Nanostructured Chitosan-Based Biomaterials for Sustained and Colon-Specific Resveratrol Release
Abstract
:1. Introduction
2. Results and Discussion
2.1. Resveratrol-Loaded Nanoparticules
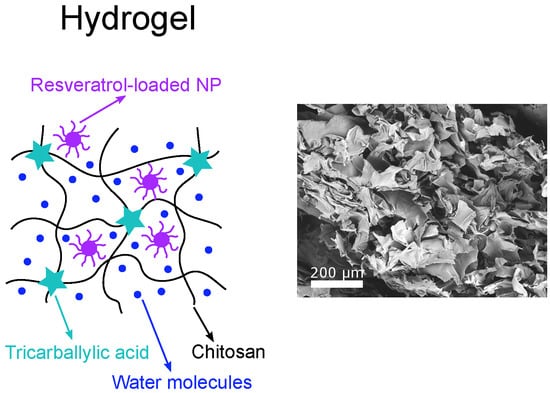
2.2. Nanostructured Chitosan-Based Composites Containing Resveratrol-Loaded NPs
2.3. Resveratrol Release Studies
3. Materials and Methods
3.1. Materials
3.2. General Methods
3.3. Micelle Formation
3.4. Preparation of Resveratrol-Loaded NPs
3.5. Experimental Design to Study the Effect of Loading Conditions on Resveratrol Encapsulation
3.6. Preparation of Nanostructured Chitosan-Based Hydrogels with Resveratrol-Loaded Nanoparticles
3.7. Release Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| TNF-α | Tumor Necrosis Factor |
| IL-12 | Interleukin-12 |
| IL-6 | Interleukin-6 |
| IBD | Inflammatory Bowel Disease |
| CD | Crohn’s Disease |
| UC | Ulcerative Colitis |
| GIT | Gastro-Intestinal Tract |
| DALYs | Disability-Adjusted Life Years |
| RES | Resveratrol |
| ADME-T | Absorption, Distribution, Metabolism, Excretion and Toxicity |
| DDS | Drug Delivery Systems |
| NPs | Nanoparticles |
| MNP | Micro-Nanoparticles |
| ADME-T | Absorption, Distribution, Metabolism, Excretion and Toxicity |
| pHEMA | poly(2-hydroxyethyl methacrylate) |
| pDMAEMA | poly(N,N-dimethylaminoethyl methacrylate) |
| EE | Encapsulation Efficiency |
| LCST | Lower Critical Solution Temperature |
| CTS | Chitosan |
| DCNa | Diclofenac Sodium |
| CEBAS-CSIC | Segura’s Center of Edaphology and Applied Biology |
| UV-Vis | Ultraviolet Visible |
| SEM | Scanning Electron Microscopy |
| CITIUS | General Research Services of the University of Seville |
| Dh | Average Diameter |
| PdI | Polydispersity Index |
| DLS | Dynamic Light Scattering |
| TGA | Thermogravimetric Analysis |
| DTG | Derivative Thermogravimetric Analysis |
| DMDOO | 1,8-dimaleimide-3,6-dioxaoctane |
| DMSO | Dimethyl sulfoxide |
References
- Calder, P.C. n-3 Polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006, 83, 1505S–1519S. [Google Scholar] [CrossRef] [PubMed]
- Bouma, G.; Strober, W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 2003, 3, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Loftus, E.V. Clinical epidemiology of inflammatory bowel disease: Incidence, prevalence, and environmental influences. Gastroenterology 2004, 126, 1504–1517. [Google Scholar] [CrossRef] [PubMed]
- Xavier, R.J.; Podolsky, D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Rakoff-Nahoum, S.; Paglino, J.; Eslami-Varzaneh, F.; Edberg, S.; Medzhitov, R. Recognition of Commensal Microflora by Toll-Like Receptors Is Required for Intestinal Homeostasis. Cell 2004, 118, 229–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seksik, P.; Sokol, H.; Lepage, P.; Vasquez, N.; Manichanh, C.; Mangin, I.; Pochart, P.; Doré, J.; Marteau, P. Review article: The role of bacteria in onset and perpetuation of inflammatory bowel disease. Aliment. Pharmacol. Ther. 2006, 24, 11–18. [Google Scholar] [CrossRef]
- Sokol, H.; Pigneur, B.; Watterlot, L.; Lakhdari, O.; Bermudez-Humaran, L.G.; Gratadoux, J.-J.; Blugeon, S.; Bridonneau, C.; Furet, J.-P.; Corthier, G.; et al. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc. Natl. Acad. Sci. USA 2008, 105, 16731–16736. [Google Scholar] [CrossRef] [Green Version]
