Omega-3 Fatty Acid-Derived Resolvin D2 Regulates Human Placental Vascular Smooth Muscle and Extravillous Trophoblast Activities
Abstract
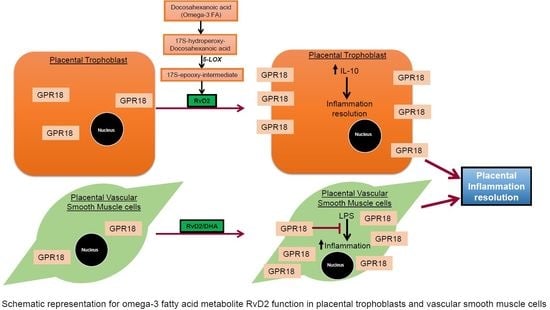
:1. Introduction
2. Results
2.1. Participant Characteristics
2.2. GPR18 Expression and Localization in Human Placental Tissue
2.3. Confirmation of GPR18 Expression at Transcript Level in Placental Cells
2.4. Effects of RvD2 on Placental Trophoblast GPR18 Expression and Function
2.5. Effects of RvD2 on Human Umbilical Artery Smooth Muscle Cell Inflammatory Response
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Human Placental Tissue Collection
4.3. Placental Tissue Processing and Staining for GPR18
4.4. Tissue Culture and Cell Treatments
4.5. Human IL-6 ELISA
4.6. Western Blotting
4.7. Real-Time PCR
4.8. Immunofluorescent Staining
4.9. Collagen Gel Contraction Assay
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| RvD2 | Resolvin D2 |
| RvD1 | Resolvin D1 |
| SPM | Specialized pro-resolving lipid mediator |
| GPR18 | G-protein coupled receptor 18 |
| DHA | Docosahexaenoic acid |
| EPA | Eicosapentaenoic acid |
| DPA | Docosapentaenoic acid |
| EVT | Extravillous trophoblast |
| NICU | Neonatal intensive care unit |
| HUASMC | Human umbilical artery smooth muscle cells |
| IL | Interleukin |
| TNFα | Tumor necrosis factor alpha |
| 17-HDHA | 17-hydroxy-docosahexaenoic acid |
| 18-HEPE | 18-hydroxy-eicosapentaenoic acid |
| αSMA | Alpha smooth muscle actin |
| LPS | Lipopolysaccharide |
| DMEM | Dulbecco’s modified Eagle’s medium |
| FBS | Fetal bovine serum |
References
- Emmett, P.M.; Jones, L.R.; Golding, J. Pregnancy diet and associated outcomes in the Avon Longitudinal Study of Parents and Children. Nutr. Rev. 2015, 73, 154–174. [Google Scholar] [CrossRef] [PubMed]
- Miyata, J.; Arita, M. Role of omega-3 fatty acids and their metabolites in asthma and allergic diseases. Allergol. Int. 2015, 64, 27–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordgren, T.M.; Lyden, E.; Anderson-Berry, A.; Hanson, C. Omega-3 Fatty Acid Intake of Pregnant Women and Women of Childbearing Age in the United States: Potential for Deficiency? Nutrients 2017, 9, 197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, M.; Hein, N.; Hanson, C.; Smith, L.M.; Anderson-Berry, A.; Richter, C.K.; Stessy Bisselou, K.; Kusi Appiah, A.; Kris-Etherton, P.; Skulas-Ray, A.C.; et al. Omega-3 Fatty Acid Intake by Age, Gender, and Pregnancy Status in the United States: National Health and Nutrition Examination Survey 2003–2014. Nutrients 2019, 11, 177. [Google Scholar] [CrossRef]
- Zhang, Z.; Fulgoni, V.L.; Kris-Etherton, P.M.; Mitmesser, S.H. Dietary intakes of EPA and DHA omega-3 fatty acids among US childbearing-age and pregnant women: an analysis of NHANES 2001–2014. Nutrients 2018, 10, 416. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.K.; Bisselou, K.S.; Nordgren, T.M.; Smith, L.; Appiah, A.K.; Hein, N.; Anderson-Berry, A.; Kris-Etherton, P.; Hanson, C.; Skulas-Ray, A.C. n-3 docosapentaenoic acid intake and relationship with plasma long-chain n-3 fatty acid concentrations in the United States: NHANES 2003–2014. Lipids 2019, 54, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Basil, M.C.; Levy, B.D. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N.; Krishnamoorthy, S.; Recchiuti, A.; Chiang, N. Novel anti-inflammatory--pro-resolving mediators and their receptors. Curr. Top. Med. Chem. 2011, 11, 629–647. [Google Scholar] [CrossRef]
