Somatostatin as an Active Substance in the Mammalian Enteric Nervous System
Abstract
:1. Introduction
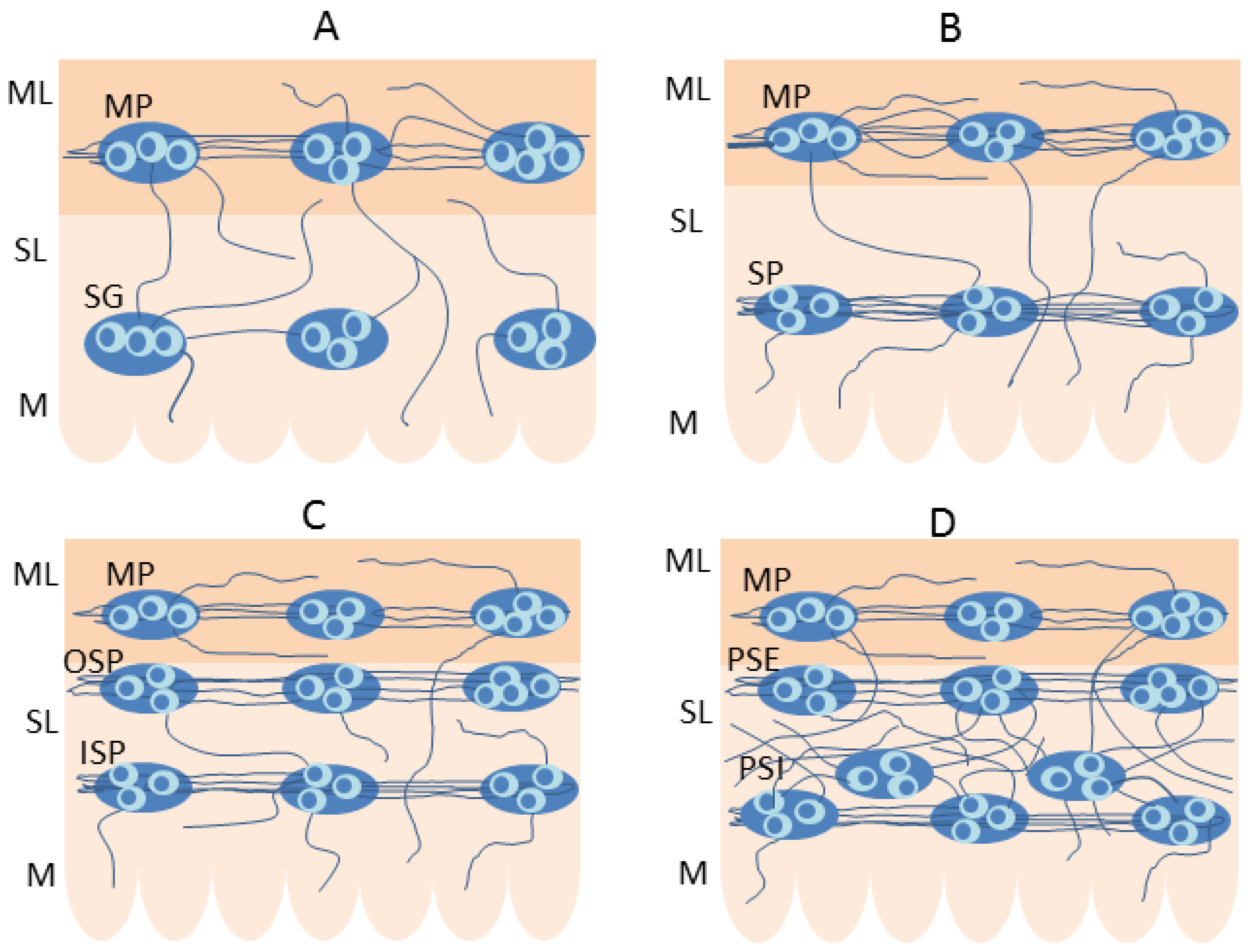
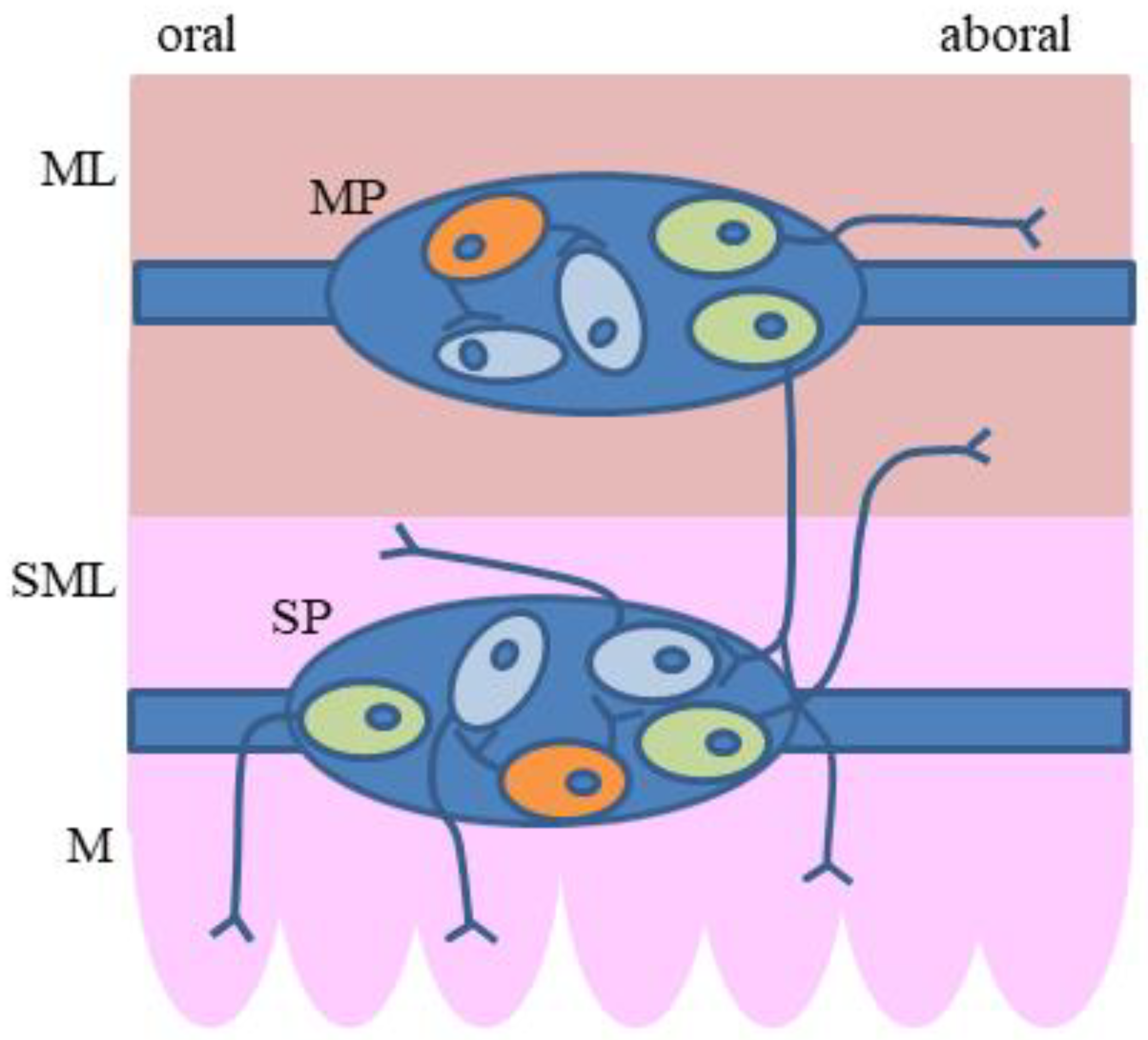
2. Organization of the Enteric Nervous System (ENS)
3. The Presence of Somatostatin (SOM) in the Enteric Neurons in Particular Mammal Species
3.1. Guinea Pig
3.2. Domestic Pig
3.3. Human
3.4. Other Mammal Species
4. Distribution of SOM Receptors in the Enteric Nervous System
5. The Plasticity of the Enteric Nervous Structures Containing Somatostatin
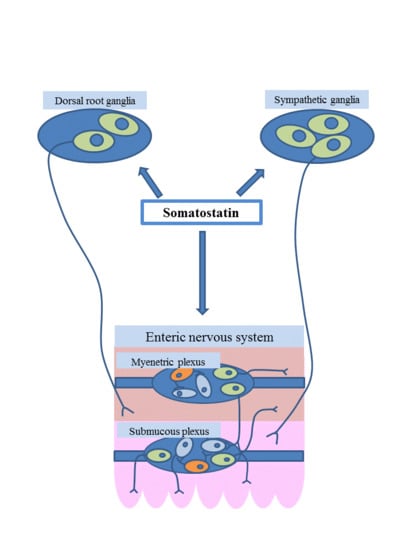
6. SOM in the Extrinsic Innervation of the Gastrointestinal (GI) Tract
7. Functions of Somatostatin in the Enteric Nervous System
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Brazeau, P.; Vale, W.; Burgus, R.; Ling, N.; Butcher, M.; Rivier, J.; Guillemin, R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive growth hormone. Science 1973, 179, 77–79. [Google Scholar] [CrossRef]
- Pradayrol, L.; Jornvall, H.; Mutt, V.; Ribet, A. N-terminally extended somatostatin: The primary structure of somatostatin-28. FEBS Lett. 1980, 1, 55–58. [Google Scholar] [CrossRef]
- Corleto, V.D.; Nasoni, S.; Panzuto, F.; Cassetta, S.; Delle Fave, G. Somatostatin receptor subtypes: Basic pharmacology and tissue distribution. Dig. Liver Dis. 2004, 36 (Suppl. S1), S8–S16. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.C.; Wheatley, T.; Ning, C. Multiple forms of immunoreactive somatostatin: Comparison of distribution in neural and nonneural tissues and portal plasma of the rat. Endocrinology 1981, 109, 1943–1949. [Google Scholar] [CrossRef] [PubMed]
- Shulkes, A. Somatostatin: Physiology and clinical applications. Baillieres Clin. Endocrinol. Metab. 1994, 8, 215–236. [Google Scholar] [CrossRef]
- Penman, E.; Wass, J.A.; Butler, M.G.; Penny, E.S.; Price, J.; Wu, P.; Rees, L.H. Distribution and characterization of immunoreactive somatostatin in human gastrointestinal tract. Regul. Pept. 1983, 7, 53–65. [Google Scholar] [CrossRef]
- Schneider, S.; Wright, C.M.; Heuckeroth, R.O. Unexpected roles for the second brain: Enteric nervous system as master regulator of bowel function. Annu. Rev. Physiol. 2019, 81, 235–259. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: Integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar]
- Makowska, K. Chemically induced inflammation and nerve damage affect the distribution of vasoactive intestinal polypeptide-like immunoreactive (VIP-LI) nervous structures in the descending colon of the domestic pig. Neurogastroenterol. Motil. 2018, 30, e13439. [Google Scholar] [CrossRef]
- Furness, J.B. Types of neurons in the enteric nervous system. J. Auton. Nerv. Syst. 2000, 81, 87–96. [Google Scholar] [CrossRef]
- Makowska, K.; Rytel, L.; Lech, P.; Osowski, A.; Kruminis-Kaszkiel, E.; Gonkowski, S. Cocaine- and amphetamine-regulated transcript (CART) peptide in the enteric nervous system of the porcine esophagus. C. R. Biol. 2018, 341, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Makowska, K.; Obremski, K.; Gonkowski, S. The impact of T-2 toxin on vasoactive intestinal polypeptide-like immunoreactive (VIP-LI) nerve structures in the wall of the porcine stomach and duodenum. Toxins 2018, 10, 138. [Google Scholar] [CrossRef] [PubMed]
- Arciszewski, M.B.; Barabasz, S.; Skobowiat, C.; Maksymowicz, W.; Majewski, M. Immunodetection of cocaine- and amphetamine-regulated transcript in the rumen, reticulum, omasum and abomasum of the sheep. Anat. Histol. Embryol. 2009, 38, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Brehmer, A.; Rupprecht, H.; Neuhuber, W. Two submucosal nerve plexus in human intestines. Histochem. Cell Biol. 2010, 133, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Szymanska, K.; Makowska, K.; Gonkowski, S. The influence of high and low doses of bisphenol A (BPA) on the enteric nervous system of the porcine ileum. Int. J. Mol. Sci. 2018, 19, 917. [Google Scholar] [CrossRef]
- Hu, H.Z.; Gao, N.; Lin, Z.; Gao, C.; Liu, S.; Ren, J.; Xia, Y.; Wood, J.D. Chemical coding and electrophysiology of enteric neurons expressing neurofilament 145 in guinea pig gastrointestinal tract. J. Comp. Neurol. 2002, 442, 189–203. [Google Scholar] [CrossRef]
- Gonkowski, S.; Całka, J. Changes in the somatostatin (SOM)-like immunoreactivity within nervous structures of the porcine descending colon under various pathological factors. Exp. Mol. Pathol. 2010, 88, 416–423. [Google Scholar] [CrossRef]
- Kustermann, A.; Neuhuber, W.; Brehmer, A. Calretinin and somatostatin immunoreactivities label different human submucosal neuron populations. Anat. Rec. 2011, 294, 858–869. [Google Scholar] [CrossRef]
