Hepatic Stellate Cells Enhance Liver Cancer Progression by Inducing Myeloid-Derived Suppressor Cells through Interleukin-6 Signaling
Abstract
:1. Introduction
2. Results
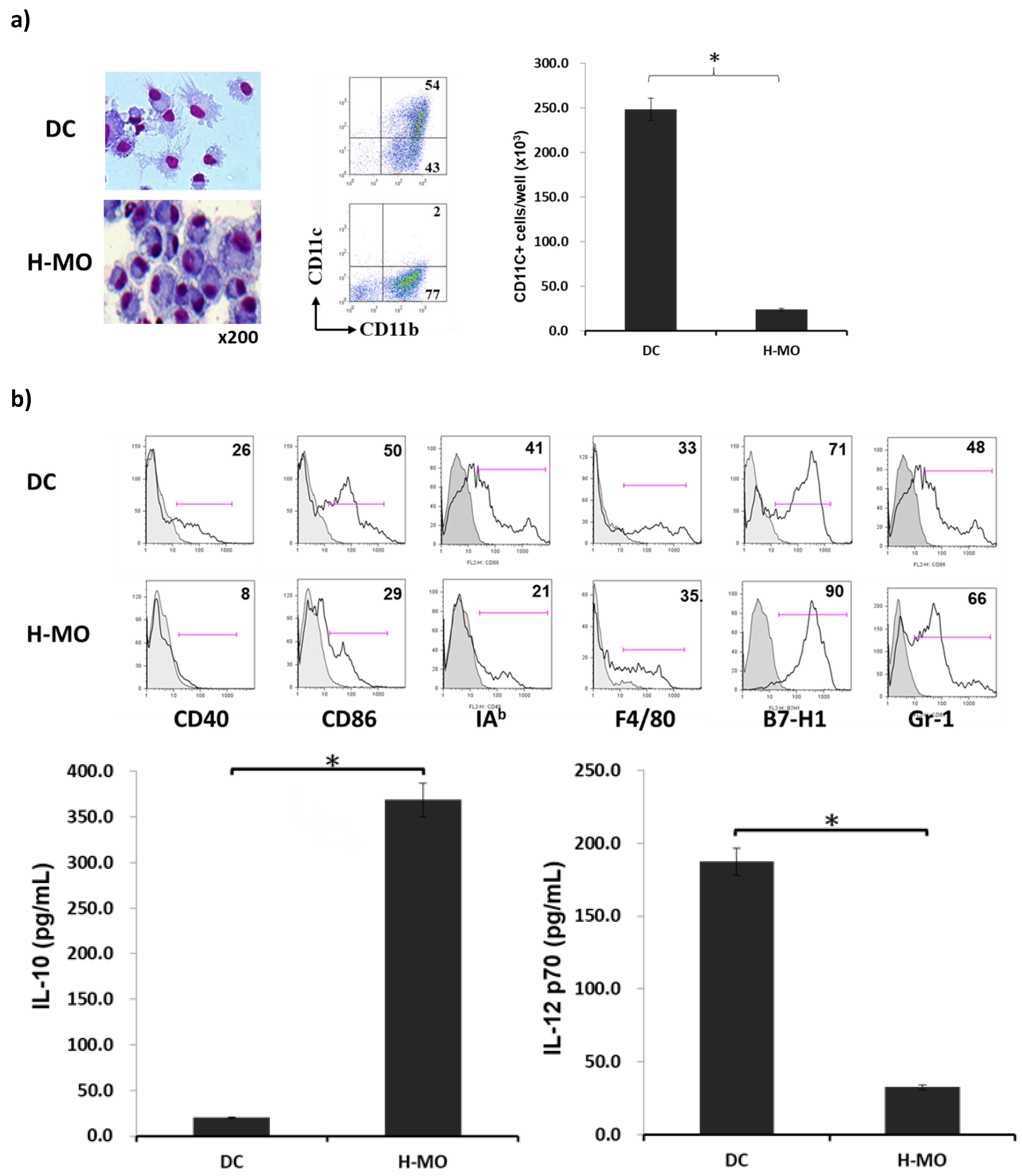
2.1. Features of HSC-Conditioned Monocytes Resemble MDSCs In Vitro
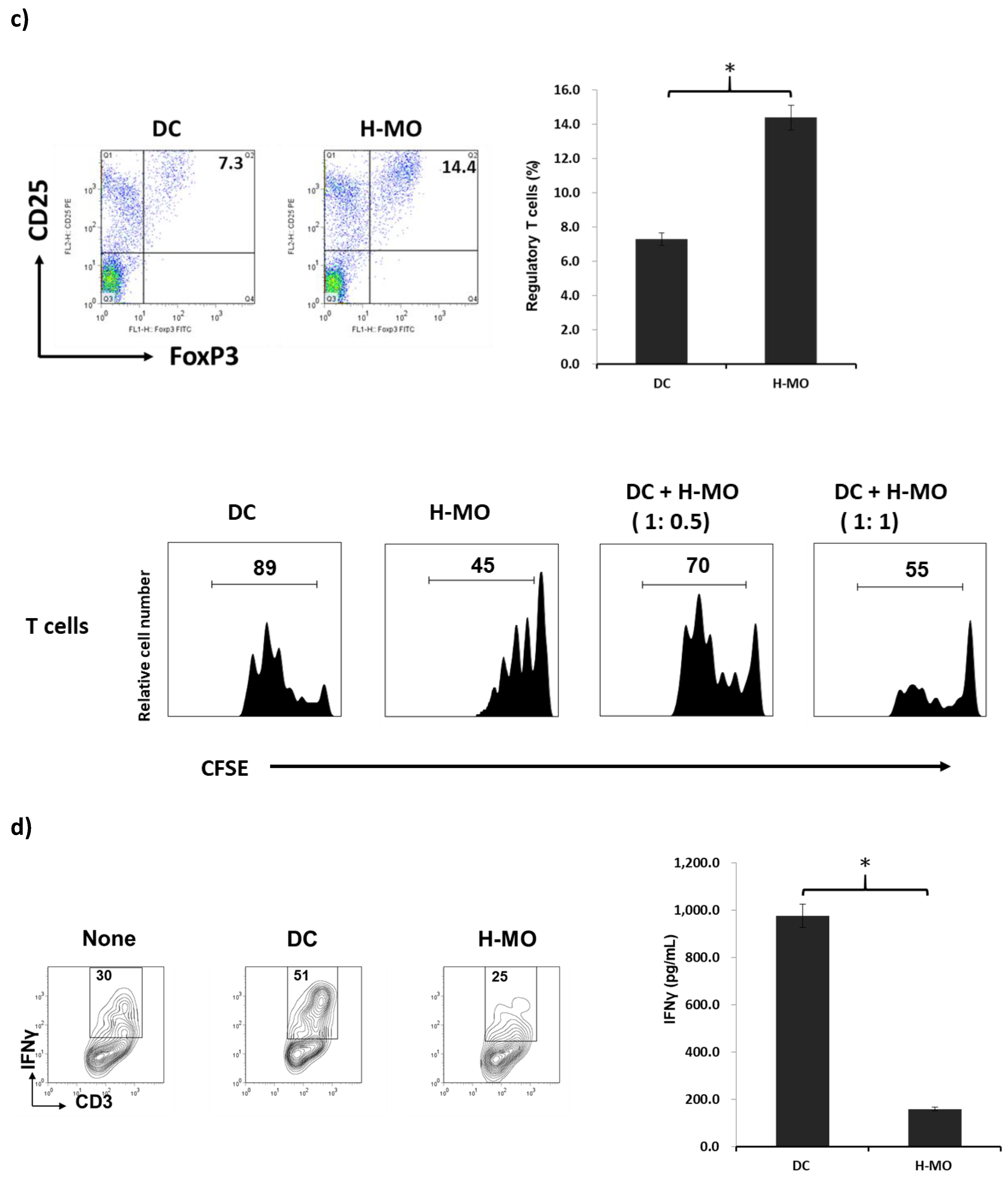
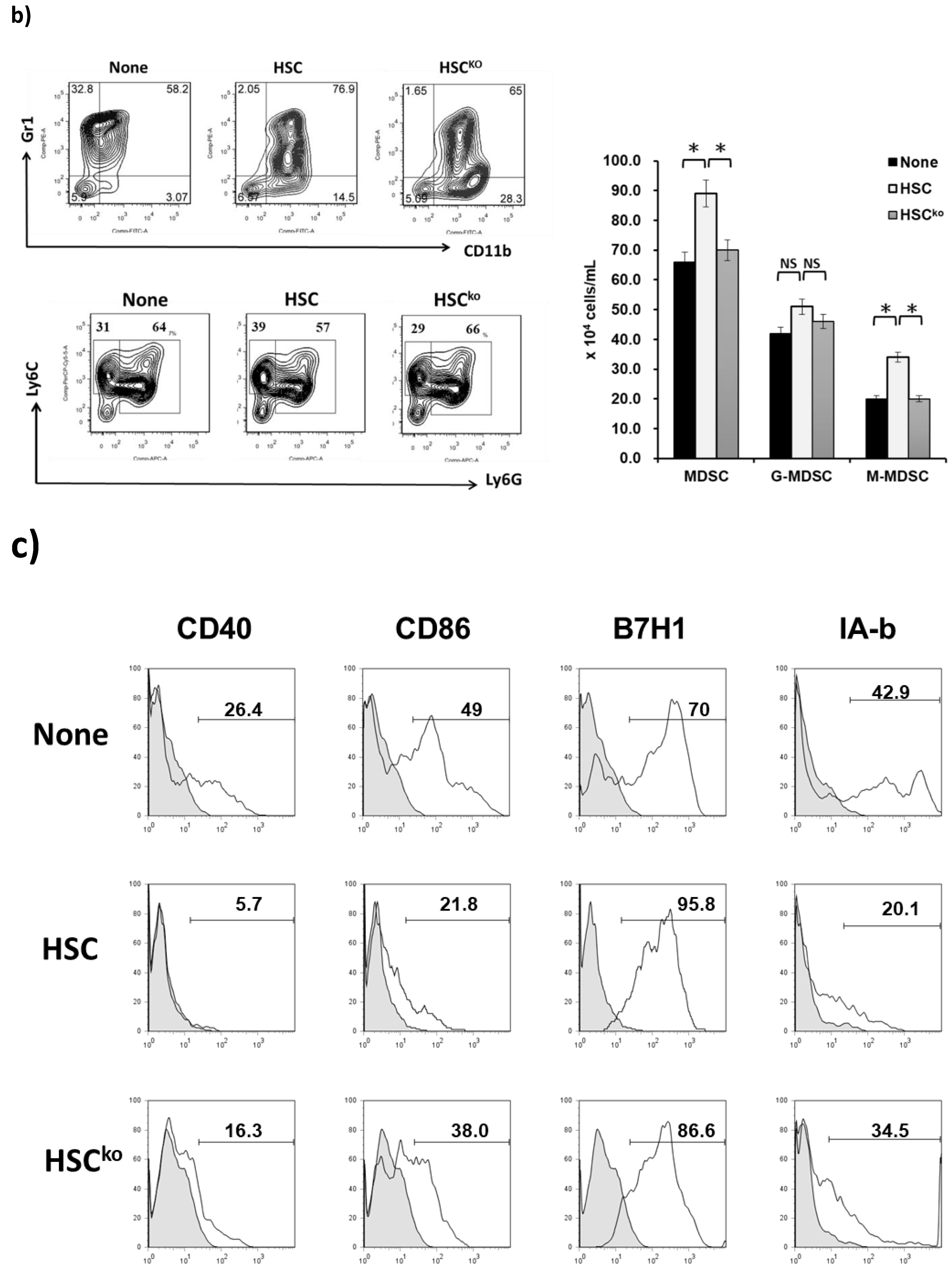
2.2. MDSCs Mediated by HSCs Display More Immunoregulatory Enzymes and Regulate T-Cell Activity in the Tumor Environment In Vitro
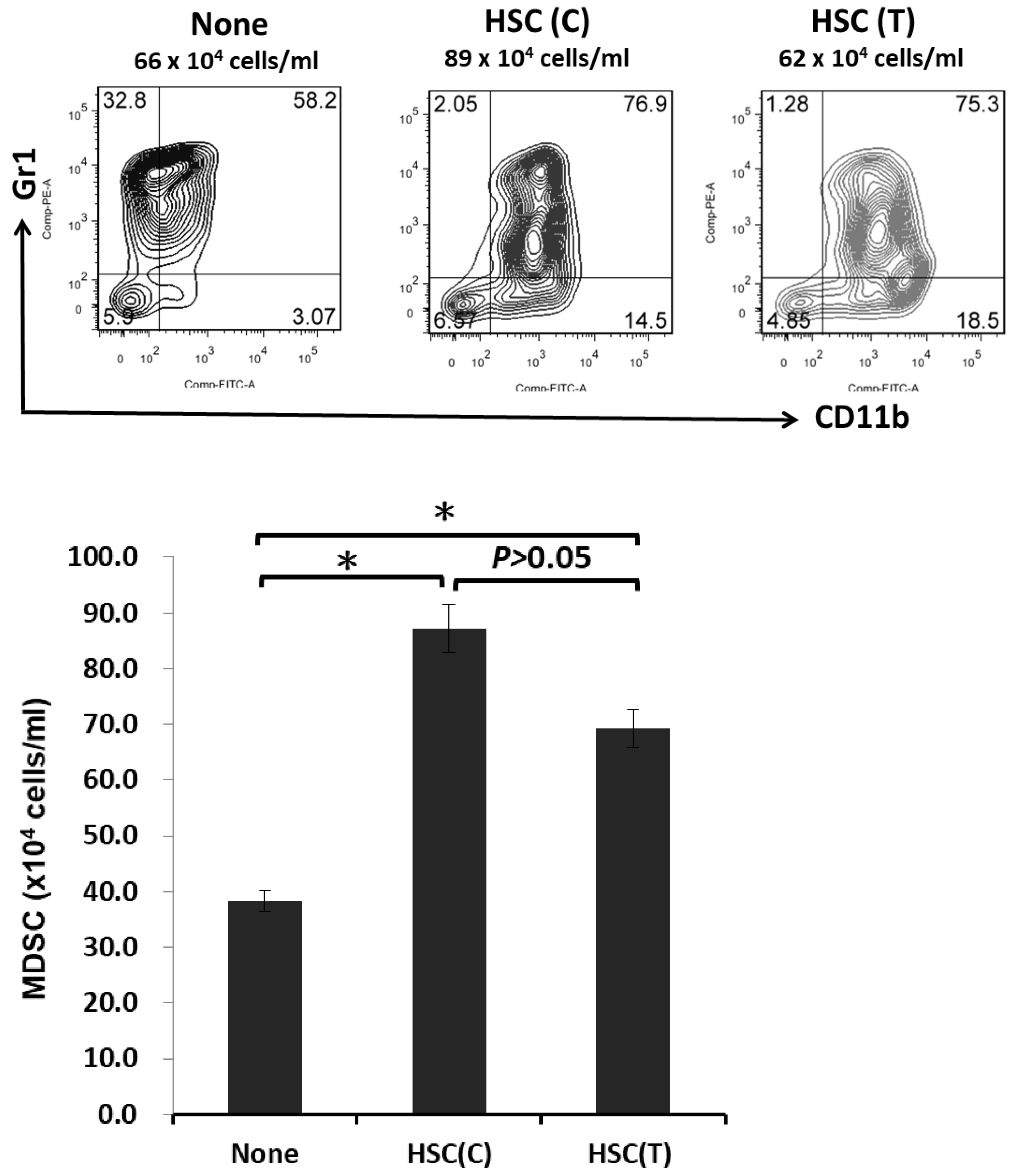
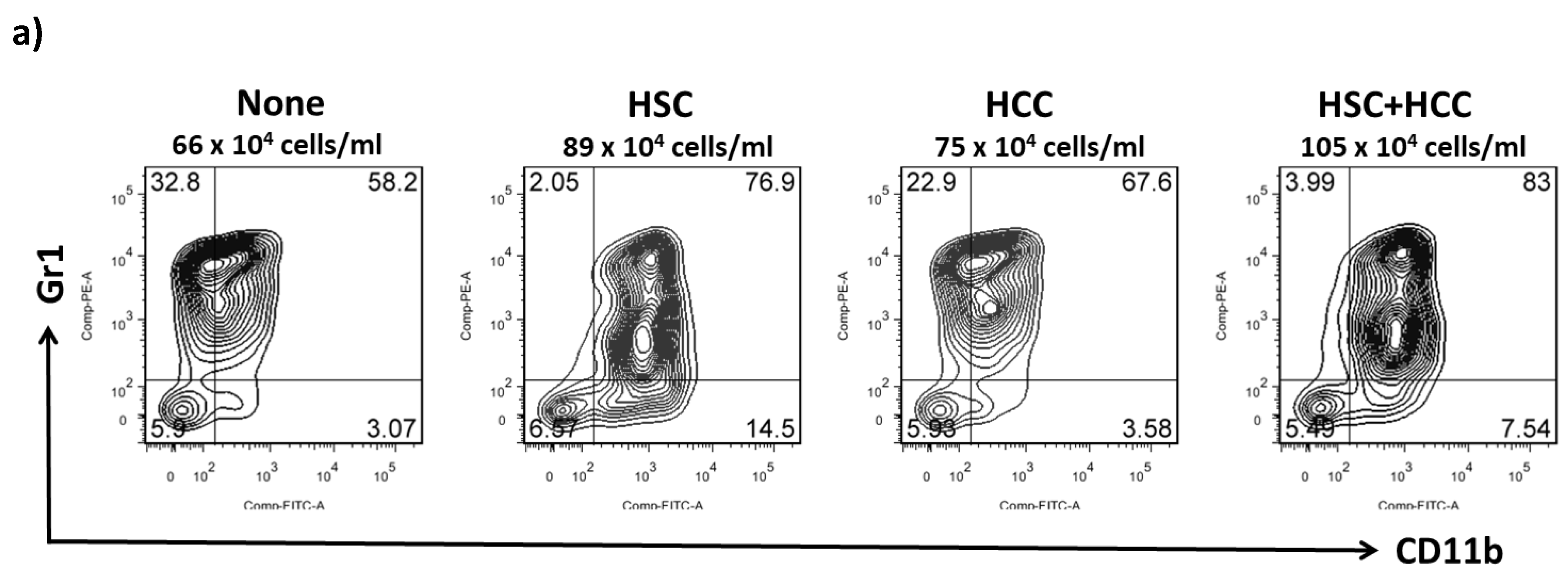
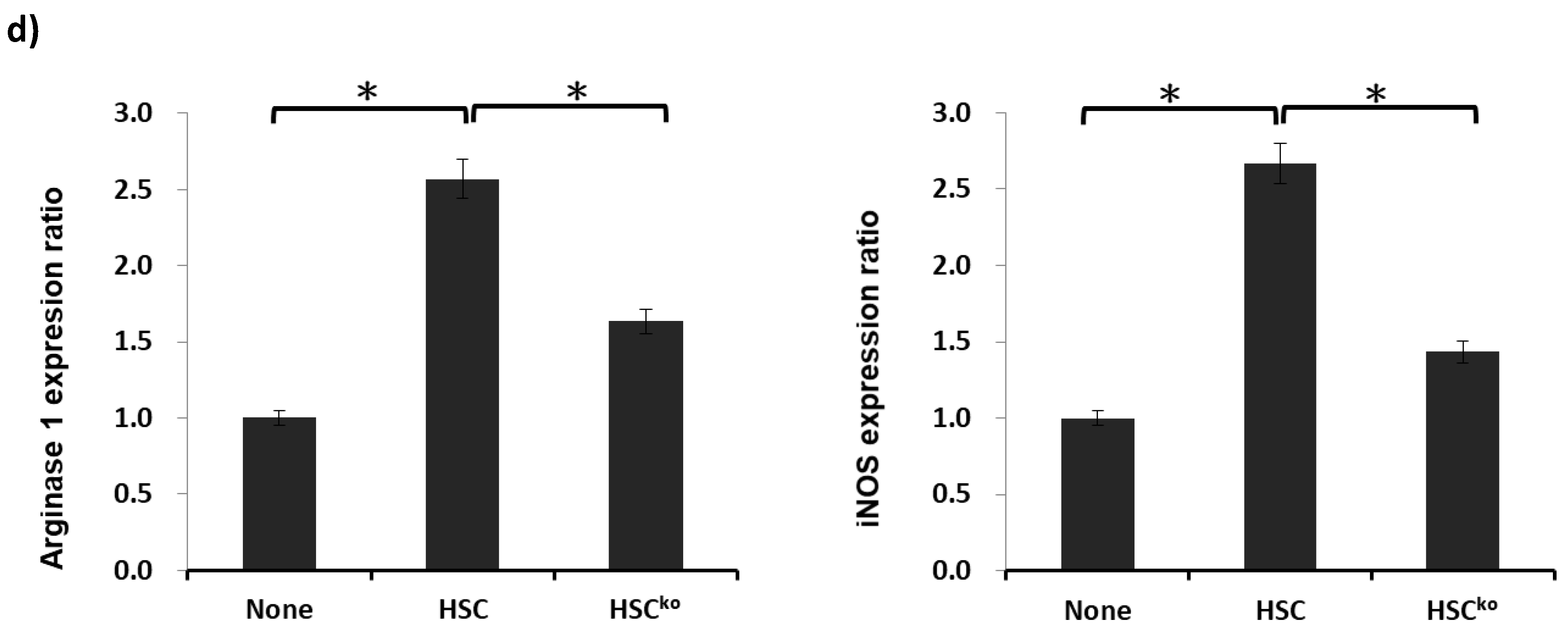
2.3. IL-6 Produced by HSCs Participates in the Development of MDSCs In Vitro
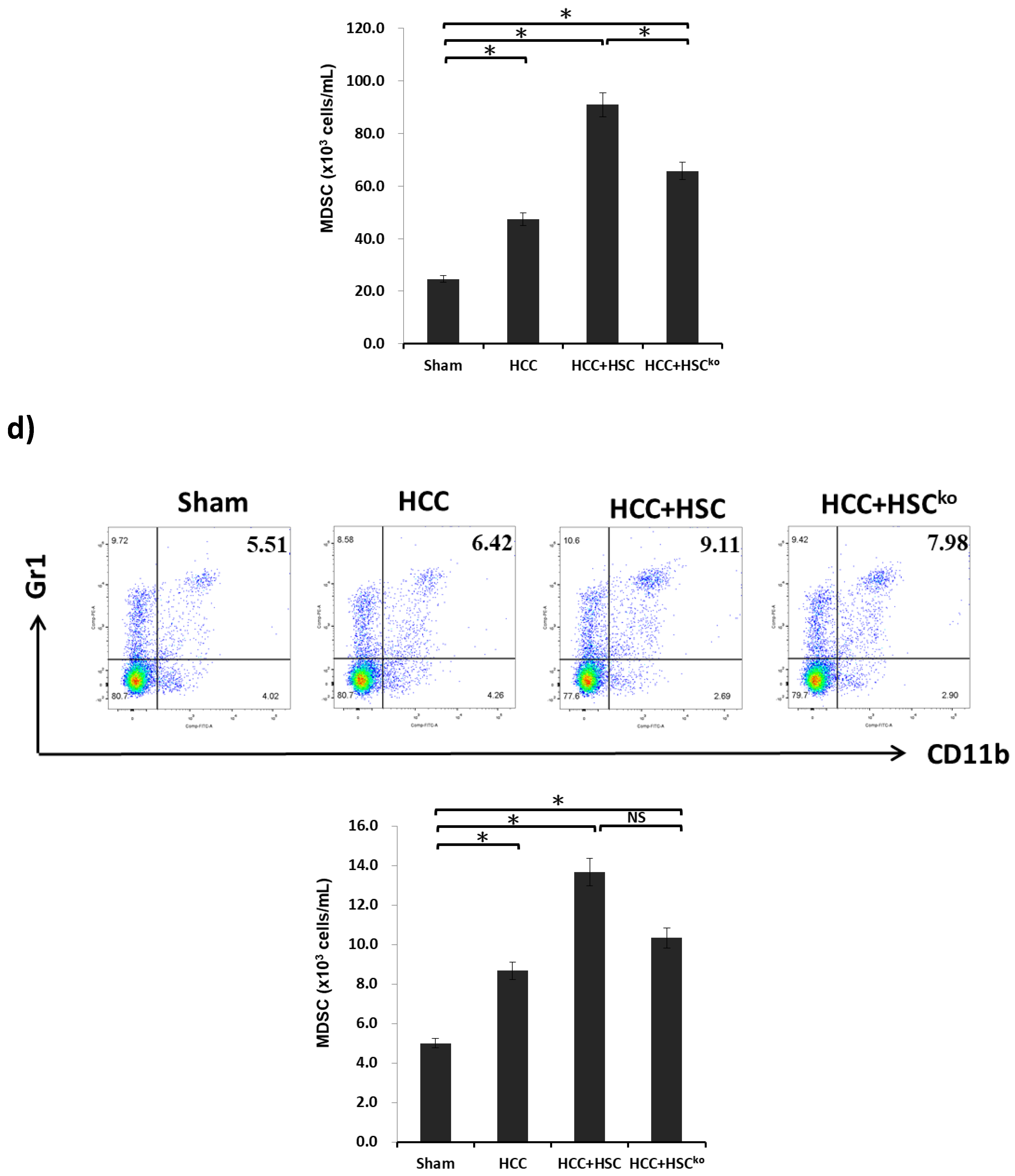
2.4. IL-6 Secreted from HSCs Expands the Population of MDSCs to Promote HCC Growth In Vivo
3. Discussion
4. Materials and Methods
4.1. Animals and Cell Line
4.2. Preparation of HSCs
4.3. Culture of DCs, MDSCs, and HSC-Conditioned MDSCs
4.4. Orthotopic Hepatic Tumor Inoculation Model
4.5. Tumor Area Calculation
4.6. Flow Cytometry Analysis
4.7. Quantitative Reverse Transcription Polymerase Chain Reaction
4.8. Immunohistochemistry Staining
4.9. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| B7H1 | B7 homolog 1 |
| BM | Bone marrow |