- Orholm, M.; Munkholm, P.; Langholz, E.; Nielsen, O.H.; Sørensen, T.I.A.; Binder, V. Familial Occurrence of Inflammatory Bowel Disease. N. Engl. J. Med. 1991, 324, 84–88. [Google Scholar] [CrossRef]
- Collnot, E.-M.; Ali, H.; Lehr, C.-M. Nano- and microparticulate drug carriers for targeting of the inflamed intestinal mucosa. J. Control. Release 2012, 161, 235–246. [Google Scholar] [CrossRef]
- Abu-Amero, K.K.; Kondkar, A.A.; Chalam, K.V. Resveratrol and ophthalmic diseases. Nutrients 2016, 8, 200. [Google Scholar] [CrossRef]
- Zordoky, B.N.M.; Robertson, I.M.; Dyck, J.R.B. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1155–1177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baur, J.A.; Sinclair, D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006, 5, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.S.; Dempsey, R.J.; Vemuganti, R. Resveratrol neuroprotection in stroke and traumatic CNS injury. Neurochem. Int. 2015, 89, 75–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastianetto, S.; Ménard, C.; Quirion, R. Neuroprotective action of resveratrol. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1195–1201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmali, N.; Baysal, O.; Harma, A.; Esenkaya, I.; Mizrak, B. Effects of Resveratrol in Inflammatory Arthritis. Inflammation 2007, 30, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Kubota, S.; Tsubota, K.; Ozawa, Y. Resveratrol prevents the development of choroidal neovascularization by modulating AMP-activated protein kinase in macrophages and other cell types. J. Nutr. Biochem. 2014, 25, 1218–1225. [Google Scholar] [CrossRef] [Green Version]
- Peñalver, P.; Belmonte-Reche, E.; Adán, N.; Caro, M.; Mateos-Martín, M.L.; Delgado, M.; González-Rey, E.; Morales, J.C. Alkylated resveratrol prodrugs and metabolites as potential therapeutics for neurodegenerative diseases. Eur. J. Med. Chem. 2018, 146, 123–138. [Google Scholar] [CrossRef]
- Cottart, C.-H.; Nivet-Antoine, V.; Laguillier-Morizot, C.; Beaudeux, J.-L. Resveratrol bioavailability and toxicity in humans. Mol. Nutr. Food Res. 2010, 54, 7–16. [Google Scholar] [CrossRef]
- Biasutto, L.; Mattarei, A.; Azzolini, M.; La Spina, M.; Sassi, N.; Romio, M.; Paradisi, C.; Zoratti, M. Resveratrol derivatives as a pharmacological tool. Ann. N. Y. Acad. Sci. 2017, 1403, 27–37. [Google Scholar] [CrossRef]
- Larrosa, M.; Tomé-Carneiro, J.; Yáñez-Gascón, M.J.; Alcántara, D.; Selma, M.V.; Beltrán, D.; García-Conesa, M.T.; Urbán, C.; Lucas, R.; Tomás-Barberán, F.; et al. Preventive Oral Treatment with Resveratrol Pro-prodrugs Drastically Reduce Colon Inflammation in Rodents. J. Med. Chem. 2010, 53, 7365–7376. [Google Scholar] [CrossRef]
- Ferris, C.; de Paz, M.V.; Aguilar-de-Leyva, A.; Caraballo, I.; Galbis, J.A. Reduction-sensitive functionalized copolyurethanes for biomedical applications. Polym. Chem. 2014, 5, 2370–2381. [Google Scholar] [CrossRef]