- Keast, J.R.; Furness, J.B.; Costa, M. Distribution of certain peptide-containing nerve fibres and endocrine cells in the gastrointestinal mucosa in five mammalian species. J. Comp. Neurol. 1985, 236, 403–422. [Google Scholar] [CrossRef]
- Pidsudko, Z.; Kaleczyc, J.; Wasowicz, K.; Sienkiewicz, W.; Majewski, M.; Zajac, W.; Lakomy, M. Distribution and chemical coding of intramural neurons in the porcine ileum during proliferative enteropathy. J. Comp. Pathol. 2008, 138, 23–31. [Google Scholar] [CrossRef]
- Wattchow, D.A.; Cass, D.T.; Furness, J.B.; Costa, M.; O’Brien, P.E.; Little, K.E.; Pitkin, J. Abnormalities of peptide-containing nerve fibers in infantile hypertrophic pyloric stenosis. Gastroenterology 1987, 92, 443–448. [Google Scholar] [CrossRef]
- Singaram, C.; Sengupta, A.; Sugarbaker, D.J.; Goyal, R.K. Peptidergic innervation of the human esophageal smooth muscle. Gastroenterology 1991, 101, 1256–1263. [Google Scholar] [CrossRef]
- Murphy, R.; Beardsley, A.M.; Furness, J.B.; Costa, M.; Oliver, J.R. Effects of fixation for immunohistochemistry on tissue concentrations of substance P and somatostatin determined by radioimmunoassay. Regul. Pept. 1982, 4, 67–74. [Google Scholar] [CrossRef]
- Beraldi, E.J.; Soares, A.; Borges, S.C.; de Souza, A.C.; Natali, M.R.; Bazotte, R.B.; Buttow, N.C. High-fat diet promotes neuronal loss in the myenteric plexus of the large intestine in mice. Dig. Dis. Sci. 2015, 60, 841–849. [Google Scholar] [CrossRef] [PubMed]
- Heiss, C.N.; Olofsson, L.E. The role of the gut microbiota in development, function and disorders of the central nervous system and the enteric nervous system. J. Neuroendocrinol. 2019, 31, e12684. [Google Scholar] [CrossRef]
- Costa, M.; Patel, Y.; Furness, J.B.; Arimura, A. Evidence that some intrinsic neurons of the intestine contain somatostatin. Neurosci. Lett. 1977, 6, 215–222. [Google Scholar] [CrossRef]
- Costa, M.; Furness, J.B.; Smith, I.J.; Davies, B.; Oliver, J. An immunohistochemical study of the projections of somatostatin-containing neurons in the guinea-pig intestine. Neuroscience 1980, 5, 841–852. [Google Scholar] [CrossRef]
- Furness, J.B.; Costa, M. Types of nerves in the enteric nervous system. Neuroscience 1980, 5, 1–20. [Google Scholar] [CrossRef]
- Liu, S.; Gao, N.; Hu, H.Z.; Wang, X.; Wang, G.D.; Fang, X.; Gao, X.; Xia, Y.; Wood, J.D. Distribution and chemical coding of corticotropin-releasing factor-immunoreactive neurons in the guinea pig enteric nervous system. J. Comp. Neurol. 2006, 494, 63–74. [Google Scholar] [CrossRef]
- Portbury, A.L.; Pompolo, S.; Furness, J.B.; Stebbing, M.J.; Kunze, W.A.; Bornstein, J.C.; Hughes, S. Cholinergic, somatostatin-immunoreactive interneurons in the guinea pig intestine: Morphology, ultrastructure, connections and projections. J. Anat. 1995, 187, 303–321. [Google Scholar]
- Poole, D.P.; Castelucci, P.; Robbins, H.L.; Chiocchetti, R.; Furness, J.B. The distribution of P2X3 purine receptor subunits in the guinea pig enteric nervous system. Auton. Neurosci. 2002, 101, 39–47. [Google Scholar] [CrossRef]
- Furness, J.B.; Costa, M.; Gibbins, I.L.; Llewellyn-Smith, I.J.; Oliver, J.R. Neurochemically similar myenteric and submucous neurons directly traced to the mucosa of the small intestine. Cell Tissue Res. 1985, 241, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B.; Bornstein, J.C.; Murphy, R.; Pompolo, S. Roles of peptides in transmission in the enteric nervous system. Trends Neurosci. 1992, 15, 66–71. [Google Scholar] [CrossRef]
- Bornstein, J.C.; Furness, J.B. Correlated electrophysiological and histochemical studies of submucous neurons and their contribution to understanding enteric neural circuits. J. Auton. Nerv. Syst. 1988, 25, 1–13. [Google Scholar] [CrossRef]
- Probert, L.; De Mey, J.; Polak, J.M. Ultrastructural localization of four different neuropeptides within separate populations of p-type nerves in the guinea pig colon. Gastroenterology 1983, 85, 1094–1104. [Google Scholar] [PubMed]
- Leander, S.; Håkanson, R.; Sundler, F. Nerves containing substance P, vasoactive intestinal polypeptide, enkephalin or somatostatin in the guinea-pig taenia coli. Distribution, ultrastructure and possible functions. Cell Tissue Res. 1981, 215, 21–39. [Google Scholar] [CrossRef] [PubMed]
- Messenger, J.P.; Furness, J.B. Projections of chemically-specified neurons in the guinea-pig colon. Arch. Histol. Cytol. 1990, 53, 467–495. [Google Scholar] [CrossRef] [PubMed]
- Schemann, M.; Schaaf, C.; Mäder, M. Neurochemical coding of enteric neurons in the guinea pig stomach. J. Comp. Neurol. 1995, 353, 161–178. [Google Scholar] [CrossRef]
- Reiche, D.; Huber, K.; Hoppe, S.; Schemann, M. Neurochemically distinct myenteric neurone populations containing calbindin have specific distribution patterns around the circumference of the gastric corpus. Cell Tissue Res. 2001, 303, 319–328. [Google Scholar]