| C3 | Complement factor 3 |
| CD | Cluster of differentiation |
| CFSE | Carboxyfluorescein succinimidyl ester |
| DC | Dendritic cell |
| ELISA | Enzyme-linked immunosorbent assay |
| FOXp3 | Forkhead box p3 |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| G-MDSC | Granulocytic MDSC |
| HCC | Hepatocellular carcinoma |
| H-MO | HSC-conditioned monocyte |
| HSC | Hepatic stellate cell |
| HSCko | IL-6 knockout HSC |
| IFNγ | Interferon gamma |
| IL | Interleukin |
| IMC | Immature myeloid cells |
| iNOS | Inducible NO synthase |
| mAb | Monoclonal Ab |
| MDSC | Myeloid-derived suppressor cell |
| M-MDSC | Monocytic MDSC |
| PI | Propidium iodide |
| ROI | Regions of interest |
| Treg | Regulatory T-cell |
| WT | Wild type |
Appendix A
References
- Tlsty, T.D.; Coussens, L.M. Tumor stroma and regulation of cancer development. Annu. Rev. Pathol. 2006, 1, 119–150. [Google Scholar] [CrossRef] [PubMed]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J. Biol. Chem. 2000, 275, 2247–2250. [Google Scholar] [CrossRef]
- Schuppan, D.; Porov, Y. Hepatic fibrosis: From bench to bedside. J. Gastroenterol. Hepatol. 2002, 17, S300–S305. [Google Scholar] [CrossRef] [PubMed]