- Xu, Y.; Zhan, C.; Fan, L.; Wang, L.; Zheng, H. Preparation of dual crosslinked alginate-chitosan blend gel beads and in vitro controlled release in oral site-specific drug delivery system. Int. J. Pharm. 2007, 336, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Giri, T.K.; Thakur, A.; Alexander, A.; Ajazuddin; Badwaik, H.; Tripathi, D.K. Modified chitosan hydrogels as drug delivery and tissue engineering systems: Present status and applications. Acta Pharm. Sin. B 2012, 2, 439–449. [Google Scholar] [CrossRef]
- Culver, H.R.; Clegg, J.R.; Peppas, N.A. Analyte-Responsive Hydrogels: Intelligent Materials for Biosensing and Drug Delivery. Acc. Chem. Res. 2017, 50, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Conde, J.; Oliva, N.; Zhang, Y.; Artzi, N. Local triple-combination therapy results in tumour regression and prevents recurrence in a colon cancer model. Nat. Mater. 2016, 15, 1128–1138. [Google Scholar] [CrossRef] [PubMed]
- Brøndsted, H.; Andersen, C.; Hovgaard, L. Crosslinked dextran—A new capsule material for colon targeting of drugs. J. Control. Release 1998, 53, 7–13. [Google Scholar] [CrossRef]
- Das, S.; Chaudhury, A.; Ng, K.-Y. Preparation and evaluation of zinc–pectin–chitosan composite particles for drug delivery to the colon: Role of chitosan in modifying in vitro and in vivo drug release. Int. J. Pharm. 2011, 406, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.R.; Nguyen, L.P.; Inayathullah, M.; Malkovskiy, A.; Habte, F.; Rajadas, J.; Habtezion, A. A Thermo-Sensitive Delivery Platform for Topical Administration of Inflammatory Bowel Disease Therapies. Gastroenterology 2015, 149, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Galbis, E.; de-Paz, M.-V.; Iglesias, N.; Lacroix, B.; Alcudia, A.; Galbis, J.A. Core cross-linked nanoparticles from self-assembling polyfma-based micelles. Encapsulation of lipophilic molecules. Eur. Polym. J. 2017, 89, 406–418. [Google Scholar] [CrossRef]
- Galbis, E.; De Paz, M.-V.; Iglesias, N.; Lucas, R.; Galbis, J.A. pH-Responsive Polymeric Nanoparticles as Drug Delivery Systems. J. Drug Des. Res. 2017, 4, 1047. [Google Scholar]
- Galbis, E.; Iglesias, N.; Lucas, R.; Tinajero-Díaz, E.; De-Paz, M.-V.; Muñoz-Guerra, S.; Galbis, J.A. Validation of Smart Nanoparticles as Controlled Drug Delivery Systems: Loading and pH-Dependent Release of Pilocarpine. ACS Omega 2018, 3, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, N.; Galbis, E.; Díaz-Blanco, M.J.; de-Paz, M.V.; Galbis, J.A. Loading studies of the anticancer drug camptothecin into dual stimuli-sensitive nanoparticles. Stability scrutiny. Int. J. Pharm. 2018, 550, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Griset, A.P.; Walpole, J.; Liu, R.; Gaffey, A.; Colson, Y.L.; Grinstaff, M.W. Expansile nanoparticles: Synthesis, characterization, and in vivo efficacy of an acid-responsive polymeric drug delivery system. J. Am. Chem. Soc. 2009, 131, 2469–2471. [Google Scholar] [CrossRef] [PubMed]
- Chouhan, R.; Bajpai, A.K. An in vitro release study of 5-fluoro-uracil (5-FU) from swellable poly-(2-hydroxyethyl methacrylate) (PHEMA) nanoparticles. J. Mater. Sci. Mater. Med. 2009, 20, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Qiao, Y.; Wang, W.; He, H.; Deng, L.; Xing, L.; Xu, J.; Liang, X.-J.; Dong, A. Poly(ε-caprolactone)-graft-poly(2-(N, N-dimethylamino) ethyl methacrylate) nanoparticles: PH dependent thermo-sensitive multifunctional carriers for gene and drug delivery. J. Mater. Chem. 2010, 20, 6935–6941. [Google Scholar] [CrossRef]
