Water-Soluble Fullerenol C60(OH)36 toward Effective Anti-Air Pollution Induced by Urban Particulate Matter in HaCaT Cell
Abstract
:1. Introduction
2. Results
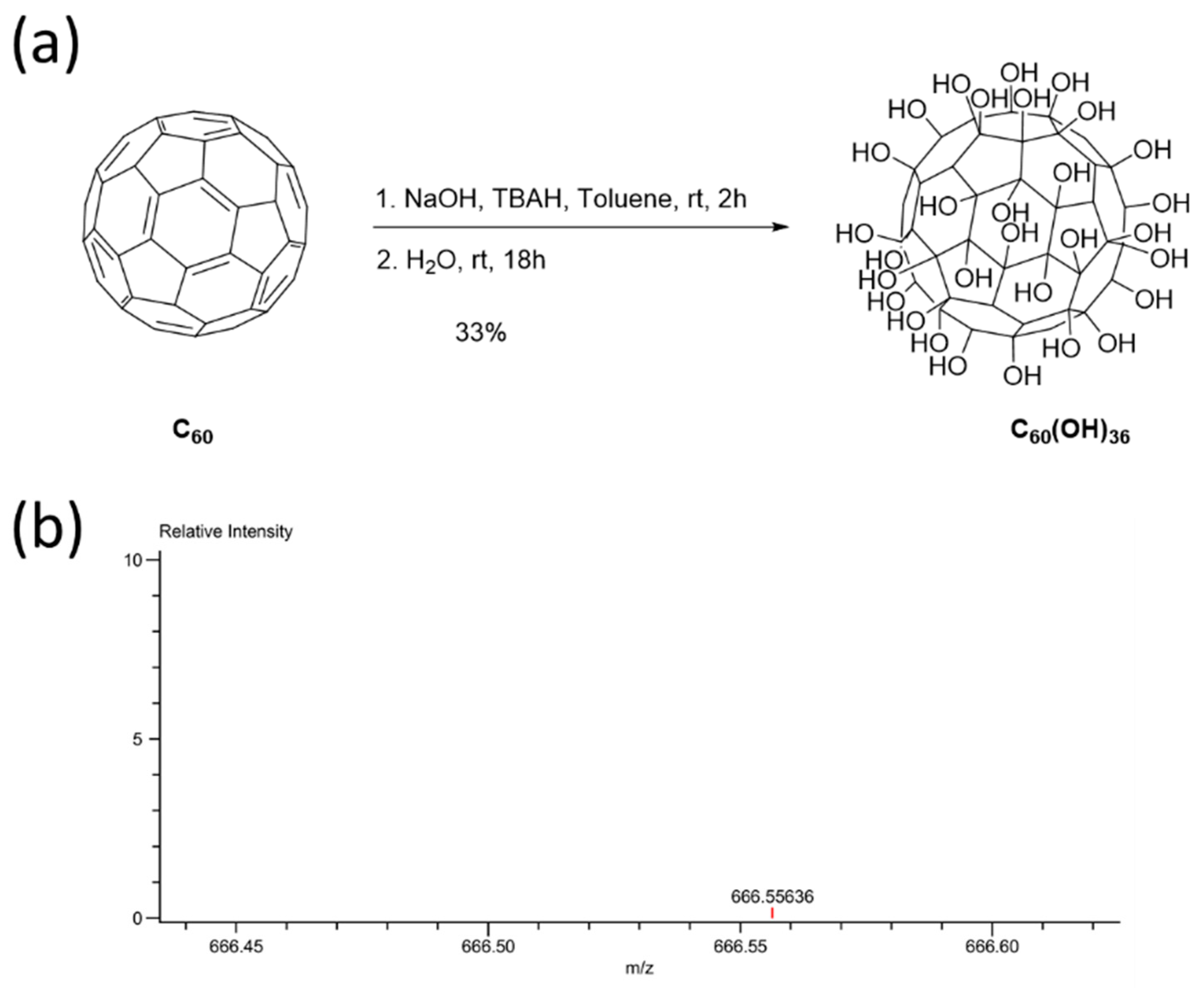
2.1. Synthesis and Characterization of Fullerenol C60(OH)36
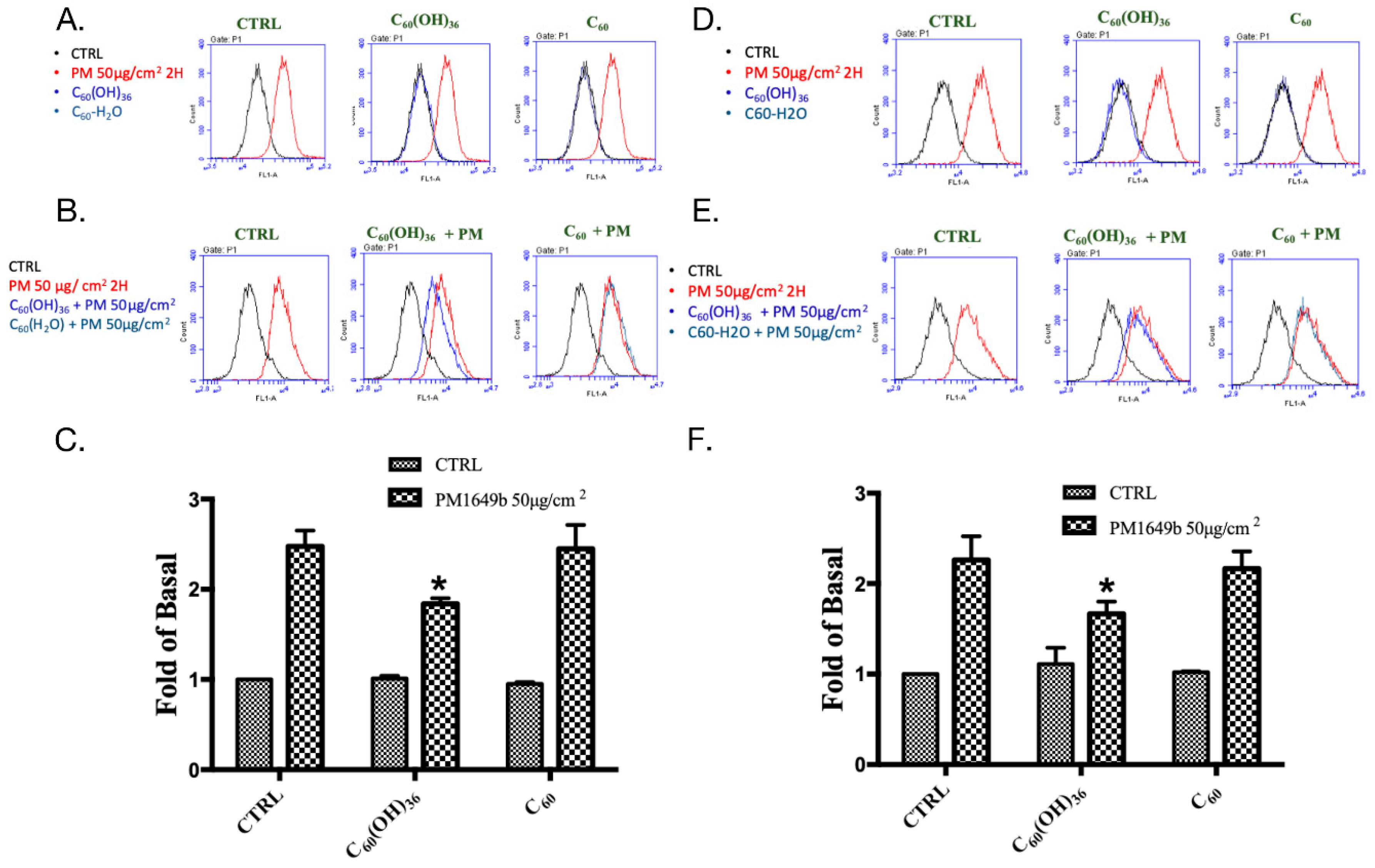
2.2. Fullerenol C60(OH)36 Blocked PM-Induced Cell Apoptosis
2.3. Fullerenol C60(OH)36 Suppressed PM-Triggered the Cellular and Mitochondrial Production of Reactive Oxygen Species
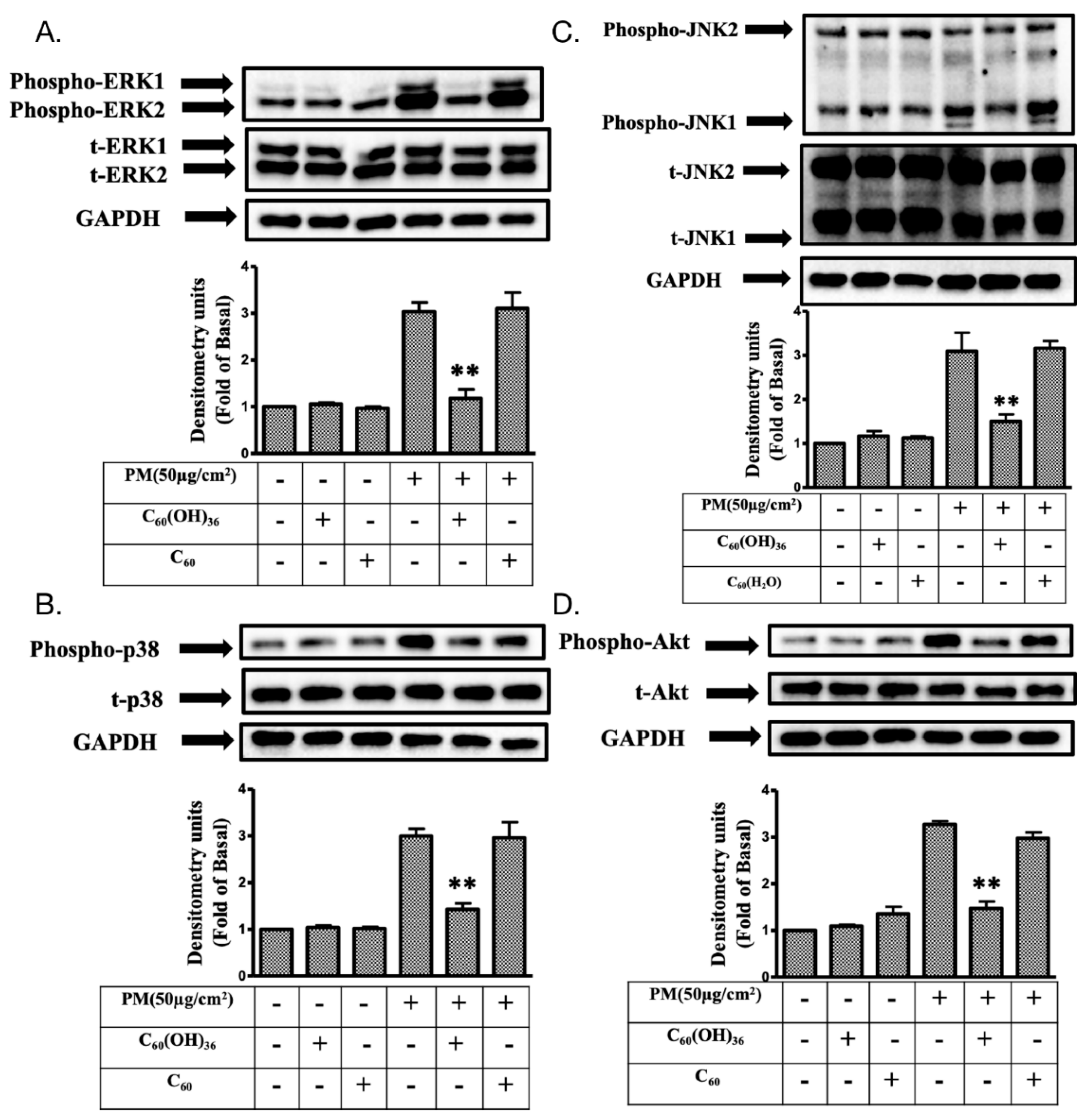