- Pfannkuche, H.; Reiche, D.; Firzlaff, U.; Sann, H.; Schemann, M. Enkephalin-immunoreactive subpopulations in the myenteric plexus of the guinea-pig fundus project primarily to the muscle and not to the mucosa. Cell Tissue Res. 1998, 294, 45–55. [Google Scholar] [CrossRef]
- Reiche, D.; Schemann, M. Mucosa of the guinea pig gastric corpus is innervated by myenteric neurones with specific neurochemical coding and projection preferences. J. Comp. Neurol. 1999, 410, 489–502. [Google Scholar] [CrossRef]
- Brown, D.R.; Timmermans, J.P. Lessons from the porcine enteric nervous system. Neurogastroenterol. Motil. 2004, 16 (Suppl. S1), 50–54. [Google Scholar] [CrossRef]
- Verma, N.; Rettenmeier, A.W.; Schmitz-Spanke, S. Recent advances in the use of Sus scrofa (pig) as a model system for proteomic studies. Proteomics 2011, 11, 776–793. [Google Scholar] [CrossRef] [PubMed]
- Brehmer, A.; Schrödl, F.; Neuhuber, W. Correlated morphological and chemical phenotyping in myenteric type V neurons of porcine ileum. J. Comp. Neurol. 2002, 453, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Timmermans, J.P.; Hens, J.; Adriaensen, D. Outer submucous plexus. An intrinsic nerve network involved in both secretory and motility processes in the intestine of large mammals and humans. Anat. Rec. 2001, 262, 71–78. [Google Scholar] [CrossRef]
- Kapp, S.; Schrödl, F.; Neuhuber, W.; Brehmer, A. Chemical coding of submucosal type V neurons in porcine ileum. Cells Tissues Organs 2006, 184, 31–41. [Google Scholar] [CrossRef]
- Gonkowski, S.; Kamińska, B.; Bossowska, A.; Korzon, M.; Landowski, P.; Majewski, M. The influence of experimental Bacteroides fragilis infection on substance P and somatostatin-immunoreactive neural elements in the porcine ascending colon - a preliminary report. Folia Morphol. 2003, 62, 455–457. [Google Scholar]
- Petto, C.; Gäbel, G.; Pfannkuche, H. Architecture and chemical coding of the inner and outer submucous plexus in the colon of piglets. PLoS ONE 2015, 10, e0133350. [Google Scholar] [CrossRef]
- Wasowicz, K.; Winnicka, A.; Kaleczyc, J.; Zalecki, M.; Podlasz, P.; Pidsudko, Z. Neuropeptides and lymphocyte populations in the porcine ileum and ileocecal lymph nodes during postnatal life. PLoS ONE 2018, 13, e0196458. [Google Scholar] [CrossRef]
- Wojtkiewicz, J.; Gonkowski, S.; Równiak, M.; Crayton, R.; Majewski, M.; Jałynski, M. Neurochemical characterization of zinc transporter 3-like immunoreactive (ZnT3(+)) neurons in the intramural ganglia of the porcine duodenum. J. Mol. Neurosci. 2012, 48, 766–776. [Google Scholar] [CrossRef]
- Kaleczyc, J.; Klimczuk, M.; Franke-Radowiecka, A.; Sienkiewicz, W.; Majewski, M.; Łakomy, M. The distribution and chemical coding of intramural neurons supplying the porcine stomach—the study on normal pigs and on animals suffering from swine dysentery. Anat. Histol. Embryol. 2007, 36, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Rytel, L.; Szymanska, K.; Gonkowski, I.; Wojtkiewicz, J. Neurochemical characterization of intramural nerve fibres in the porcine oesophagus. Anat. Histol. Embryol. 2018, 47, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Godlewski, J.; Kaleczyc, J. Somatostatin, substance P and calcitonin gene-related peptide-positive intramural nerve structures of the human large intestine affected by carcinoma. Folia Histochem. Cytobiol. 2010, 48, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Tsuto, T.; Okamura, H.; Fukui, K.; Obata-Tsuto, H.L.; Terubayashi, H.; Yanagihara, J.; Iwai, N.; Majima, S.; Yanaihara, N.; Ibata, Y. Immunohistochemical investigations of gut hormones in the colon of patients with Hirschsprung’s disease. J. Pediatr. Surg. 1985, 20, 266–270. [Google Scholar] [CrossRef]
- Smith, V.C.; Dhatt, N.; Buchan, A.M. The innervation of the human antro-pyloric region: Organization and composition. Can. J. Physiol. Pharmacol. 2001, 79, 905–918. [Google Scholar] [CrossRef] [PubMed]
- Beyer, J.; Jabari, S.; Rau, T.T.; Neuhuber, W.; Brehmer, A. Substance P- and choline acetyltransferase immunoreactivities in somatostatin-containing, human submucosal neurons. Histochem. Cell Biol. 2013, 140, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Zetzmann, K.; Strehl, J.; Geppert, C.; Kuerten, S.; Jabari, S.; Brehmer, A. Calbindin D28k-immunoreactivity in human enteric neurons. Int. J. Mol. Sci. 2018, 19, 194. [Google Scholar] [CrossRef] [PubMed]
- Brehmer, A.; Croner, R.; Dimmler, A.; Papadopoulos, T.; Schrödl, F.; Neuhuber, W. Immunohistochemical characterization of putative primary afferent (sensory) myenteric neurons in human small intestine. Auton. Neurosci. 2004, 112, 49–59. [Google Scholar] [CrossRef] [PubMed]