- Dinu, I.A.; Duskey, J.T.; Car, A.; Palivan, C.G.; Meier, W. Engineered non-toxic cationic nanocarriers with photo-triggered slow-release properties. Polym. Chem. 2016, 7, 3451–3464. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, P. Reduction-responsive core-shell-corona micelles based on triblock copolymers: Novel synthetic strategy, characterization, and application as a tumor microenvironment-responsive drug delivery system. ACS Appl. Mater. Interfaces 2015, 7, 166–174. [Google Scholar] [CrossRef]
- Vivek, R.; Nipun Babu, V.; Thangam, R.; Subramanian, K.S.; Kannan, S. PH-responsive drug delivery of chitosan nanoparticles as Tamoxifen carriers for effective anti-tumor activity in breast cancer cells. Colloids Surf. B Biointerfaces 2013, 111, 117–123. [Google Scholar] [CrossRef]
- Gou, M.; Zheng, X.; Men, K.; Zhang, J.; Wang, B.; Lv, L.; Wang, X.; Zhao, Y.; Luo, F.; Chen, L.; et al. Self-assembled hydrophobic honokiol loaded MPEG-PCL diblock copolymer micelles. Pharm. Res. 2009, 26, 2164–2173. [Google Scholar] [CrossRef]
- Tripathi, A.; Gupta, R.; Saraf, S.A. PLGA nanoparticles of anti tubercular drug: Drug loading and release studies of a water in-soluble drug. Int. J. PharmTech Res. 2010, 2, 2116–2123. [Google Scholar]
- Gupta, H.; Aqil, M.; Khar, R.K.; Ali, A.; Bhatnagar, A.; Mittal, G. Biodegradable levofloxacin nanoparticles for sustained ocular drug delivery. J. Drug Target. 2011, 19, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Wang, Y.; Zhang, J.; Zhan, X.; Zhu, S.; Yang, H.; Wang, G. Multiple stimuli-responsive polymeric micelles for controlled release. Soft Matter 2013, 9, 370–373. [Google Scholar] [CrossRef]
- Sakloetsakun, D.; Bernkop-Schnürch, A. Thiolated chitosans. J. Drug Deliv. Sci. Technol. 2010, 20, 63–69. [Google Scholar] [CrossRef]
- Thanou, M.; Verhoef, J.C.; Junginger, H.E. Chitosan and its derivatives as intestinal absorption enhancers. Adv. Drug Deliv. Rev. 2001, 50, S91–S101. [Google Scholar] [CrossRef]
- Ferris, C.; Casas, M.; Lucero, M.J.; de Paz, M.V.; Jimenez-Castellanos, M.R. Synthesis and characterization of a novel chitosan-N-acetyl-homocysteine thiolactone polymer using MES buffer. Carbohydr. Polym. 2014, 111, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Lucero, M.J.; Ferris, C.; Sanchez-Gutierrez, C.A.; Jimenez-Castellanos, M.R.; de-Paz, M.-V. Novel aqueous chitosan-based dispersions as efficient drug delivery systems for topical use. Rheological, textural and release studies. Carbohydr. Polym. 2016, 151, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef]
- Köping-Höggard, M.; Tubulekas, I.; Guan, H.; Edwards, K.; Nilsson, M.; Varum, K.M.; Artursson, P. Chitosan as a nonviral gene delivery system. Structure—Property relationships and characteristics compared with polyethylenimine in vitro and after lung administration in vivo. Gene Ther. 2001, 8, 1108–1121. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef]