2.4. Fullerenol C60(OH)36 Reduced the PM-Induced Phosphorylation of Mitogen-Activated Protein Kinases (MAPKs) and AKT Proteins in HaCaT Cells
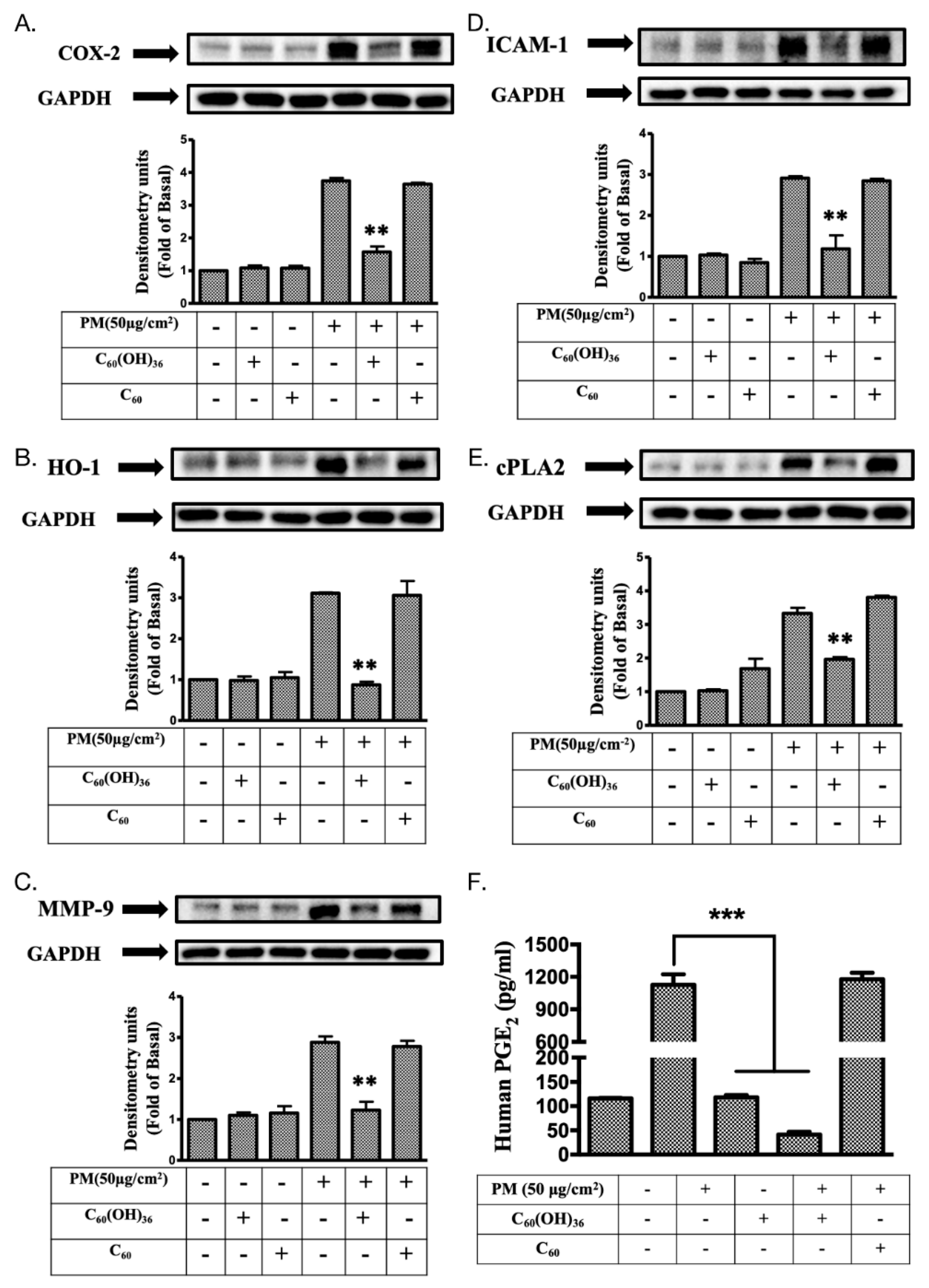
2.5. Fullerenol C60(OH)36 Suppressed PM-Induced Inflammatory Protein Expression
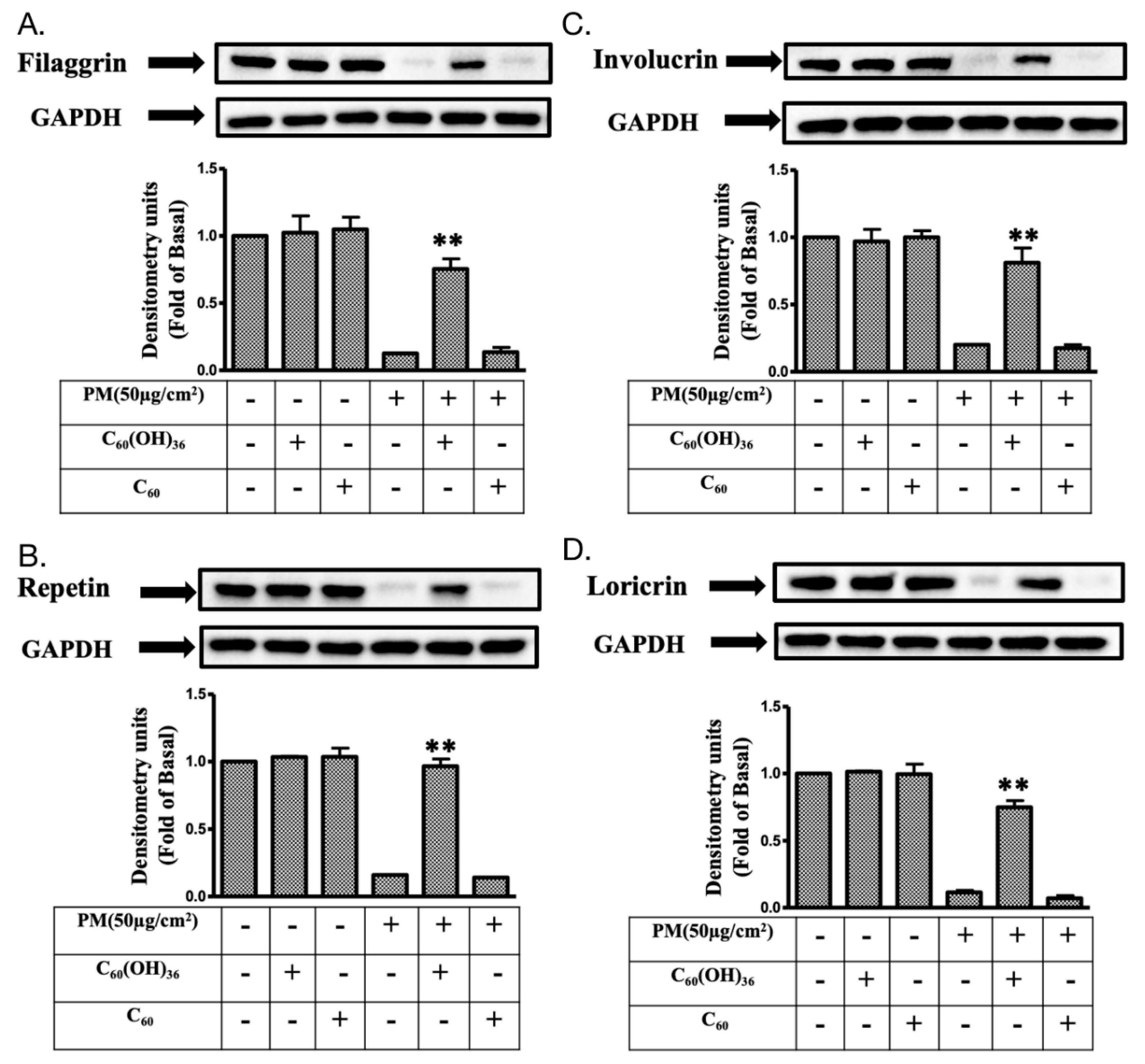
2.6. Fullerenol C60(OH)36 Could Maintain the Levels of Proteins Exhibiting Protective Effects towards PM-Exposed Keratinocytes
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Thin-Layer Chromatography, Mass and Spectrometer Assay
4.3. Transmission Electron Microscopy Assay