- Rockey, D.C. The cellular pathogenesis of portal hypertension: Stellate cell contractility, endothelin, and nitric oxide. Hepatology 1997, 25, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.C.; Chen, C.H.; Liang, X.; Wang, L.; Gandhi, C.R.; Fung, J.J.; Lu, L.; Qian, S. Inhibition of T-cell responses by hepatic stellate cells via B7-H1 mediated T-cell apoptosis. Hepatology 2004, 40, 1312–1321. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Yang, H.R.; Wang, L.; Wildey, G.M.; Fung, J.J.; Qian, S.; Lu, L. Hepatic stellate cells preferentially expand allogeneic CD4+CD25+FoxP3+ regulatory T-cells in an IL-2-dependent manner. Transplantation 2008, 86, 1492–1502. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.S.; Hsieh, C.C.; Charles, R.; Wang, L.; Wagner, T.; Fung, J.J.; Qian, S.; Lu, L. Myeloid-derived suppressor cells protect islet transplants by B7-H1 mediated enhancement of T regulatory cells. Transplantation 2012, 93, 272–282. [Google Scholar] [CrossRef]
- Hsieh, C.C.; Chou, H.S.; Yang, H.R.; Lin, F.; Bhatt, S.; Qin, J.; Wang, L.; Fung, J.J.; Qian, S.; Lu, L. The role of complement component 3 (C3) in differentiation of myeloid-derived suppressor cells. Blood 2013, 121, 1760–1768. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Ormandy, L.A.; Hillemann, T.; Wedemeyer, H.; Manns, M.P.; Greten, T.F.; Korangy, F. Increased populations of regulatory T-cells in peripheral blood of patients with hepatocellular carcinoma. Cancer Res. 2005, 65, 2457–2464. [Google Scholar] [CrossRef] [PubMed]
- Hoechst, B.; Ormandy, L.A.; Ballmaier, M.; Lehner, F.; Kruger, C.; Manns, M.P.; Greten, T.F.; Korangy, F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4+CD25+Foxp3+ T-cells. Gastroenterology 2008, 135, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T. Interleukin-6: From basic science to medicine—40 years in immunology. Annu. Rev. Immunol. 2005, 23, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Schafer, Z.T.; Brugge, J.S. IL-6 involvement in epithelial cancers. J. Clin. Invest. 2007, 117, 3660–3663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Orlando, G.; Soker, S.; Wood, K. Operational tolerance after liver transplantation. J. Hepatol. 2009, 50, 1247–1257. [Google Scholar] [CrossRef]
- Chen, C.H.; Kuo, L.M.; Chang, Y.; Wu, W.; Goldbach, C.; Ross, M.A.; Stolz, D.B.; Chen, L.; Fung, J.J.; Lu, L.; et al. In vivo immune modulatory activity of hepatic stellate cells in mice. Hepatology 2006, 44, 1171–1181. [Google Scholar] [CrossRef]