- Chiang, N.; Dalli, J.; Colas, R.A.; Serhan, C.N. Identification of resolvin D2 receptor mediating resolution of infections and organ protection. J. Exp. Med. 2015, 212, 1203–1217. [Google Scholar] [CrossRef] [Green Version]
- Chiang, N.; de la Rosa, X.; Libreros, S.; Serhan, C.N. Novel Resolvin D2 receptor axis in infectious inflammation. J. Immunol. 2017, 198, 842–851. [Google Scholar] [CrossRef]
- Weiss, G.A.; Troxler, H.; Klinke, G.; Rogler, D.; Braegger, C.; Hersberger, M. High levels of anti-inflammatory and pro-resolving lipid mediators lipoxins and resolvins and declining docosahexaenoic acid levels in human milk during the first month of lactation. Lipids Health Dis. 2013, 12, 89. [Google Scholar] [CrossRef] [PubMed]
- Arnardottir, H.; Orr, S.K.; Dalli, J.; Serhan, C.N. Human milk proresolving mediators stimulate resolution of acute inflammation. Mucosal Immunol. 2016, 9, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Mas, E.; Croft, K.D.; Zahra, P.; Barden, A.; Mori, T.A. Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin. Chem. 2012, 58, 1476–1484. [Google Scholar] [CrossRef] [PubMed]
- Mozurkewich, E.L.; Greenwood, M.; Clinton, C.; Berman, D.; Romero, V.; Djuric, Z.; Qualls, C.; Gronert, K. Pathway Markers for pro-resolving lipid mediators in maternal and umbilical cord blood: a secondary analysis of the mothers, omega-3, and mental health study. Front. Pharmacol. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Keelan, J.A.; Mas, E.; D’Vaz, N.; Dunstan, J.A.; Li, S.; Barden, A.E.; Mark, P.J.; Waddell, B.J.; Prescott, S.L.; Mori, T.A. Effects of maternal n-3 fatty acid supplementation on placental cytokines, pro-resolving lipid mediators and their precursors. Reproduction 2015, 149, 171–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordgren, T.M.; Anderson Berry, A.; Van Ormer, M.; Zoucha, S.; Elliott, E.; Johnson, R.; McGinn, E.; Cave, C.; Rilett, K.; Weishaar, K.; et al. Omega-3 fatty acid supplementation, pro-resolving mediators, and clinical outcomes in maternal-infant Pairs. Nutrients 2019, 11, 98. [Google Scholar] [CrossRef]
- Strunk, T.; Inder, T.; Wang, X.; Burgner, D.; Mallard, C.; Levy, O. Infection-induced inflammation and cerebral injury in preterm infants. Lancet.Infect. Dis. 2014, 14, 751–762. [Google Scholar] [CrossRef] [Green Version]
- Dessardo, N.S.; Dessardo, S.; Mustac, E.; Banac, S.; Petrovic, O.; Peter, B. Chronic lung disease of prematurity and early childhood wheezing: Is foetal inflammatory response syndrome to blame? Early Hum. Dev. 2014, 90, 493–499. [Google Scholar] [CrossRef]
- See, V.H.L.; Mas, E.; Prescott, S.L.; Beilin, L.J.; Burrows, S.; Barden, A.E.; Huang, R.C.; Mori, T.A. Effects of prenatal n-3 fatty acid supplementation on offspring resolvins at birth and 12 years of age: A double-blind, randomised controlled clinical trial. Br. J. Nutr. 2017, 118, 971–980. [Google Scholar] [CrossRef]
