Cellular Mechanisms of Angiogenesis in Neonatal Rat Models of Retinal Neurodegeneration
Abstract
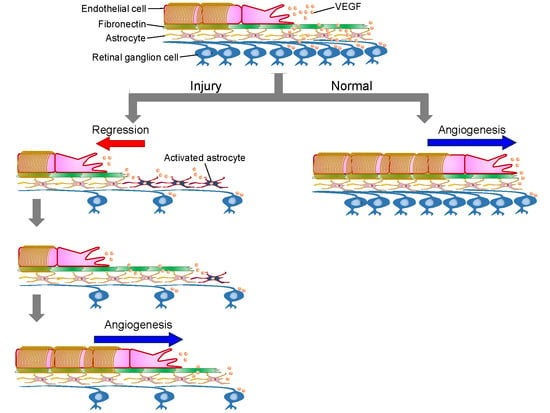
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Retinal Neurodegenerative Injury Models
4.3. Histological Assessment of The Retina
4.4. Immunohistochemistry
4.5. Assessment of Retinal Vasculature
4.6. Changes in Astrocytes and Fibronectins
4.7. Role of VEGF in Re-Vascularization in Retinal Neurodegenerative Injury Models
4.8. Distribution of VEGF in The Retina
4.9. Data Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hollmann, M.; Heinemann, S. Cloned glutamate receptors. Annu. Rev. Neurosci. 1994, 17, 31–108. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Mishina, M. Neurotransmitter receptors. Structure and function of the NMDA receptor channel. Neuropharmacology 1995, 34, 1219–1237. [Google Scholar] [CrossRef]
- Lam, T.T.; Abler, A.S.; Kwong, J.M.; Tso, M.O. N-methyl-D-aspartate (NMDA)--induced apoptosis in rat retina. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2391–2397. [Google Scholar] [PubMed]
- Zhang, X.; Cheng, M.; Chintala, S.K. Kainic acid-mediated upregulation of matrix metalloproteinase-9 promotes retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2004, 45, 2374–2383. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, B.S.; Chintala, S.K. Inhibition of reactive gliosis attenuates excitotoxicity-mediated death of retinal ganglion cells. PLoS ONE 2011, 6, e18305. [Google Scholar] [CrossRef] [PubMed]
- Kiagiadaki, F.; Thermos, K. Effect of intravitreal administration of somatostatin and sst2 analogs on AMPA-induced neurotoxicity in rat retina. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3080–3089. [Google Scholar] [CrossRef] [PubMed]
- Honjo, M.; Tanihara, H.; Kido, N.; Inatani, M.; Okazaki, K.; Honda, Y. Expression of ciliary neurotrophic factor activated by retinal Müller cells in eyes with NMDA- and kainic acid-induced neuronal death. Investig. Ophthalmol. Vis. Sci. 2000, 41, 552–560. [Google Scholar]
- Morizane, C.; Adachi, K.; Furutani, I.; Fujita, Y.; Akaike, A.; Kashii, S.; Honda, Y. N(omega)-nitro-L-arginine methyl ester protects retinal neurons against N-methyl-D-aspartate-induced neurotoxicity in vivo. Eur. J. Pharmacol. 1997, 328, 45–49. [Google Scholar] [CrossRef]
- Vorwerk, C.K.; Hyman, B.T.; Miller, J.W.; Husain, D.; Zurakowski, D.; Huang, P.L.; Fishman, M.C.; Dreyer, E.B. The role of neuronal and endothelial nitric oxide synthase in retinal excitotoxicity. Investig. Ophthalmol. Vis. Sci. 1997, 38, 2038–2044. [Google Scholar]
- Nakazawa, T.; Takahashi, H.; Nishijima, K.; Shimura, M.; Fuse, N.; Tamai, M.; Hafezi-Moghadam, A.; Nishida, K. Pitavastatin prevents NMDA induced retinal ganglion cell death by suppressing leukocyte recruitment. J. Neurochem. 2007, 100, 1018–1031. [Google Scholar] [CrossRef]
- Zheng, L.; Gong, B.; Hatala, D.A.; Kern, T.S. Retinal ischemia and reperfusion causes capillary degeneration: Similarities to diabetes. Investig. Ophthalmol. Vis. Sci. 2007, 48, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Al-Gayyar, M.M.; Abdelsaid, M.A.; Matragoon, S.; Pillai, B.A.; El-Remessy, A.B. Neurovascular protective effect of FeTPPs in N-methyl-D-aspartate model: Similarities to diabetes. Am. J. Pathol. 2010, 177, 1187–1197. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Nakahara, T.; Hoshino, M.; Mori, A.; Sakamoto, K.; Ishii, K. Retinal blood vessels are damaged in a rat model of NMDA-induced retinal degeneration. Neurosci. Lett. 2010, 485, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Al-Gayyar, M.M.; Abdelsaid, M.A.; Matragoon, S.; Pillai, B.A.; El-Remessy, A.B. Thioredoxin interacting protein is a novel mediator of retinal inflammation and neurotoxicity. Br. J. Pharmacol. 