- Yang, H.R.; Chou, H.S.; Gu, X.; Wang, L.; Brown, K.E.; Fung, J.J.; Lu, L.; Qian, S. Mechanistic insights into immunomodulation by hepatic stellate cells in mice: A critical role of interferon-c signaling. Hepatology 2009, 50, 1981–1991. [Google Scholar] [CrossRef]
- Wang, C.Q.; Sun, H.T.; Gao, X.M.; Ren, N.; Sheng, Y.Y.; Wang, Z.; Zheng, Y.; Wei, J.W.; Zhang, K.L.; Yu, X.X.; et al. Interleukin-6 enhances cancer stemness and promotes metastasis of hepatocellular carcinoma via up-regulating osteopontin expression. Am. J. Cancer Res. 2016, 6, 1873–1889. [Google Scholar]
- Xu, Y.; Zhao, W.; Xu, J.; Li, J.; Hong, Z.; Yin, Z.; Wang, X. Activated hepatic stellate cells promote liver cancer by induction of myeloid-derived suppressor cells through cyclooxygenase-2. Oncotarget 2016, 7, 8866–8878. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kusmartsev, S.; Eruslanov, E.; Kübler, H.; Tseng, T.; Sakai, Y.; Su, Z.; Kaliberov, S.; Heiser, A.; Rosser, C.; Dahm, P.; et al. Oxidative stress regulates expression of VEGFR1 in myeloid cells: Link to tumor-induced immune suppression in renal cell carcinoma. J. Immunol. 2008, 181, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Markiewski, M.M.; DeAngelis, R.A.; Benencia, F.; Ricklin-Lichtsteiner, S.K.; Koutoulaki, A.; Gerard, C.; Coukos, G.; Lambris, J.D. Modulation of the antitumor immune response by complement. Nat. Immunol. 