- Yamashita, A.; Kawana, K.; Tomio, K.; Taguchi, A.; Isobe, Y.; Iwamoto, R.; Masuda, K.; Furuya, H.; Nagamatsu, T.; Nagasaka, K.; et al. Increased tissue levels of omega-3 polyunsaturated fatty acids prevents pathological preterm birth. Sci. Rep. 2013, 3, 3113. [Google Scholar] [CrossRef]
- Germain, A.M.; Carvajal, J.; Sanchez, M.; Valenzuela, G.J.; Tsunekawa, H.; Chuaqui, B. Preterm labor: Placental pathology and clinical correlation. Obs. Gynecol. 1999, 94, 284–289. [Google Scholar] [CrossRef]
- Kelly, R.; Holzman, C.; Senagore, P.; Wang, J.; Tian, Y.; Rahbar, M.H.; Chung, H. Placental vascular pathology findings and pathways to preterm delivery. Am. J. Epidemiol. 2009, 170, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Catov, J.M.; Scifres, C.M.; Caritis, S.N.; Bertolet, M.; Larkin, J.; Parks, W.T. Neonatal outcomes following preterm birth classified according to placental features. Am. J. Obs. Gynecol. 2017, 216. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Kingdom, J.; Burton, G.J.; Cindrova-Davies, T. Placental Stem Villus Arterial Remodeling Associated with Reduced Hydrogen Sulfide Synthesis Contributes to Human Fetal Growth Restriction. Am. J. Pathol. 2017, 187, 908–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lagana, A.S.; Giordano, D.; Loddo, S.; Zoccali, G.; Vitale, S.G.; Santamaria, A.; Buemi, M.; D’Anna, R. Decreased Endothelial Progenitor Cells (EPCs) and increased Natural Killer (NK) cells in peripheral blood as possible early markers of preeclampsia: A case-control analysis. Arch. Gynecol. Obs. 2017, 295, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Chiofalo, B.; Lagana, A.S.; Vaiarelli, A.; La Rosa, V.L.; Rossetti, D.; Palmara, V.; Valenti, G.; Rapisarda, A.M.C.; Granese, R.; Sapia, F.; et al. Do miRNAs Play a Role in Fetal Growth Restriction? A Fresh Look to a Busy Corner. Biomed. Res. Int. 2017, 2017, 6073167. [Google Scholar] [CrossRef] [PubMed]
- Lagana, A.S.; Vitale, S.G.; Sapia, F.; Valenti, G.; Corrado, F.; Padula, F.; Rapisarda, A.M.C.; D’Anna, R. miRNA expression for early diagnosis of preeclampsia onset: Hope or hype? J. Matern Fetal Neonatal Med. 2018, 31, 817–821. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.Y.; Liu, Z.H.; Zhu, Q.; Wen, S.; Yang, C.X.; Fu, Z.J.; Sun, T. Resolvin D2 relieving radicular pain is associated with regulation of inflammatory mediators, Akt/GSK-3beta signal pathway and GPR18. Neurochem. Res. 2018, 43, 2384–2392. [Google Scholar] [CrossRef]
- Chen, C.Y.; Liu, C.C.; Chen, C.P. Omega-3 polyunsaturated fatty acids reduce preterm labor by inhibiting trophoblast cathepsin S and inflammasome activation. Clin. Sci. 2018, 132, 2221–2239. [Google Scholar] [CrossRef]