- Pang, Y.; Li, Y.; Zhao, H.; Zheng, J.; Xu, H.; Wei, G.; Hao, J.; Cui, F. Bioadhesive polysaccharide in protein delivery system: Chitosan nanoparticles improve the intestinal absorption of insulin in vivo. Int. J. Pharm. 2002, 249, 139–147. [Google Scholar]
- Sahoo, D.; Sahoo, S.; Mohanty, P.; Sasmal, S.; Nayak, P.L. Chitosan: A New Versatile Bio-polymer for Various Applications. Des. Monomers Polym. 2009, 12, 377–404. [Google Scholar] [CrossRef]
- Shamji, M.F.; Hwang, P.; Bullock, R.W.; Adams, S.B.; Nettles, D.L.; Setton, L.A. Release and activity of anti-TNFα therapeutics from injectable chitosan preparations for local drug delivery. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 90B, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, N.; Galbis, E.; Valencia, C.; De-Paz, M.-V.; Galbis, J.A. Reversible pH-sensitive chitosan-based hydrogels. Influence of dispersion composition on rheological properties and sustained drug delivery. Polymers 2018, 10, 392. [Google Scholar] [CrossRef]
- Ferrari, F.; Bertoni, M.; Caramella, C.; La Manna, A. Description and validation of an apparatus for gel strength measurements. Int. J. Pharm. 1994, 109, 115–124. [Google Scholar] [CrossRef]
- Favi, P.M.; Benson, R.S.; Neilsen, N.R.; Hammonds, R.L.; Bates, C.C.; Stephens, C.P.; Dhar, M.S. Cell proliferation, viability, and in vitro differentiation of equine mesenchymal stem cells seeded on bacterial cellulose hydrogel scaffolds. Mater. Sci. Eng. C 2013, 33, 1935–1944. [Google Scholar] [CrossRef] [PubMed]
- Palma Santana, B.; Nedel, F.; Perelló Ferrúa, C.; Marques e Silva, R.; Fernandes da Silva, A.; Demarco, F.F.; Lenin Villarreal Carreño, N. Comparing different methods to fix and to dehydrate cells on alginate hydrogel scaffolds using scanning electron microscopy. Microsc. Res. Tech. 2015, 78, 553–561. [Google Scholar] [CrossRef] [PubMed]
- Yomota, C.; Miyazaki, T.; Okada, S. Determination of the viscometric constants for chitosan and the application of universal calibration procedure in its gel permeation chromatography. Colloid Polym. Sci. 1993, 271, 76–82. [Google Scholar] [CrossRef]
- Campiñez, M.D.; Benito, E.; Romero-Azogil, L.; Aguilar-de-Leyva, Á.; García-Martín, M.d.G.; Galbis, J.A.; Caraballo, I. Development and characterization of new functionalized polyurethanes for sustained and site-specific drug release in the gastrointestinal tract. Eur. J. Pharm. Sci. 2017, 100, 285–295. [Google Scholar] [CrossRef]
- Galbis, E.; de Paz, M.V.; McGuinness, K.L.; Angulo, M.; Valencia, C.; Galbis, J.A. Tandem ATRP/Diels-Alder synthesis of polyHEMA-based hydrogels. Polym. Chem. 2014, 5, 5391–5402. [Google Scholar] [CrossRef]
- Ahnazarova, S.; Kafarov, V.; Rep’ev, A. Experiment Optimization in Chemistry and Chemical Engineering; Mir Publishers: Moscow, Russia, 1982. [Google Scholar]
- Montgomery, D.C. Diseño y Análisis de Experimentos; Editorial Iberoamericana: México D.F., Mexico, 1991. [Google Scholar]
- Schellekens, R.C.A.; Stuurman, F.E.; van der Weert, F.H.J.; Kosterink, J.G.W.; Frijlink, H.W. A novel dissolution method relevant to intestinal release behaviour and its application in the evaluation of modified release mesalazine products. Eur. J. Pharm. Sci. 2007, 30, 15–20. [Google Scholar] [CrossRef]