2008, 9, 1225–1235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunt, S.K.; Yang, L.; Sinha, P.; Clements, V.K.; Leips, J.; Ostrand-Rosenberg, S. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 2007, 67, 10019–10026. [Google Scholar] [CrossRef]
- Yang, L.; DeBusk, L.M.; Fukuda, K.; Fingleton, B.; Green-Jarvis, B.; Shyr, Y.; Matrisian, L.M.; Carbone, D.P.; Lin, P.C. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 2004, 6, 409–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinha, P.; Clements, V.K.; Bunt, S.K.; Albelda, S.M.; Ostrand-Rosenberg, S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J. Immunol. 2007, 179, 977–983. [Google Scholar] [CrossRef]
- Pan, P.Y.; Wang, G.X.; Yin, B.; Ozao, J.; Ku, T.; Divino, C.M.; Chen, S.H. Reversion of immune tolerance in advanced malignancy: Modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood 2008, 111, 219–228. [Google Scholar] [CrossRef]
- Serafini, P.; Carbley, R.; Noonan, K.A.; Tan, G.; Bronte, V.; Borrello, I. High-Dose GM-CSF-Producing Vaccines Impair the Immune Response Through the Recruitment of Myeloid Suppressor Cells. Cancer Res. 2004, 64, 6337–6343. [Google Scholar] [CrossRef]