- Anand, S.; Young, S.; Esplin, M.S.; Peaden, B.; Tolley, H.D.; Porter, T.F.; Varner, M.W.; D’Alton, M.E.; Jackson, B.J.; Graves, S.W. Detection and confirmation of serum lipid biomarkers for preeclampsia using direct infusion mass spectrometry. J. Lipid Res. 2016, 57, 687–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldenberg, R.L.; Hauth, J.C.; Andrews, W.W. Intrauterine infection and preterm delivery. N. Engl. J. Med. 2000, 342, 1500–1507. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, L.F.; Chaiworapongsa, T.; Romero, R. Intrauterine infection and prematurity. Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Anton, L.; Brown, A.G.; Parry, S.; Elovitz, M.A. Lipopolysaccharide induces cytokine production and decreases extravillous trophoblast invasion through a mitogen-activated protein kinase-mediated pathway: Possible mechanisms of first trimester placental dysfunction. Hum. Reprod. 2012, 27, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Kingdom, J.; Huppertz, B.; Seaward, G.; Kaufmann, P. Development of the placental villous tree and its consequences for fetal growth. Eur. J. Obs. Gynecol. Reprod. Biol. 2000, 92, 35–43. [Google Scholar] [CrossRef]
- Byrne, T.J. A “cure” for preeclampsia: Improving neonatal outcomes by overcoming excess fetal placental vascular resistance. Med. Hypotheses 2015, 85, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Makikallio, K.; Kaukola, T.; Tuimala, J.; Kingsmore, S.F.; Hallman, M.; Ojaniemi, M. Umbilical artery chemokine CCL16 is associated with preterm preeclampsia and fetal growth restriction. Cytokine 2012, 60, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Pascoal, L.B.; Bombassaro, B.; Ramalho, A.F.; Coope, A.; Moura, R.F.; Correa-da-Silva, F.; Ignacio-Souza, L.; Razolli, D.; de Oliveira, D.; Catharino, R.; et al. Resolvin RvD2 reduces hypothalamic inflammation and rescues mice from diet-induced obesity. J. Neuroinflammation 2017, 14, 5. [Google Scholar] [CrossRef]
- Elagizi, A.; Lavie, C.J.; Marshall, K.; DiNicolantonio, J.J.; O’Keefe, J.H.; Milani, R.V. Omega-3 Polyunsaturated Fatty Acids and Cardiovascular Health: A Comprehensive Review. Prog. Cardiovasc. Dis. 2018, 61, 76–85. [Google Scholar] [CrossRef] [Green Version]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Arter. Thromb. Vasc. Biol. 2003, 23, e20–e30. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M.; Harris, W.S.; Appel, L.J. Omega-3 fatty acids and cardiovascular disease: New recommendations from the American Heart Association. Arter. Thromb. Vasc. Biol. 2003, 23, 151–152. [Google Scholar] [CrossRef] [PubMed]
- Miyahara, T.; Runge, S.; Chatterjee, A.; Chen, M.; Mottola, G.; Fitzgerald, J.M.; Serhan, C.N.; Conte, M.S. D-series resolvin attenuates vascular smooth muscle cell activation and neointimal hyperplasia following vascular injury. Faseb. J. 2013, 27, 2220–2232. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Gong, Y.; Zhang, R.; Piao, L.; Li, X.; Liu, Q.; Yan, S.; Shen, Y.; Guo, S.; Zhu, M.; et al. Resolvin E1 attenuates injury-induced vascular neointimal formation by inhibition of inflammatory responses and vascular smooth muscle cell migration. Faseb J. 2018, 32, 5413–5425. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, S.K.; Stringham, B.A.; Mohr, A.M.; Wehrkamp, C.J.; Lu, S.; Phillippi, M.A.; Harrison-Findik, D.; Mott, J.L. FoxO3 increases miR-34a to cause palmitate-induced cholangiocyte lipoapoptosis. J. Lipid Res. 2017, 58, 866–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carr, H.S.; Morris, C.A.; Menon, S.; Song, E.H.; Frost, J.A. Rac1 controls the subcellular localization of the Rho guanine nucleotide exchange factor Net1A to regulate focal adhesion formation and cell spreading. Mol. Cell Biol. 2013, 33, 622–634. [Google Scholar] [CrossRef]