4.4. Hydro-Diameter Size and Zeta Potential
4.5. Synthesis of Fullerenol C60(OH)36
4.6. Cell Culture Conditions
4.7. Cell Apoptosis Assay
4.8. Cellular and Mitochondrial ROS Measurement
4.9. Immunoblotting
4.10. Prostaglandin E2 (PGE2) Production Measurement
4.11. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| COX-2 | Cyclooxygenase |
| cPLA2 | Cytosolic phospholipases A2 |
| ERK | Extracellular regulated protein kinase |
| FITC | Fluorescein isothiocyanate |
| GAPDH | Glyceraldehyde 3-phosphate dehydrogenase |
| HO-1 | Heme oxygenase-1 |
| ICAM-1 | Intercellular adhesion molecular-1 |
| JNK | c-Jun N-terminal kinase |
| MAPK | Mitogen-activated protein kinase |
| MMP-9 | Metalloproteinase-9 |
| PM | Particulate matter |
| PGE2 | Prostaglandin E2 |
| ROS | Reactive oxygen species |
References
- A Prüss-Ustün, J.W.; Corvalán, C.; Bos, R.; Neira, M. Preventing disease through healthy environments: A global assessment of the burden of disease from environmental risks. Available online: https://www.who.int/quantifying_ehimpacts/publications/preventing-disease/en/ (accessed on 2 May 2019).