- Gabrilovich, D.; Ishida, T.; Oyama, T.; Ran, S.; Kravtsov, V.; Nadaf, S.; Carbone, D.P. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 1998, 92, 4150–4166. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Hernandez, C.P.; Quiceno, D.; Dubinett, S.M.; Zabaleta, J.; Ochoa, J.B.; Gilbert, J.; Ochoa, A.C. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J. Exp. Med. 2005, 202, 931–939. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, P.C.; Ochoa, A.C. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: Mechanisms and therapeutic perspectives. Immunol. Rev. 2008, 222, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.K.; Sinha, P.; Clements, V.K.; Rodriguez, P.; Ostrand-Rosenberg, S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010, 70, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Talmadge, J.E.; Gabrilovich, D.I. History of myeloid-derived suppressor cells. Nat. Rev. Cancer 2013, 13, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N. Engl. J. Med. 1993, 328, 1828–1835. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Rudert, W.A.; Qian, S.; McCaslin, D.; Fu, F.; Rao, A.S.; Trucco, M.; Fung, J.J.; Starzl, T.E.; Thomson, A.W. Growth of donor-derived dendritic cells from the bone marrow of murine liver allograft recipients in response to granulocyte/macrophage colony-stimulating factor. J. Exp. Med. 1995, 182, 379–387. [Google Scholar] [CrossRef]