- Ulu, A.; Oh, W.; Zuo, Y.; Frost, J.A. Stress-activated MAPKs and CRM1 regulate the subcellular localization of Net1A to control cell motility and invasion. J. Cell Sci. 2018, 131. [Google Scholar] [CrossRef]
- Song, E.H.; Oh, W.; Ulu, A.; Carr, H.S.; Zuo, Y.; Frost, J.A. Acetylation of the RhoA GEF Net1A controls its subcellular localization and activity. J. Cell Sci. 2015, 128, 913–922. [Google Scholar] [CrossRef] [Green Version]
- Benoit, C.; Gu, Y.; Xhang, Y.; Alexander, J.S.; Wang, Y. Contractility of Placental Vascular Smooth Muscle Cells in Response to Stimuli Produced by the Placenta: Roles of ACE vs. Non-ACE and AT1 vs. AT2 in Placental Vessel Cells. Placenta 2008, 29, 503–509. [Google Scholar] [CrossRef] [Green Version]









| Maternal/Infant Characteristics | N | Mean (SD) |
|---|---|---|
| Maternal Age, years | 26 | 27.65 (5.16) |
| Maternal pre-pregnancy BMI, m/kg2 (self-report) | 17 | 29.50 (7.81) |
| Maternal DHA intake, grams | 24 | 0.15 (0.14) |
| Maternal total omega-3 fatty acid intake, grams | 24 | 2.18 (0.90) |
| Birth CGA, weeks | 26 | 39.04 (2.46) |
| Infant Birthweight, grams | 26 | 3357.31 (668.45) |
| Infant Birth Head Circumference, cm | 25 | 33.86 (2.72) |
| Infant Birth Length, cm | 25 | 49.12 (3.80) |
| N | % | |
| Race: | ||
| White | 14 | 53.85 |
| African American | 7 | 26.92 |
| Hispanic/Latino | 2 | 7.69 |
| Asian/Pacific Islander | 1 | 3.85 |
| Native American | 0 | 0 |
| Other | 2 | 7.69 |
| Infant Sex (M/F) | 17 male 9 female | 65.38 34.62 |
| Maternal n-3 Supplement Use (Y/N) | 8 | 30.77 |
| Infant NICU Admission at Birth (Y/N) | 8 | 30.77 |
| Preterm Delivery (<37 weeks gestation) (Y/N) | 3 | 11.54 |
| Suspected or confirmed chorioamnionitis (Y/N) | 1 | 3.85 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ulu, A.; Sahoo, P.K.; Yuil-Valdes, A.G.; Mukherjee, M.; Van Ormer, M.; Muthuraj, P.G.; Thompson, M.; Anderson Berry, A.; Hanson, C.K.; Natarajan, S.K.; et al. Omega-3 Fatty Acid-Derived Resolvin D2 Regulates Human Placental Vascular Smooth Muscle and Extravillous Trophoblast Activities. Int. J. Mol. Sci. 2019, 20, 4402. https://doi.org/10.3390/ijms20184402
Ulu A, Sahoo PK, Yuil-Valdes AG, Mukherjee M, Van Ormer M, Muthuraj PG, Thompson M, Anderson Berry A, Hanson CK, Natarajan SK, et al. Omega-3 Fatty Acid-Derived Resolvin D2 Regulates Human Placental Vascular Smooth Muscle and Extravillous Trophoblast Activities. International Journal of Molecular Sciences. 2019; 20(18):4402. https://doi.org/10.3390/ijms20184402
Chicago/Turabian StyleUlu, Arzu, Prakash K. Sahoo, Ana G. Yuil-Valdes, Maheswari Mukherjee, Matthew Van Ormer, Philma Glora Muthuraj, Maranda Thompson, Ann Anderson Berry, Corrine K. Hanson, Sathish Kumar Natarajan, and et al. 2019. "Omega-3 Fatty Acid-Derived Resolvin D2 Regulates Human Placental Vascular Smooth Muscle and Extravillous Trophoblast Activities" International Journal of Molecular Sciences 20, no. 18: 4402. https://doi.org/10.3390/ijms20184402