- Lawal, A.O. Air particulate matter induced oxidative stress and inflammation in cardiovascular disease and atherosclerosis: The role of Nrf2 and AhR-mediated pathways. Toxicol. Lett. 2017, 270, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Haikerwal, A.; Akram, M.; Monaco, A.D.; Smith, K.; Sim, M.R.; Meyer, M.; Tonkin, A.M.; Abramson, M.J.; Dennekamp, M. Impact of Fine Particulate Matter (PM2.5) Exposure during Wildfires on Cardiovascular Health Outcomes. J. Am. Heart Assoc. 2015, 4, e001653. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.A.; Nel, A.E. Particulate matter and atherosclerosis: Role of particle size, composition and oxidative stress. Part. Fibre Toxicol. 2009, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Rao, X.; Zhong, J.; Brook, R.D.; Rajagopalan, S. Effect of Particulate Matter Air Pollution on Cardiovascular Oxidative Stress Pathways. Antioxid. Redox Signal. 2018, 28, 797–818. [Google Scholar] [CrossRef] [PubMed]
- Drakaki, E.; Dessinioti, C.; Antoniou, C.V. Air pollution and the skin. Front. Environ. Sci. 2014, 2. [Google Scholar] [CrossRef] [Green Version]
- Parrado, C.; Mercado-Saenz, S.; Perez-Davo, A.; Gilaberte, Y.; Gonzalez, S.; Juarranz, A. Environmental Stressors on Skin Aging. Mechanistic Insights. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Ahn, M.J.; Kang, K.A.; Ryu, Y.S.; Hyun, Y.J.; Shilnikova, K.; Zhen, A.X.; Jeong, J.W.; Choi, Y.H.; Kang, H.K.; et al. Particulate matter 2.5 damages skin cells by inducing oxidative stress, subcellular organelle dysfunction, and apoptosis. Arch. Toxicol. 2018, 92, 2077–2091. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Park, E.H.; Schauer, J.J.; Yi, S.-M.; Heo, J. Reactive oxygen species (ROS) activity of ambient fine particles (PM2.5) measured in Seoul, Korea. Environ. Int. 2018, 117, 276–283. [Google Scholar] [CrossRef]
- Lee, C.W.; Lin, Z.C.; Hu, S.C.; Chiang, Y.C.; Hsu, L.F.; Lin, Y.C.; Lee, I.T.; Tsai, M.H.; Fang, J.Y. Urban particulate matter down-regulates filaggrin via COX2 expression/PGE2 production leading to skin barrier dysfunction. Sci. Rep. 2016, 6, 27995. [Google Scholar] [CrossRef] [Green Version]
- Mousavi, S.Z.; Nafisi, S.; Maibach, H.I. Fullerene nanoparticle in dermatological and cosmetic applications. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 1071–1087. [Google Scholar] [CrossRef]
- Lai, C.-H.; Lin, C.-Y.; Wu, H.-T.; Chan, H.-S.; Chuang, Y.-J.; Chen, C.-T.; Lin, C.-C. Galactose Encapsulated Multifunctional Nanoparticle for HepG2 Cell Internalization. Adv. Funct. Mater. 2010, 20, 3948–3958. [Google Scholar] [CrossRef]
- Lai, C.-H.; Chang, T.-C.; Chuang, Y.-J.; Tzou, D.-L.; Lin, C.-C. Stepwise Orthogonal Click Chemistry toward Fabrication of Paclitaxel/Galactose Functionalized Fluorescent Nanoparticles for HepG2 Cell Targeting and Delivery. Bioconjug. Chem. 2013, 24, 1698–1709. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162. [Google Scholar] [CrossRef]
- Krätschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A new form of carbon. Nature 1990, 347, 354. [Google Scholar] [CrossRef]
- Krusic, P.J.; Wasserman, E.; Keizer, P.N.; Morton, J.R.; Preston, K.F. Radical Reactions of C60. Science 1991, 254, 1183–1185. [Google Scholar] [CrossRef] [PubMed]
- Tokuyama, H.; Yamago, S.; Nakamura, E.; Shiraki, T.; Sugiura, Y. Photoinduced biochemical activity of fullerene carboxylic acid. J. Am. Chem. Soc. 1993, 115, 7918–7919. [Google Scholar] [CrossRef]
- Castro, E.; Hernandez Garcia, A.; Zavala, G.; Echegoyen, L. Fullerenes in Biology and Medicine. J. Mater. Chem. B 2017, 5, 6523–6535. [Google Scholar] [CrossRef] [PubMed]
- Semenov, K.N.; Charykov, N.A.; Postnov, V.N.; Sharoyko, V.V.; Vorotyntsev, I.V.; Galagudza, M.M.; Murin, I.V. Fullerenols: Physicochemical properties and applications. Prog. Solid State Chem. 2016, 44, 59–74. [Google Scholar] [CrossRef]
- Fluri, F.; Grünstein, D.; Cam, E.; Ungethuem, U.; Hatz, F.; Schäfer, J.; Samnick, S.; Israel, I.; Kleinschnitz, C.; Orts-Gil, G.; et al. Fullerenols and glucosamine fullerenes reduce infarct volume and cerebral inflammation after ischemic stroke in normotensive and hypertensive rats. Exp. Neurol. 2015, 265, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Grebowski, J.; Kazmierska, P.; Litwinienko, G.; Lankoff, A.; Wolszczak, M.; Krokosz, A. Fullerenol C60(OH)36 protects human erythrocyte membrane against high-energy electrons. Biochim. Biophys. Acta (Bba) - Biomembr. 2018, 1860, 1528–1536. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-W.; Lin, Z.-C.; Hsu, L.-F.; Fang, J.-Y.; Chiang, Y.-C.; Tsai, M.-H.; Lee, M.-H.; Li, S.-Y.; Hu, S.C.-S.; Lee, I.T.; et al. Eupafolin ameliorates COX-2 expression and PGE2 production in particulate pollutants-exposed human keratinocytes through ROS/MAPKs pathways. J. Ethnopharmacol. 2016, 189, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.C.; Lee, C.W.; Tsai, M.H.; Ko, H.H.; Fang, J.Y.; Chiang, Y.C.; Liang, C.J.; Hsu, L.F.; Hu, S.C.; Yen, F.L. Eupafolin nanoparticles protect HaCaT keratinocytes from particulate matter-induced inflammation and oxidative stress. Int. J. Nanomed. 2016, 11, 3907–3926. [Google Scholar] [CrossRef]
