions in a murine model of primary Sjogren’s syndrome. PLoS ONE 2014, 9, e98390. [Google Scholar] [CrossRef]
- Ueta, M.; Sotozono, C.; Yokoi, N.; Kinoshita, S. Rebamipide suppresses PolyI:C-stimulated cytokine production in human conjunctival epithelial cells. J. Ocul. Pharmacol. Ther. 2013, 29, 688–693. [Google Scholar] [CrossRef]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Choi, E.Y.; Kang, H.G.; Lee, C.H.; Yeo, A.; Noh, H.M.; Gu, N.; Kim, M.J.; Song, J.S.; Kim, H.C.; Lee, H.K. Langerhans cells prevent subbasal nerve damage and upregulate neurotrophic factors in dry eye disease. PLoS ONE 2017, 12, e0176153. [Google Scholar] [CrossRef]
- Yamazaki, R.; Yamazoe, K.; Yoshida, S.; Hatou, S.; Inagaki, E.; Okano, H.; Tsubota, K.; Shimmura, S. The Semaphorin 3A inhibitor SM-345431 preserves corneal nerve and epithelial integrity in a murine dry eye model. Sci. Rep. 2017, 7, 15584. [Google Scholar] [CrossRef] [Green Version]
- Stepp, M.A.; Pal-Ghosh, S.; Tadvalkar, G.; Williams, A.; Pflugfelder, S.C.; de Paiva, C.S. Reduced intraepithelial corneal nerve density and sensitivity accompany desiccating stress and aging in C57BL/6 mice. Exp. Eye Res. 2018, 169, 91–98. [Google Scholar] [CrossRef]
- Miwa, H.; Osada, T.; Nagahara, A.; Ohkusa, T.; Hojo, M.; Tomita, T.; Hori, K.; Matsumoto, T.; Sato, N. Effect of a gastro-protective agent, rebamipide, on symptom improvement in patients with functional dyspepsia: A double-blind placebo-controlled study in Japan. J. Gastroenterol. Hepatol. 2006, 21, 1826–1831. [Google Scholar] [CrossRef]
- Naito, Y.; Yoshikawa, T.; Tanigawa, T.; Sakurai, K.; Yamasaki, K.; Uchida, M.; Kondo, M. Hydroxyl radical scavenging by rebamipide and related compounds: Electron paramagnetic resonance study. Free Radic Biol. Med. 1995, 18, 117–123. [Google Scholar] [CrossRef]
- Moon, S.J.; Woo, Y.J.; Jeong, J.H.; Park, M.K.; Oh, H.J.; Park, J.S.; Kim, E.K.; Cho, M.L.; Park, S.H.; Kim, H.Y.; et al. Rebamipide attenuates pain severity and cartilage degeneration in a rat model of osteoarthritis by downregulating oxidative damage and catabolic activity in chondrocytes. Osteoarthritis Cartilage 2012, 20, 1426–1438. [Google Scholar] [CrossRef] [Green Version]
- Galor, A.; Moein, H.R.; Lee, C.; Rodriguez, A.; Felix, E.R.; Sarantopoulos, K.D.; Levitt, R.C. Neuropathic pain and dry eye. Ocul. Surf. 2018, 16, 31–44. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Shimmura, S.; Ono, M.; Shinozaki, K.; Toda, I.; Takamura, E.; Mashima, Y.; Tsubota, K. Sodium hyaluronate eyedrops in the treatment of dry eyes. Br. J. Ophthalmol. 1995, 79, 1007–1011. [Google Scholar] [CrossRef]
- Oliveira-Soto, L.; Efron, N. Morphology of corneal nerves using confocal microscopy. Cornea 2001, 20, 374–384. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simsek, C.; Kojima, T.; Nakamura, S.; Dogru, M.; Tsubota, K. The Effects of Rebamipide 2% Ophthalmic Solution Application on Murine Subbasal Corneal Nerves After Environmental Dry Eye Stress. Int. J. Mol. Sci. 2019, 20, 4031. https://doi.org/10.3390/ijms20164031
Simsek C, Kojima T, Nakamura S, Dogru M, Tsubota K. The Effects of Rebamipide 2% Ophthalmic Solution Application on Murine Subbasal Corneal Nerves After Environmental Dry Eye Stress. International Journal of Molecular Sciences. 2019; 20(16):4031. https://doi.org/10.3390/ijms20164031
Chicago/Turabian StyleSimsek, Cem, Takashi Kojima, Shigeru Nakamura, Murat Dogru, and Kazuo Tsubota. 2019. "The Effects of Rebamipide 2% Ophthalmic Solution Application on Murine Subbasal Corneal Nerves After Environmental Dry Eye Stress" International Journal of Molecular Sciences 20, no. 16: 4031. https://doi.org/10.3390/ijms20164031
APA StyleSimsek, C., Kojima, T., Nakamura, S., Dogru, M., & Tsubota, K. (2019). The Effects of Rebamipide 2% Ophthalmic Solution Application on Murine Subbasal Corneal Nerves After Environmental Dry Eye Stress. International Journal of Molecular Sciences, 20(16), 4031. https://doi.org/10.3390/ijms20164031