- De Fontgalland, D.; Wattchow, D.A.; Costa, M.; Brookes, S.J. Immunohistochemical characterization of the innervation of human colonic mesenteric and submucosal blood vessels. Neurogastroenterol. Motil. 2008, 20, 1212–1226. [Google Scholar] [CrossRef]
- Aggestrup, S.; Uddman, R.; Jensen, S.L.; Håkanson, R.; Sundler, F.; Schaffalitzky de Muckadell, O.; Emson, P. Regulatory peptides in lower esophageal sphincter of pig and man. Dig. Dis. Sci. 1986, 31, 1370–1375. [Google Scholar] [CrossRef]
- Uemura, S.; Pompolo, S.; Furness, J.B.; Hardy, K.J. Nitric oxide synthase in neurons of the human gall-bladder and its colocalization with neuropeptides. J. Gastroenterol. Hepatol. 1997, 12, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Buchan, A.M.; Baimbridge, K.G. Distribution and co-localization of calbindin D28k with VIP and neuropeptide Y but not somatostatin, galanin and substance P in the enteric nervous system of the rat. Peptides 1988, 9, 333–338. [Google Scholar] [CrossRef]
- Willard, A.L.; Nishi, R. Neuropeptides mark functionally distinguishable cholinergic enteric neurons. Brain Res. 1987, 422, 163–167. [Google Scholar] [CrossRef]
- Pataky, D.M.; Curtis, S.B.; Buchan, A.M. The co-localization of neuropeptides in the submucosa of the small intestine of normal Wistar and non-diabetic BB rats. Neuroscience 1990, 36, 247–254. [Google Scholar] [CrossRef]
- Heinicke, E.A.; Kiernan, J.A. An immunohistochemical study of the myenteric plexus of the colon in the rat and mouse. J. Anat. 1990, 170, 51–62. [Google Scholar] [PubMed]
- Kristensson, E.; Themner-Persson, A.; Ekblad, E. Survival and neurotransmitter plasticity in cultured rat colonic myenteric neurons. Regul. Pept. 2007, 140, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.D.; Thacker, M.; Castelucci, P.; Bagyánszki, M.; Epstein, M.L.; Furness, J.B. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res. 2008, 334, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Mongardi Fantaguzzi, C.; Thacker, M.; Chiocchetti, R.; Furness, J.B. Identification of neuron types in the submucosal ganglia of the mouse ileum. Cell Tissue Res. 2009, 336, 179–189. [Google Scholar] [CrossRef]
- Fehér, E.; Görcs, T.; Burnstock, G. Somatostatin-immunoreactive nerve fibers in close association with capillaries in the small intestine. Peptides 1989, 10, 945–949. [Google Scholar] [CrossRef]
- Ekblad, E.; Winther, C.; Ekman, R.; Hakanson, R.; Sundler, F. Projections of peptide-containing neuronsin rat small intestine. Neuroscience 1987, 20, 169–188. [Google Scholar] [CrossRef]
- Ekblad, E.; Ekman, R.; Hakanson, R.; Sundler, F. Projections of peptide-containing neurons in rat colon. Neuroscience 1988, 27, 655–674. [Google Scholar] [CrossRef]
- Ji, R.; Zhu, J.; Wang, D.; Sui, Q.Q.; Knight, G.E.; Burnstock, G.; Yuan, H.; Xiang, Z. Expression of P2X1 receptors in somatostatin-containing cells in mouse gastrointestinal tract and pancreatic islets of both mouse and human. Purinergic Signal. 2018, 14, 285–298. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Yamada, K.; Matsuda, Y.; Onozuka, M.; Yamamoto, T. CXCL14-like immunoreactivity exists in somatostatin-containing endocrine cells, and in the lamina propria and submucosal somatostatinergic nervous system of mouse alimentary tract. Acta Histochem. Cytochem. 2017, 50, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Lolova, I.; Itzev, D.; Davidoff, M. Immunocytochemical localization of substance P, methionine-enkephalin and somatostatin in the cat intestinal wall. J. Neural Transm. 1984, 60, 71–88. [Google Scholar] [CrossRef] [PubMed]
- Daniel, E.E.; Costa, M.; Furness, J.B.; Keast, J.R. Peptide neurons in the canine small intestine. J. Comp. Neurol. 1985, 237, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Buchan, A.M.; Barber, D.L. Neurotensin-containing neurons in the canine enteric innervation. Neurosci. Lett. 1987, 76, 13–17. [Google Scholar] [CrossRef]
- Buchan, A.M.; Doyle, A.D.; Accili, E. Canine jejunal submucosa cultures: Characterization and release of neural somatostatin. Can. J. Physiol. Pharmacol. 1990, 68, 705–710. [Google Scholar] [CrossRef] [PubMed]
- Daniel, E.E.; Furness, J.B.; Costa, M.; Belbeck, L. The projections of chemically identified nerve fibres in canine ileum. Cell Tissue Res. 1987, 247, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Van Aswegen, G.; Schoeman, J.H.; De Vos, V.; Van Noorden, S. The oesophagus and stomach of the African elephant: A histological, immunocytochemical and immunofluorescence study. Onderstepoort. J. Vet. Res. 1994, 61, 223–229. [Google Scholar]
- Sternini, C.; Wong, H.; Wu, S.V.; de Giorgio, R.; Yang, M.; Reeve, J., Jr.; Brecha, N.C.; Walsh, J.H. Somatostatin 2A receptor is expressed by enteric neurons, and by interstitial cells of Cajal and enterochromaffin-like cells of the gastrointestinal tract. J. Comp. Neurol. 1997, 386, 396–408. [Google Scholar] [CrossRef]