- Huang, P.H.; Tseng, C.H.; Lin, C.Y.; Lee, C.W.; Yen, F.L. Preparation, characterizations and anti-pollutant activity of 7,3’,4’-trihydroxyisoflavone nanoparticles in particulate matter-induced HaCaT keratinocytes. Int. J. Nanomed. 2018, 13, 3279–3293. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, A.; Srdjenovic, B.; Seke, M.; Petrovic, D.; Injac, R.; Mrdjanovic, J. Review of Synthesis and Antioxidant Potential of Fullerenol Nanoparticles. J. Nanomater. 2015, 2015, 15. [Google Scholar] [CrossRef]
- Roy, P.; Bag, S.; Chakraborty, D.; Dasgupta, S. Exploring the Inhibitory and Antioxidant Effects of Fullerene and Fullerenol on Ribonuclease A. ACS Omega 2018, 3, 12270–12283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narayanapillai, S.; Agarwal, C.; Tilley, C.; Agarwal, R. Silibinin is a potent sensitizer of UVA radiation-induced oxidative stress and apoptosis in human keratinocyte HaCaT cells. Photochem. Photobiol. 2012, 88, 1135–1140. [Google Scholar] [CrossRef]
- Fujisaki, H.; Futaki, S.; Yamada, M.; Sekiguchi, K.; Hayashi, T.; Ikejima, T.; Hattori, S. Respective optimal calcium concentrations for proliferation on type I collagen fibrils in two keratinocyte line cells, HaCaT and FEPE1L-8. Regen. Ther. 2018, 8, 73–79. [Google Scholar] [CrossRef]
- Tzirakis, M.D.; Orfanopoulos, M. Radical Reactions of Fullerenes: From Synthetic Organic Chemistry to Materials Science and Biology. Chem. Rev. 2013, 113, 5262–5321. [Google Scholar] [CrossRef]
- Son, Y.; Cheong, Y.K.; Kim, N.H.; Chung, H.T.; Kang, D.G.; Pae, H.O. Mitogen-Activated Protein Kinases and Reactive Oxygen Species: How Can ROS Activate MAPK Pathways? J. Signal. Transduct. 2011, 2011, 792639. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Kang, Z.; Jiang, S.; Zhao, J.; Yan, S.; Xu, F.; Xu, J. Effects of Ambient Fine Particles PM2.5 on Human HaCaT Cells. Int. J. Environ. Res. Public Health 2017, 14. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, K.; Shirakawa, S.; Kobayashi, N.; Aoshima, H.; Oshima, T. Facile and scalable synthesis of a highly hydroxylated water-soluble fullerenol as a single nanoparticle. Nano Res. 2011, 4, 204–215. [Google Scholar] [CrossRef]
- TSAI, M.-C.; CHEN, Y.H.; CHIANG, L.Y. Polyhydroxylated C60, Fullerenol, a Novel Free-radical Trapper, Prevented Hydrogen Peroxide- and Cumene Hydroperoxide-elicited Changes in Rat Hippocampus In-vitro. J. Pharm. Pharmacol. 1997, 49, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.-S.; Chen, W.-J.; Chiang, L.-Y. Free Radical Scavenging Activity of Fullerenol on the Ischemia-reperfusion Intestine in Dogs. World J. Surg. 2000, 24, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.S.; Chen, Y.; Chen, W.J.; Chang, K.J.; Chiang, L.Y. Free radical scavenging activity of fullerenol on grafts after small bowel transplantation in dogs. Transplant. Proc. 2000, 32, 1272–1274. [Google Scholar] [CrossRef]
- Beelen, R.; Raaschou-Nielsen, O.; Stafoggia, M.; Andersen, Z.J.; Weinmayr, G.; Hoffmann, B.; Wolf, K.; Samoli, E.; Fischer, P.; Nieuwenhuijsen, M.; et al. Effects of long-term exposure to air pollution on natural-cause mortality: An analysis of 22 European cohorts within the multicentre ESCAPE project. Lancet (Lond. Engl.) 2014, 383, 785–795. [Google Scholar] [CrossRef]
- Shah, A.S.; Langrish, J.P.; Nair, H.; McAllister, D.A.; Hunter, A.L.; Donaldson, K.; Newby, D.E.; Mills, N.L. Global association of air pollution and heart failure: A systematic review and meta-analysis. Lancet (Lond. Engl.) 2013, 382, 1039–1048. [Google Scholar] [CrossRef]
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [CrossRef]
- Brandes, R.P.; Weissmann, N.; Schroder, K. Nox family NADPH oxidases: Molecular mechanisms of activation. Free Radic. Biol. Med. 2014, 76, 208–226. [Google Scholar] [CrossRef]