2011, 164, 170–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda, K.; Nakahara, T.; Mori, A.; Sakamoto, K.; Ishii, K. Protective effects of TGF-β inhibitors in a rat model of NMDA-induced retinal degeneration. Eur. J. Pharmacol. 2013, 699, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, T.; Mori, A.; Kurauchi, Y.; Sakamoto, K.; Ishii, K. Neurovascular interactions in the retina: Physiological and pathological roles. J. Pharmacol. Sci. 2013, 123, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Newman, E.A. Glial cell regulation of neuronal activity and blood flow in the retina by release of gliotransmitters. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370. [Google Scholar] [CrossRef]
- Sapieha, P.; Sirinyan, M.; Hamel, D.; Zaniolo, K.; Joyal, J.S.; Cho, J.H.; Honoré, J.C.; Kermorvant-Duchemin, E.; Varma, D.R.; Tremblay, S.; et al. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat. Med. 2008, 14, 1067–1076. [Google Scholar] [CrossRef]
- Asano, D.; Nakahara, T.; Mori, A.; Sakamoto, K.; Ishii, K. Regression of retinal capillaries following N-methyl-D-aspartate-induced neurotoxicity in the neonatal rat retina. J. Neurosci. Res. 2015, 93, 380–390. [Google Scholar] [CrossRef]
- Stone, J.; Chan-Ling, T.; Pe’er, J.; Itin, A.; Gnessin, H.; Keshet, E. Roles of vascular endothelial growth factor and astrocyte degeneration in the genesis of retinopathy of prematurity. Investig. Ophthalmol. Vis. Sci. 1996, 37, 290–299. [Google Scholar]
- Downie, L.E.; Pianta, M.J.; Vingrys, A.J.; Wilkinson-Berka, J.L.; Fletcher, E.L. Neuronal and glial cell changes are determined by retinal vascularization in retinopathy of prematurity. J. Comp. Neurol. 2007, 504, 404–417. [Google Scholar] [CrossRef] [PubMed]
- Ruhrberg, C.; Bautch, V.L. Neurovascular development and links to disease. Cell Mol. Life Sci. 2013, 70, 1675–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, M.L.; Wu, C.H.; Jiang-Shieh, Y.F.; Shieh, J.Y.; Wen, C.Y. Reactive changes of retinal astrocytes and Müller glial cells in kainate-induced neuroexcitotoxicity. J. Anat. 2007, 210, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Liou, G.I.; Behzadian, M.A.; Caldwell, R.B. Astrocytes modulate retinal vasculogenesis: Effects on fibronectin expression. J. Cell Sci. 1994, 107 Pt 9, 2499–2508. [Google Scholar]
- Dorrell, M.I.; Aguilar, E.; Friedlander, M. Retinal vascular development is mediated by endothelial filopodia, a preexisting astrocytic template and specific R-cadherin adhesion. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3500–3510. [Google Scholar] [PubMed]
- Stenzel, D.; Lundkvist, A.; Sauvaget, D.; Busse, M.; Graupera, M.; van der Flier, A.; Wijelath, E.S.; Murray, J.; Sobel, M.; Costell, M.; et al. Integrin-dependent and -independent functions of astrocytic fibronectin in retinal angiogenesis. Development 2011, 138, 4451–4463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stone, J.; Itin, A.; Alon, T.; Pe’er, J.; Gnessin, H.; Chan-Ling, T.; Keshet, E. Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J. Neurosci. 1995, 15 Pt 1, 4738–4747. [Google Scholar] [CrossRef]
- Nakamura, K.; Yamamoto, A.; Kamishohara, M.; Takahashi, K.; Taguchi, E.; Miura, T.; Kubo, K.; Shibuya, M.; Isoe, T. KRN633: A selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase that suppresses tumor angiogenesis and growth. Mol. Cancer Ther. 2004, 3, 1639–1649. [Google Scholar]
- Hartveit, E.; Brandstätter, J.H.; Sassoè-Pognetto, M.; Laurie, D.J.; Seeburg, P.H.; Wässle, H. Localization and developmental expression of the NMDA receptor subunit NR2A in the mammalian retina. J. Comp. Neurol. 1994, 348, 570–582. [Google Scholar] [CrossRef] [Green Version]