- Lu, L.; McCaslin, D.; Starzl, T.E.; Thomson, A.W. Bone marrow-derived dendritic cell progenitors (NLDCs 145+, MHC class II+, B7-1dim. B7-2-) induce alloantigen-specific hyporesponsiveness in murine T lymphocytes. Transplantation 1995, 60, 1539–1545. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, C.-C.; Hung, C.-H.; Chiang, M.; Tsai, Y.-C.; He, J.-T. Hepatic Stellate Cells Enhance Liver Cancer Progression by Inducing Myeloid-Derived Suppressor Cells through Interleukin-6 Signaling. Int. J. Mol. Sci. 2019, 20, 5079. https://doi.org/10.3390/ijms20205079
Hsieh C-C, Hung C-H, Chiang M, Tsai Y-C, He J-T. Hepatic Stellate Cells Enhance Liver Cancer Progression by Inducing Myeloid-Derived Suppressor Cells through Interleukin-6 Signaling. International Journal of Molecular Sciences. 2019; 20(20):5079. https://doi.org/10.3390/ijms20205079
Chicago/Turabian StyleHsieh, Ching-Chuan, Chien-Hui Hung, Meihua Chiang, Yu-Chin Tsai, and Jie-Teng He. 2019. "Hepatic Stellate Cells Enhance Liver Cancer Progression by Inducing Myeloid-Derived Suppressor Cells through Interleukin-6 Signaling" International Journal of Molecular Sciences 20, no. 20: 5079. https://doi.org/10.3390/ijms20205079