- Day, B.J. Antioxidant therapeutics: Pandora’s box. Free Radic. Biol. Med. 2014, 66, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Brocker, C.; Koppaka, V.; Chen, Y.; Jackson, B.C.; Matsumoto, A.; Thompson, D.C.; Vasiliou, V. Aldehyde dehydrogenases in cellular responses to oxidative/electrophilic stress. Free Radic. Biol. Med. 2013, 56, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Devasagayam, T.P.; Tilak, J.C.; Boloor, K.K.; Sane, K.S.; Ghaskadbi, S.S.; Lele, R.D. Free radicals and antioxidants in human health: Current status and future prospects. J. Assoc. Phys. India 2004, 52, 794–804. [Google Scholar]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Farmer, E.E.; Mueller, M.J. ROS-mediated lipid peroxidation and RES-activated signaling. Annu. Rev. Plant. Biol. 2013, 64, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Zhao, Z.; Goto, S.; Koltai, E. Age-associated neurodegeneration and oxidative damage to lipids, proteins and DNA. Mol. Asp. Med. 2011, 32, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, S.; Ahn, D.U. Protein oxidation: Basic principles and implications for meat quality. Crit. Rev. Food Sci. Nutr. 2013, 53, 1191–1201. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Whyand, T.; Hurst, J.R.; Beckles, M.; Caplin, M.E. Pollution and respiratory disease: Can diet or supplements help? A review. Respir. Res. 2018, 19, 79. [Google Scholar] [CrossRef]
- Baati, T.; Bourasset, F.; Gharbi, N.; Njim, L.; Abderrabba, M.; Kerkeni, A.; Szwarc, H.; Moussa, F. The prolongation of the lifespan of rats by repeated oral administration of [60] fullerene. Biomaterials 2012, 33, 4936–4946. [Google Scholar] [CrossRef]
- Galvan, Y.P.; Alperovich, I.; Zolotukhin, P.; Prazdnova, E.; Mazanko, M.; Belanova, A.; Chistyakov, V. Fullerenes as Anti-Aging Antioxidants. Curr. Aging Sci. 2017, 10, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed]
- Schoop, V.M.; Mirancea, N.; Fusenig, N.E. Epidermal organization and differentiation of HaCaT keratinocytes in organotypic coculture with human dermal fibroblasts. J. Investig. Derm. 1999, 112, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Cargnello, M.; Roux, P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiol. Mol. Biol. Rev. 2011, 75, 50–83. [Google Scholar] [CrossRef] [PubMed]
- Busca, R.; Pouyssegur, J.; Lenormand, P. ERK1 and ERK2 Map Kinases: Specific Roles or Functional Redundancy? Front. Cell Dev. Biol. 2016, 4, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bode, A.M.; Dong, Z. The functional contrariety of JNK. Mol. Carcinog. 2007, 46, 591–598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, H.; Harris, R.; Coloff, J.L.; Jin, J.Y.; Leshin, B.; Miliani de Marval, P.; Tao, S.; Rathmell, J.C.; Hall, R.P.; Zhang, J.Y. The c-Jun NH2-terminal kinase 2 plays a dominant role in human epidermal neoplasia. Cancer Res. 2010, 70, 3080–3088. [Google Scholar] [CrossRef] [PubMed]
- Sabapathy, K.; Wagner, E.F. JNK2: A negative regulator of cellular proliferation. Cell Cycle 2004, 3, 1520–1523. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, X.; Han, J.; Fan, Z.; Sadia, S.; Zhang, R.; Guo, Y.; Jiang, Y.; Wu, Y. AKT and its related molecular feature in aged mice skin. PLoS ONE 2017, 12, e0178969. [Google Scholar] [CrossRef] [PubMed]
- Castilho, R.M.; Squarize, C.H.; Gutkind, J.S. Exploiting PI3K/mTOR signaling to accelerate epithelial wound healing. Oral Dis. 2013, 19, 551–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.L.; Liu, C.S.; Zhao, R.; Wang, L.L.; Li, S.S.; Liu, M.; Zhang, M.; Jiang, S.K.; Tian, Z.L.; Wang, M.; et al. Effects of PI3K/Akt Pathway in Wound Healing Process of Mice Skin. Fa Yi Xue Za Zhi 2016, 32, 7–12. [Google Scholar] [PubMed]
- Di-Poi, N.; Ng, C.Y.; Tan, N.S.; Yang, Z.; Hemmings, B.A.; Desvergne, B.; Michalik, L.; Wahli, W. Epithelium-mesenchyme interactions control the activity of peroxisome proliferator-activated receptor beta/delta during hair follicle development. Mol. Cell Biol. 2005, 25, 1696–1712. [Google Scholar] [CrossRef] [PubMed]
- Paine, A.; Eiz-Vesper, B.; Blasczyk, R.; Immenschuh, S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharm. 2010, 80, 1895–1903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, R.L.; Yang, C.C.; Lee, I.T.; Lin, C.C.; Chi, P.L.; Hsiao, L.D.; Yang, C.M. Lipopolysaccharide induces ICAM-1 expression via a c-Src/NADPH oxidase/ROS-dependent NF-kappaB pathway in human pulmonary alveolar epithelial cells. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L639–L657. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Lin, W.N.; Cho, R.L.; Wang, C.Y.; Hsiao, L.D.; Yang, C.M. TNF-alpha-Induced cPLA2 Expression via NADPH Oxidase/Reactive Oxygen Species-Dependent NF-kappaB Cascade on Human Pulmonary Alveolar Epithelial Cells. Front. Pharm. 2016, 7, 447. [Google Scholar] [CrossRef]