- Brandstätter, J.H.; Koulen, P.; Wässle, H. Selective synaptic distribution of kainate receptor subunits in the two plexiform layers of the rat retina. J. Neurosci. 1997, 17, 9298–9307. [Google Scholar] [CrossRef]
- Gründer, T.; Kohler, K.; Kaletta, A.; Guenther, E. The distribution and developmental regulation of NMDA receptor subunit proteins in the outer and inner retina of the rat. J. Neurobiol. 2000, 44, 333–342. [Google Scholar] [CrossRef]
- Hack, I.; Koulen, P.; Peichl, L.; Brandstätter, J.H. Development of glutamatergic synapses in the rat retina: The postnatal expression of ionotropic glutamate receptor subunits. Vis. Neurosci. 2002, 19, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Bai, N.; Aida, T.; Yanagisawa, M.; Katou, S.; Sakimura, K.; Mishina, M.; Tanaka, K. NMDA receptor subunits have different roles in NMDA-induced neurotoxicity in the retina. Mol. Brain 2013, 6, 34. [Google Scholar] [CrossRef] [PubMed]
- Pierce, E.A.; Avery, R.L.; Foley, E.D.; Aiello, L.P.; Smith, L.E. Vascular endothelial growth factor/vascular permeability factor expression in a mouse model of retinal neovascularization. Proc. Natl. Acad. Sci. USA 1995, 92, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Nakano, A.; Asano, D.; Kondo, R.; Mori, A.; Arima, S.; Ushikubo, H.; Sakamoto, K.; Nagamitsu, T.; Ishii, K.; Nakahara, T. Retinal neuronal cell loss prevents abnormal retinal vascular growth in a rat model of retinopathy of prematurity. Exp. Eye Res. 2018, 168, 115–127. [Google Scholar] [CrossRef]
- Asami, Y.; Nakahara, T.; Asano, D.; Kurauchi, Y.; Mori, A.; Sakamoto, K.; Ishii, K. Age-dependent changes in the severity of capillary degeneration in rat retina following N-methyl-D-aspartate-induced neurotoxicity. Curr. Eye Res. 2015, 40, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Asano, D.; Morita, A.; Mori, A.; Sakamoto, K.; Ishii, K.; Nakahara, T. Involvement of matrix metalloproteinases in capillary degeneration following NMDA-induced neurotoxicity in the neonatal rat retina. Exp. Eye Res. 2019, 182, 101–108. [Google Scholar] [CrossRef]
- Morita, A.; Sawada, S.; Mori, A.; Arima, S.; Sakamoto, K.; Nagamitsu, T.; Nakahara, T. Establishment of an abnormal vascular patterning model in the mouse retina. J. Pharmacol. Sci. 2018, 136, 177–188. [Google Scholar] [CrossRef]
- Nakano, A.; Nakahara, T.; Mori, A.; Ushikubo, H.; Sakamoto, K.; Ishii, K. Short-term treatment with VEGF receptor inhibitors induces retinopathy of prematurity-like abnormal vascular growth in neonatal rats. Exp. Eye Res. 2016, 143, 120–131. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asano, D.; Hokazono, M.; Hirano, S.; Morita, A.; Nakahara, T. Cellular Mechanisms of Angiogenesis in Neonatal Rat Models of Retinal Neurodegeneration. Int. J. Mol. Sci. 2019, 20, 4759. https://doi.org/10.3390/ijms20194759
Asano D, Hokazono M, Hirano S, Morita A, Nakahara T. Cellular Mechanisms of Angiogenesis in Neonatal Rat Models of Retinal Neurodegeneration. International Journal of Molecular Sciences. 2019; 20(19):4759. https://doi.org/10.3390/ijms20194759
Chicago/Turabian StyleAsano, Daiki, Masaki Hokazono, Shogo Hirano, Akane Morita, and Tsutomu Nakahara. 2019. "Cellular Mechanisms of Angiogenesis in Neonatal Rat Models of Retinal Neurodegeneration" International Journal of Molecular Sciences 20, no. 19: 4759. https://doi.org/10.3390/ijms20194759
APA StyleAsano, D., Hokazono, M., Hirano, S., Morita, A., & Nakahara, T. (2019). Cellular Mechanisms of Angiogenesis in Neonatal Rat Models of Retinal Neurodegeneration. International Journal of Molecular Sciences, 20(19), 4759. https://doi.org/10.3390/ijms20194759