- Krempels, K.; Hunyady, B.; O’Carroll, A.M.; Mezey, E. Distribution of somatostatin receptor messenger RNAs in the rat gastrointestinal tract. Gastroenterology 1997, 112, 1948–1960. [Google Scholar] [CrossRef] [PubMed]
- Rettenbacher, M.; Reubi, J.C. Localization and characterization of neuropeptide receptors in human colon. Naunyn Schmiedebergs Arch. Pharmacol. 2001, 364, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Van Op den Bosch, J.; van Nassauw, L.; Lantermann, K.; van Marck, E.; Timmermans, J.P. Effect of intestinal inflammation on the cell-specific expression of somatostatin receptor subtypes in the murine ileum. Neurogastroenterol. Motil. 2007, 19, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Reubi, J.C.; Laissue, J.A.; Waser, B.; Steffen, D.L.; Hipkin, R.W.; Schonbrunn, A. SSTR2A in myenteric and submucosal plexus cells Immunohistochemical detection of somatostatin sst2a receptors in the lymphatic, smooth muscular, and peripheral nervous systems of the human gastrointestinal tract: Facts and artifacts. J. Clin. Endocrinol. Metab. 1999, 84, 2942–2950. [Google Scholar] [PubMed]
- Foong, J.P.; Parry, L.J.; Gwynne, R.M.; Bornstein, J.C. 5-HT(1A), SST(1), and SST(2) receptors mediate inhibitory postsynaptic potentials in the submucous plexus of the guinea pig ileum. Am. J. Physiol. Gastrointest. Liver Physiol. 2010, 298, G384–G394. [Google Scholar] [CrossRef] [PubMed]
- Berlucchi, G.; Buchtel, H.A. Neuronal plasticity: Historical roots and evolution of meaning. Exp. Brain Res. 2009, 192, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Hübener, M.; Bonhoeffer, T. Neuronal plasticity: Beyond the critical period. Cell 2014, 159, 727–737. [Google Scholar] [CrossRef] [PubMed]
- Brasileiro, A.D.; Garcia, L.P.; de Carvalho da Silva, S.; Rocha, L.B.; Pedrosa, A.L.; Vieira, A.S.; da Silva, V.J.D.; Rodrigues, A.R.A. Effects of diabetes mellitus on myenteric neuronal density and sodium channel expression in the rat ileum. Brain Res. 2019, 1708, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Makowska, K.; Gonkowski, S. Age and sex-dependent differences in the neurochemical characterization of calcitonin gene-related peptide-like immunoreactive (CGRP-LI) nervous structures in the porcine descending colon. Int. J. Mol. Sci. 2019, 20, 1024. [Google Scholar] [CrossRef] [PubMed]
- Vasina, V.; Barbara, G.; Talamonti, L.; Stanghellini, V.; Corinaldesi, R.; Tonini, M.; De Ponti, F.; De Giorgio, R. Enteric neuroplasticity evoked by inflammation. Auton. Neurosci. 2006, 126, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Buchan, A.M. Effect of diabetes in the BB Wistar rat on the peptidergic component of the enteric innervation. Digestion 1990, 46 (Suppl. S2), 142–147. [Google Scholar] [CrossRef]
- Schmidt, R.E.; Plurad, D.A.; Roth, K.A. Effects of chronic experimental streptozotocin-induced diabetes on the noradrenergic and peptidergic innervation of the rat alimentary tract. Brain Res. 1988, 458, 353–360. [Google Scholar] [CrossRef]
- Fox, D.A.; Herman, J.R.; Bass, P. Differentiation between myenteric plexus and longitudinal muscle of the rat jejunum as the site of action of putative enteric neurotransmitters. Eur. J. Pharmacol. 1986, 131, 39–47. [Google Scholar] [CrossRef]
- Saffrey, M.J.; Burnstock, G. Distribution of peptide-immunoreactive nerves in the foetal and newborn guinea-pig caecum. Cell Tissue Res. 1988, 253, 105–114. [Google Scholar] [CrossRef]
- Buchan, A.M.; Curtis, S.B.; Lund, P.K.; Pederson, R.A. Effect of massive small bowel resection on components of the peptidergic innervation of the rat small intestine. Digestion 1990, 46 (Suppl. S2), 134–141. [Google Scholar] [CrossRef]
- Rytel, L.; Całka, J. Neuropeptide profile changes in sensory neurons after partial prepyloric resection in pigs. Ann. Anat. 2016, 206, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Chiocchetti, R.; Grandis, A.; Bombardi, C.; Lucchi, M.L.; Dal Lago, D.T.; Bortolami, R.; Furness, J.B. Extrinsic and intrinsic sources of calcitonin gene-related peptide immunoreactivity in the lamb ileum: A morphometric and neurochemical investigation. Cell Tissue Res. 2006, 323, 183–196. [Google Scholar] [CrossRef] [PubMed]
- Skobowiat, C.; Calka, J.; Majewski, M. Axotomy induced changes in neuronal plasticity of sympathetic chain ganglia (SChG) neurons supplying descending colon in the pig. Exp. Mol. Pathol. 2011, 90, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Browning, K.N.; Travagli, R.A. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr. Physiol. 2014, 4, 1339–1368. [Google Scholar] [PubMed]
- Su, H.C.; Bishop, A.E.; Power, R.F.; Hamada, Y.; Polak, J.M. Dual intrinsic and extrinsic origins of CGRP- and NPY-immunoreactive nerves of rat gut and pancreas. J. Neurosci. 1987, 7, 2674–2687. [Google Scholar] [CrossRef]