- Chuang, D.Y.; Simonyi, A.; Kotzbauer, P.T.; Gu, Z.; Sun, G.Y. Cytosolic phospholipase A2 plays a crucial role in ROS/NO signaling during microglial activation through the lipoxygenase pathway. J. Neuroinflamm. 2015, 12, 199. [Google Scholar] [CrossRef] [PubMed]
- Vile, G.F.; Basu-Modak, S.; Waltner, C.; Tyrrell, R.M. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc. Natl. Acad. Sci. USA 1994, 91, 2607–2610. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.L.; Ho, Y.C.; Lin, C.Y.; Yet, S.F. Heme oxygenase-1 in inflammation and cardiovascular disease. Am. J. Cardiovasc. Dis. 2011, 1, 150–158. [Google Scholar]
- Turkseven, S.; Kruger, A.; Mingone, C.J.; Kaminski, P.; Inaba, M.; Rodella, L.F.; Ikehara, S.; Wolin, M.S.; Abraham, N.G. Antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H701–H707. [Google Scholar] [CrossRef]
- Muller, W.A. Mechanisms of leukocyte transendothelial migration. Annu. Rev. Pathol. 2011, 6, 323–344. [Google Scholar] [CrossRef]
- Viac, J.; Schmitt, D.; Claudy, A. Adhesion molecules and inflammatory dermatoses. Allerg. Immunol. (Paris) 1994, 26, 274–277. [Google Scholar] [PubMed]
- Singer, K.H.; Tuck, D.T.; Sampson, H.A.; Hall, R.P. Epidermal keratinocytes express the adhesion molecule intercellular adhesion molecule-1 in inflammatory dermatoses. J. Investig. Derm. 1989, 92, 746–750. [Google Scholar] [CrossRef] [Green Version]
- Murakami, M.; Kuwata, H.; Amakasu, Y.; Shimbara, S.; Nakatani, Y.; Atsumi, G.; Kudo, I. Prostaglandin E2 amplifies cytosolic phospholipase A2- and cyclooxygenase-2-dependent delayed prostaglandin E2 generation in mouse osteoblastic cells. Enhancement by secretory phospholipase A2. J. Biol. Chem. 1997, 272, 19891–19897. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.T.; Lee, C.W.; Tung, W.H.; Wang, S.W.; Lin, C.C.; Shu, J.C.; Yang, C.M. Cooperation of TLR2 with MyD88, PI3K, and Rac1 in lipoteichoic acid-induced cPLA2/COX-2-dependent airway inflammatory responses. Am. J. Pathol. 2010, 176, 1671–1684. [Google Scholar] [CrossRef] [PubMed]
- Mruwat, R.; Cohen, Y.; Yedgar, S. Phospholipase A(2) inhibition as potential therapy for inflammatory skin diseases. Immunotherapy 2013, 5, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Soubhye, J.; van Antwerpen, P.; Dufrasne, F. Targeting Cytosolic Phospholipase A2alpha for Novel Anti-Inflammatory Agents. Curr. Med. Chem. 2018, 25, 2418–2447. [Google Scholar] [CrossRef] [PubMed]
- Sandilands, A.; Sutherland, C.; Irvine, A.D.; McLean, W.H. Filaggrin in the frontline: Role in skin barrier function and disease. J. Cell Sci. 2009, 122, 1285–1294. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.H.; Choi, D.; Chun, Y.J.; Noh, M. Keratinocyte-derived IL-24 plays a role in the positive feedback regulation of epidermal inflammation in response to environmental and endogenous toxic stressors. Toxicol. Appl. Pharmacol. 2014, 280, 199–206. [Google Scholar] [CrossRef] [PubMed]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-W.; Chi, M.-C.; Peng, K.-T.; Chiang, Y.-C.; Hsu, L.-F.; Yan, Y.-L.; Li, H.-Y.; Chen, M.-C.; Lee, I.-T.; Lai, C.-H. Water-Soluble Fullerenol C60(OH)36 toward Effective Anti-Air Pollution Induced by Urban Particulate Matter in HaCaT Cell. Int. J. Mol. Sci. 2019, 20, 4259. https://doi.org/10.3390/ijms20174259
Lee C-W, Chi M-C, Peng K-T, Chiang Y-C, Hsu L-F, Yan Y-L, Li H-Y, Chen M-C, Lee I-T, Lai C-H. Water-Soluble Fullerenol C60(OH)36 toward Effective Anti-Air Pollution Induced by Urban Particulate Matter in HaCaT Cell. International Journal of Molecular Sciences. 2019; 20(17):4259. https://doi.org/10.3390/ijms20174259
Chicago/Turabian StyleLee, Chiang-Wen, Miao-Ching Chi, Kuo-Ti Peng, Yao-Chang Chiang, Lee-Fen Hsu, Yi-Ling Yan, Hsing-Yen Li, Ming-Chun Chen, I-Ta Lee, and Chian-Hui Lai. 2019. "Water-Soluble Fullerenol C60(OH)36 toward Effective Anti-Air Pollution Induced by Urban Particulate Matter in HaCaT Cell" International Journal of Molecular Sciences 20, no. 17: 4259. https://doi.org/10.3390/ijms20174259
APA StyleLee, C.-W., Chi, M.-C., Peng, K.-T., Chiang, Y.-C., Hsu, L.-F., Yan, Y.-L., Li, H.-Y., Chen, M.-C., Lee, I.-T., & Lai, C.-H. (2019). Water-Soluble Fullerenol C60(OH)36 toward Effective Anti-Air Pollution Induced by Urban Particulate Matter in HaCaT Cell. International Journal of Molecular Sciences, 20(17), 4259. https://doi.org/10.3390/ijms20174259