| Experimental Parameters | Non Cross-Linked Loaded NPs | |||
| RES/Polymer Ratio | Temperature (°C) | Sample | Formulation Code | EE (%) |
| 0.25 | 25 | 1 | NonXr-RES/Pol0.25-T25 | 6.80 |
| 0.25 | 25 | 2 | NonXr-RES/Pol0.25-T32 | 29.51 |
| 0.25 | 25 | 3 | NonXr-RES/Pol0.25-T39 | 33.82 |
| 0.50 | 32 | 4 | NonXr-RES/Pol0.5-T25 | 23.87 |
| 0.50 | 32 | 5 | NonXr-RES/Pol0.5-T32 | 36.00 |
| 0.50 | 32 | 6 | NonXr-RES/Pol0.5-T32 | 38.18 |
| 0.50 | 32 | 7 | NonXr-RES/Pol0.5-T39 | 49.37 |
| 0.75 | 39 | 8 | NonXr-RES/Pol0.75-T25 | 18.31 |
| 0.75 | 39 | 9 | NonXr-RES/Pol0.75-T32 | 37.54 |
| 0.75 | 39 | 10 | NonXr-RES/Pol0.75-T39 | 48.10 |
| Experimental Parameters | Cross-Linked Loaded NPs | |||
| RES/Polymer Ratio | Temperature (°C) | Sample | Formulation Code | EE (%) |
| 0.25 | 25 | 11 | Xr-RES/Pol0.25-T25 | 9.51 |
| 0.25 | 25 | 12 | Xr-RES/Pol0.25-T32 | 43.74 |
| 0.25 | 25 | 13 | Xr-RES/Pol0.25-T39 | 49.01 |
| 0.50 | 32 | 14 | Xr-RES/Pol0.5-T25 | 12.57 |
| 0.50 | 32 | 15 | Xr-RES/Pol0.5-T32 | 38.00 |
| 0.50 | 32 | 16 | Xr-RES/Pol0.5-T32 | 40.09 |
| 0.50 | 32 | 17 | Xr-RES/Pol0.5-T39 | 36.19 |
| 0.75 | 39 | 18 | Xr-RES/Pol0.75-T25 | 28.46 |
| 0.75 | 39 | 19 | Xr-RES/Pol0.75-T32 | 43.12 |
| 0.75 | 39 | 20 | Xr-RES/Pol0.75-T39 | 44.34 |
| Equation | R2 | Df | F | P | Std. Error |
|---|---|---|---|---|---|
| 0.96 | 4.5 | 29.03 | 0.001 | 3.69 | |
| 0.97 | 4.5 | 49.96 | 0.003 | 2.74 |
| Unloaded Samples [31] | Resveratrol-Loaded NPs | |||||||
|---|---|---|---|---|---|---|---|---|
| Degree of Crosslinking | Sample | Z-av (± SD) | PdI (± SD) | Size (± SD) (Dh. nm) | Sample | Z-av (± SD) | PdI (± SD) | Size (± SD) (Dh. nm) |
| (nm) | (nm) | |||||||
| Non-Xr | S-01 | 177 (± 1) | 0.14 (± 0.02) | 210 (± 80) | RES-Non-Xr | 115 (± 1) | 0.46 (± 0.01) | 170 (± 90) |
| Xr 20% | S-02 | 108 (± 1) | 0.33 (± 0.01) | 130 (± 70) | RES-Xr | 121 (± 1) | 0.27 (± 0.01) | 170 (± 90) |
| Sample | Spreadability (diameter, cm)a | TGAb | |||||
|---|---|---|---|---|---|---|---|
| t 1 min | t 30 min | Δdiameter (%) | °Td (°C) | maxTd (°C) | ΔW (%) | Mass Residue at 650 °C (%) | |
| CTS | - | 110 | 72/297 | 9/58 | 30 | ||
| Xr-CTS-RES | 5 | 8.2 | 64 | 98 | 62/281 | 24/43 | 32 |
| Non-Xr-CTS-RES | 5.6 | 8.9 | 59 | 99 | 111/281 | 24/42 | 32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias, N.; Galbis, E.; Díaz-Blanco, M.J.; Lucas, R.; Benito, E.; de-Paz, M.-V. Nanostructured Chitosan-Based Biomaterials for Sustained and Colon-Specific Resveratrol Release. Int. J. Mol. Sci. 2019, 20, 398. https://doi.org/10.3390/ijms20020398
Iglesias N, Galbis E, Díaz-Blanco MJ, Lucas R, Benito E, de-Paz M-V. Nanostructured Chitosan-Based Biomaterials for Sustained and Colon-Specific Resveratrol Release. International Journal of Molecular Sciences. 2019; 20(2):398. https://doi.org/10.3390/ijms20020398
Chicago/Turabian StyleIglesias, Nieves, Elsa Galbis, M. Jesús Díaz-Blanco, Ricardo Lucas, Elena Benito, and M.-Violante de-Paz. 2019. "Nanostructured Chitosan-Based Biomaterials for Sustained and Colon-Specific Resveratrol Release" International Journal of Molecular Sciences 20, no. 2: 398. https://doi.org/10.3390/ijms20020398