- Li, C.; Zhu, Y.; Shenoy, M.; Pai, R.; Liu, L.; Pasricha, P.J. Anatomical and functional characterization of a duodeno-pancreatic neural reflex that can induce acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2013, 304, G490–G500. [Google Scholar] [CrossRef] [PubMed]
- Won, M.H.; Matsuo, K.; Oh, Y.S.; Kitoh, J. Brainstem topology of the vagal motoneurons projecting to the esophagus and stomach in the house musk shrew, Suncus murinus. J. Auton. Nerv. Syst. 1998, 68, 171–181. [Google Scholar] [CrossRef]
- Million, M.; Wang, L.; Martinez, V.; Taché, Y. Differential Fos expression in the paraventricular nucleus of the hypothalamus, sacral parasympathetic nucleus and colonic motor response to water avoidance stress in Fischer and Lewis rats. Brain Res. 2000, 877, 345–353. [Google Scholar] [CrossRef]
- Ganko, M.; Całka, J. Prolonged acetylsalicylic-acid-supplementation-induced gastritis affects the chemical coding of the stomach innervating vagal efferent neurons in the porcine dorsal motor vagal nucleus (DMX). J. Mol. Neurosci. 2014, 54, 188–198. [Google Scholar] [CrossRef]
- Wojtkiewicz, J.; Rowniak, M.; Crayton, R.; Barczewska, M.; Bladowski, M.; Robak, A.; Pidsudko, Z.; Majewski, M. Inflammation-induced changes in the chemical coding pattern of colon-projecting neurons in the inferior mesenteric ganglia of the pig. J. Mol. Neurosci. 2012, 46, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Wojtkiewicz, J.; Rowniak, M.; Gonkowski, S.; Crayton, R.; Majewski, M.; Robak, A.; Bialkowska, J.; Barczewska, M. Proliferative enteropathy (PE)-induced changes in the calbindin-immunoreactive (CB-IR) neurons of inferior mesenteric ganglion supplying the descending colon in the pig. J. Mol. Neurosci. 2012, 48, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Palus, K.; Bulc, M.; Calka, J. Changes in somatostatin-like immunoreactivity in the sympathetic neurons projecting to the prepyloric area of the porcine stomach induced by selected pathological conditions. BioMed Res. Int. 2017, 2017, 9037476. [Google Scholar] [CrossRef]
- Hokfelt, T.; Elfvin, L.G.; Elde, R.; Schultzberg, M.; Goldstein, M.; Luft, R. Occurrence of somatostatin-like immunoreactivity in some peripheral sympathetic noradrenergic neurons. Proc. Natl. Acad. Sci. USA 1977, 4, 3587–3591. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Furness, J.B. Somatostatin is present in a subpopulation of noradrenergic nerve fibres supplying the intestine. Neuroscience 1984, 13, 911–919. [Google Scholar] [CrossRef]
- Chery-Croze, S.; Bosshard, A.; Martin, H.; Cuber, J.C.; Charnay, Y.; Chayvialle, J.A. Peptide immunocytochemistry in afferent neurons from lower gut in rats. Peptides 1988, 9, 873–881. [Google Scholar] [CrossRef]
- Traub, R.J.; Hutchcroft, K.; Gebhart, G.F. The peptide content of colonic afferents decreases following colonic inflammation. Peptides 1999, 20, 267–273. [Google Scholar] [CrossRef]
- Skobowiat, C.; Gonkowski, S.; Calka, J. Phenotyping of sympathetic chain ganglia (SChG) neurons in porcine colitis. J. Vet. Med. Sci. 2010, 72, 1269–1274. [Google Scholar] [CrossRef] [PubMed]
- Bossowska, A.; Józefowicz, A.; Wojtkiewicz, J.; Gonkowski, S.; Kaleczyc, J.; Pidsusko, Z.; Majewski, M. Influence of proliferative enteropathy on expression pattern of somatostatin (SOM) and galanin (GAL) in extrinsic primary afferent neurons supplying the porcine descending colon. Pol. J. Vet. Sci. 2004, 7, 21–23. [Google Scholar]
- Guillemin, R. Somatostatin inhibits the release of acetylcholine induced electrically in the myenteric plexus. Endocrinology 1976, 99, 1653–1654. [Google Scholar] [CrossRef] [PubMed]
- Guillemin, R. Some thoughts on current research with somatostatin. Metabolism 1978, 27, 1453–1461. [Google Scholar] [CrossRef]
- Williams, J.T.; North, R.A. Inhibition of firing of myenteric neurons by somatostatin. Brain Res. 1978, 155, 165–168. [Google Scholar] [CrossRef]
- Furness, J.B.; Costa, M. Actions of somatostatin on excitatory and inhibitory nerves in the intestine. Eur. J. Pharmacol. 1979, 56, 69–74. [Google Scholar] [CrossRef]
- Ormsbee, H.S.; Koehler, S.L.; Telford, G.L. Somatostatin inhibits motilin-induced interdigestive contractile activity in the dog. Am. J. Dig. Div. 1978, 23, 781–787. [Google Scholar] [CrossRef]
- Johansson, C.; Efendic, S.; Wisén, O.; Uvnäs-Wallensten, K.; Luft, R. Effects of short-time somatostatin infusion on the gastric and intestinal propulsion in humans. Scand. J. Gastroenterol. 1978, 13, 481–483. [Google Scholar] [CrossRef]
- Katayama, Y.; North, R.A. The action of somatostatin on neurones of the myenteric plexus of the guinea-pig ileum. J. Physiol. 1980, 303, 315–323. [Google Scholar] [CrossRef]
- Ishikawa, K.; Ozaki, T. Distribution of several gut neuropeptides and their effects on motor activity in muscularis mucosae of guinea-pig proximal colon. J. Auton. Nerv. Syst. 1997, 64, 91–100. [Google Scholar] [CrossRef]
- Yau, W.M.; Lingle, P.F.; Youther, M.L. Modulation of cholinergic neurotransmitter release from myenteric plexus by somatostatin. Peptides 1983, 4, 49–53. [Google Scholar] [CrossRef]
- Teitelbaum, D.H.; O’Dorisio, T.M.; Perkins, W.E.; Gaginella, T.S. Somatostatin modulation of peptide-induced acetylcholine release in guinea pig ileum. Am. J. Physiol. 1984, 246, G509–G514. [Google Scholar] [CrossRef]
- Yau, W.M.; Dorsett, J.A.; Youther, M.L. Inhibitory peptidergic neurons: Functional difference between somatostatin and enkephalin in myenteric plexus. Am. J. Physiol. 1986, 250, G60–G63. [Google Scholar] [CrossRef] [PubMed]
- Wiley, J.; Owyang, C. Somatostatin inhibits cAMP-mediated cholinergic transmission in the myenteric plexus. Am. J. Physiol. 1987, 253, G607–G612. [Google Scholar] [CrossRef]
- Kowal, V.; Wiley, J.W.; Owyang, C. Differential action of somatostatin on peptide-induced release of acetylcholine. Am. J. Physiol. 1989, 257, G221–G225. [Google Scholar] [CrossRef]
- Mihara, S.; North, R.A.; Surprenant, A. Somatostatin increases an inwardly rectifying potassium conductance in guinea-pig submucous plexus neurons. J. Physiol. 1987, 390, 335–355. [Google Scholar] [CrossRef]
- Tatsumi, H.; Costa, M.; Schimerlik, M.; North, R.A. Potassium conductance increased by noradrenaline, opioids, somatostatin, and G-proteins: Whole-cell recording from guinea pig submucous neurons. J. Neurosci. 1990, 10, 1675–1682. [Google Scholar] [CrossRef]
- Grider, J.R. Somatostatin release from isolated ganglia of the myenteric plexus. Am. J. Physiol. 1989, 257, G313–G315. [Google Scholar] [CrossRef]
- Pawlik, W.W.; Gustaw, P.; Czarnobilski, K.; Sendur, R.; Konturek, S.J. Effects of somatostatin on intestinal circulation and oxygen consumption. Acta Physiol. Hung. 1989, 74, 277–283. [Google Scholar]
- Reubi, J.C.; Mazzucchelli, L.; Laissue, J.A. Intestinal vessels express a high density of somatostatin receptors in human inflammatory bowel disease. Gastroenterology 1994, 106, 951–959. [Google Scholar] [CrossRef]
- Ten Bokum, A.M.; Hofland, L.J.; van Hagen, P.M. Somatostatin and somatostatin receptors in the immune system: A review. Eur. Cytokine Netw. 2000, 11, 161–176. [Google Scholar] [PubMed]
- Rosskopf, D.; Schürks, M.; Manthey, I.; Joisten, M.; Busch, S.; Siffert, W. Signal transduction of somatostatin in human B lymphoblasts. Am. J. Physiol. Cell Physiol. 2003, 284, C179–C190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ameri, P.; Ferone, D. Diffuse endocrine system, neuroendocrine tumors and immunity: What’s new? Neuroendocrinology 2012, 95, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Hasler, W.L.; Soudah, H.C.; Owyang, C. A somatostatin analogue inhibits afferent pathways mediating perception of rectal distention. Gastroenterology 1993, 104, 1390–1397. [Google Scholar] [CrossRef]
- O’Donnell, L.J.; Watson, A.J.; Cameron, D.; Farthing, M.J. Effect of octreotide on mouth-to-caecum transit time in healthy subjects and in the irritable bowel syndrome. Aliment. Pharmacol. Ther. 1990, 4, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.; Cann, P.A.; Read, N.W.; Holdsworth, C.D. Effect of two new antisecretory drugs on fluid and electrolyte transport in a patient with secretory diarrhoea. Gut 1986, 27, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.C.; Williams, N.S.; King, R.F.; Barker, M.C. Effects of a long-acting somatostatin analogue in patients with severe ileostomy diarrhoea. Br. J. Surg. 1986, 73, 128–131. [Google Scholar] [CrossRef]
- Chowers, Y.; Cahalon, L.; Lahav, M.; Schor, H.; Tal, R.; Bar-Meir, S.; Levite, M. Somatostatin through its specific receptor inhibits spontaneous and TNF-alpha and bacteria-induced IL-8 and IL-1 beta secretion from intestinal epithelial cells. J. Immunol. 2000, 165, 2955–2961. [Google Scholar] [CrossRef]
- Cury, D.H.; Costa, J.E.; Irika, K.; Mijji, L.; Garcez, A.; Buchiguel, C.; Silva, I.; Sipahi, A. Protective effect of octreotide and infliximab in an experimental model of indomethacin-induced inflammatory bowel disease. Dig. Dis. Sci. 2008, 53, 2516–2520. [Google Scholar] [CrossRef]
- Yarman, S.; Yalın, G.Y.; Dogansen, S.C.; Canbaz, B.; Tanrıkulu, S.; Akyuz, F. Double benefit of long-acting somatostatin analogs in a patient with coexistence of acromegaly and ulcerative colitis. J. Clin. Pharm. Ther. 2016, 41, 559–562. [Google Scholar] [CrossRef] [PubMed]
- Martelli, L.; Colard, A.; Fontaine, F.; Deflandre, J.; Bastens, B.; Louis, E. Evaluation of the efficacy of octreotide LAR in the treatment of Crohn’s disease associated refractory diarrhea. Scand. J. Gastroenterol. 2017, 52, 564–569. [Google Scholar] [CrossRef] [PubMed]
- Li, S.C.; Martijn, C.; Cui, T.; Essaghir, A.; Luque, R.M.; Demoulin, J.B.; Castaño, J.P.; Öberg, K.; Giandomenico, V. The somatostatin analogue octreotide inhibits growth of small intestine neuroendocrine tumour cells. PLoS ONE 2012, 7, e48411. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.; Lester, P.; Rodrigues, L. Medical therapy of malignant bowel obstruction with octreotide, dexamethasone, and metoclopramide. Am. J. Hosp. Palliat. Care 2016, 33, 407–410. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Yonemitsu, Y.; Saito, S.; Itoh, H.; Onohara, T.; Fukuda, A.; Takai, M.; Maehara, Y. A somatostatin analogue, octreotide, ameliorates intestinal ischemia-reperfusion injury through the early induction of heme oxygenase-1. J. Surg. Res. 2012, 175, 350–358. [Google Scholar] [CrossRef] [PubMed]

| Part of the Gastrointestinal Tract | Localization of Somatostatin | References |
|---|---|---|
| Human | ||
| oesophagus | intramural nerve fibres, neuronal cells located in the myenteric plexus and submucous ganglia, | [21,22] |
| stomach | neuronal cells in the myenteric plexus and submucous ganglia located in the pyloric region and antrum, intraganglionic nerve fibres, nerves in the muscular and mucosal layers nerves in the muscularis mucosae | [19,21,55] |
| small intestine | neuronal cells located in the myenteric plexus, plexus submucous externus and plexus submucous internus, intraganglionic nerves nerves in the muscular and mucosal layers | [18,19,54,57,58] |
| large intestine | neuronal cells located in the myenteric plexus, plexus submucous externus and plexus submucous internus, intraganglionic nerve fibres in all types of enteric plexuses, nerve fibres in the muscular and mucosal layers, | [18,19,53,59] |
| Domestic pig | ||
| oesophagus | rare intramural nerve fibres in the muscular and mucosal layer, | [52] |
| stomach | single neurons in the submucous ganglia, rare nerves in the muscular and mucosal layers, | [51] |
| small intestine | - neuronal cells in the myenteric, outer submucous and inner submucous plexuses, intraganglionic nerve fibres, nerve fibres in the muscular and mucosal layers, | [20,49,50] |
| large intestine | neuronal cells located in the myenteric plexus, plexus submucous externus and plexus submucous internus, intraganglionic nerve fibres in all types of enteric plexuses, nerve fibres in the muscular and mucosal layers, | [17,47,48] |
| Guinea pig | ||
| oesophagus | rare nerve fibres in muscularis mucosae and myenteric plexus | [19] |
| stomach | neuronal cells and nerve fibres in the myenteric plexus nerve fibres in the muscular layer nerve fibres in the mucosal layer | [38,39,40,41] |
| small intestine | neuronal cells in the myenteric and submucous plexus nerves in the enteric ganglia, nerve fibres in the mucosal and myenteric layers | [16,26,27,28,29,30,31,32,33,34] |
| large intestine | neuronal cells located in the myenteric and submucous plexus, intraganglionic nerve fibres, nerve fibres located in the muscular and mucosal layers, | [16,27,35,36,37] |
| Stimulus | Character of Changes | References |
|---|---|---|
| Human | ||
| Hirschsprung disease | ⭤ | [54] |
| Hypertrophic pyloric stenosis | ⭤ | [21] |
| Colonic cancer | ⭣ | [53] |
| Guinea pig | ||
| Embryo-foetal development | ⭡ | [93] |
| Extrinsic denervation | ⭣ | [26] |
| Rat | ||
| Bowel resection | ⭤ | [95] |
| Experimental diabetes | ⭣ | [91,92] |
| Benzalkonium chloride administration | ⭣ | [92] |
| Domestic pig | ||
| Experimental Bacteroides fragilis infection | ⭡⭣ | [47] |
| Proliferative enteropathy in the ileum | ⭡ | [20] |
| Proliferative enteropathy in the colon | ⭡⭣ | [17] |
| Extrinsic denervation (colon) | ⭡⭣ | [17] |
| Chemically induced colitis | ⭡⭣ | [17] |
| Swine dysentery (stomach) | ⭤ | [51] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonkowski, S.; Rytel, L. Somatostatin as an Active Substance in the Mammalian Enteric Nervous System. Int. J. Mol. Sci. 2019, 20, 4461. https://doi.org/10.3390/ijms20184461
Gonkowski S, Rytel L. Somatostatin as an Active Substance in the Mammalian Enteric Nervous System. International Journal of Molecular Sciences. 2019; 20(18):4461. https://doi.org/10.3390/ijms20184461
Chicago/Turabian StyleGonkowski, Slawomir, and Liliana Rytel. 2019. "Somatostatin as an Active Substance in the Mammalian Enteric Nervous System" International Journal of Molecular Sciences 20, no. 18: 4461. https://doi.org/10.3390/ijms20184461
APA StyleGonkowski, S., & Rytel, L. (2019). Somatostatin as an Active Substance in the Mammalian Enteric Nervous System. International Journal of Molecular Sciences, 20(18), 4461. https://doi.org/10.3390/ijms20184461