The G9a Histone Methyltransferase Inhibitor BIX-01294 Modulates Gene Expression during Plasmodium falciparum Gametocyte Development and Transmission
Abstract
:1. Introduction
2. Results and Discussion
2.1. P. falciparum SET-Domain-Containing HMTs Are Expressed in Gametocytes
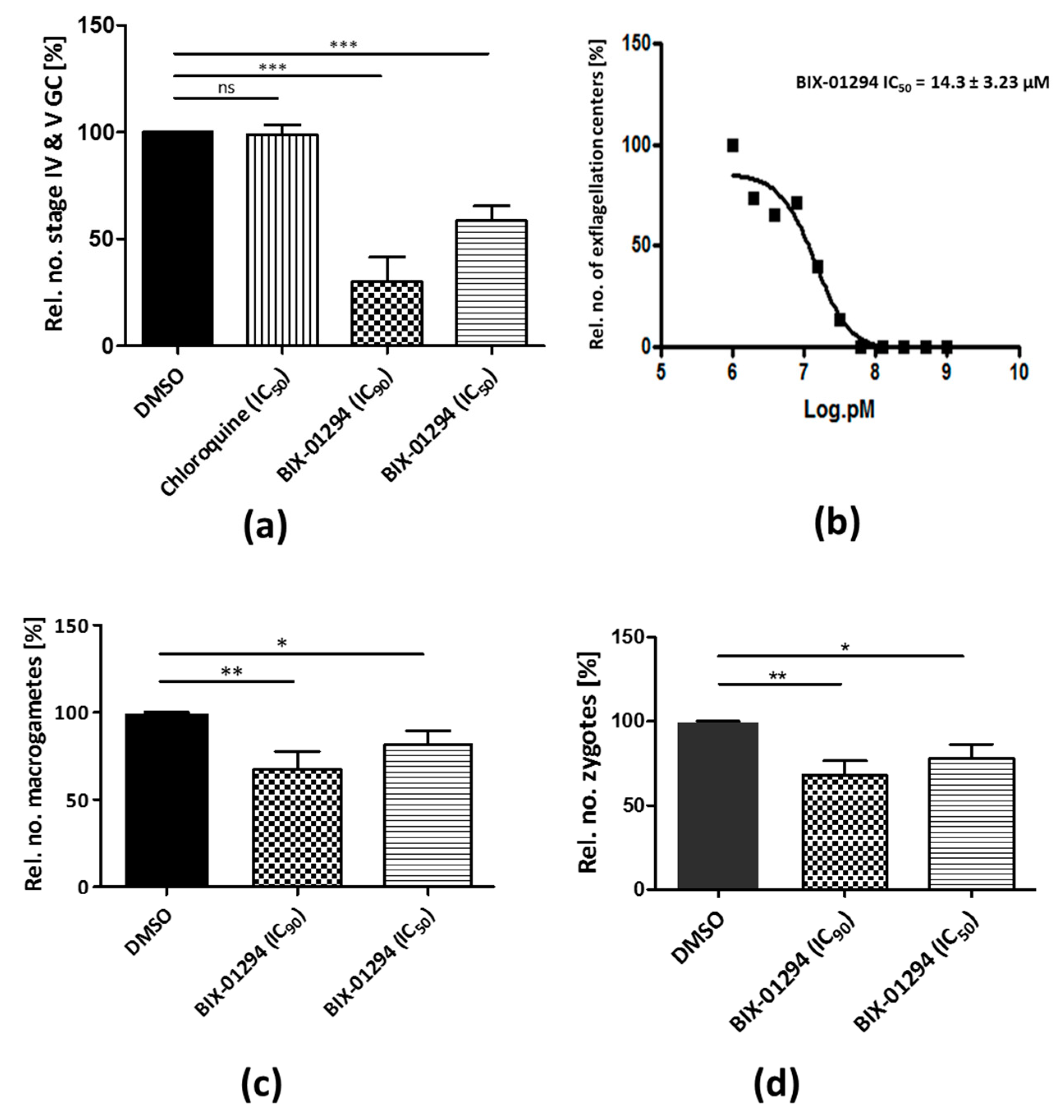
2.2. BIX-01294 Treatment Impairs P. falciparum Asexual Blood Stage Replication and Sexual Development
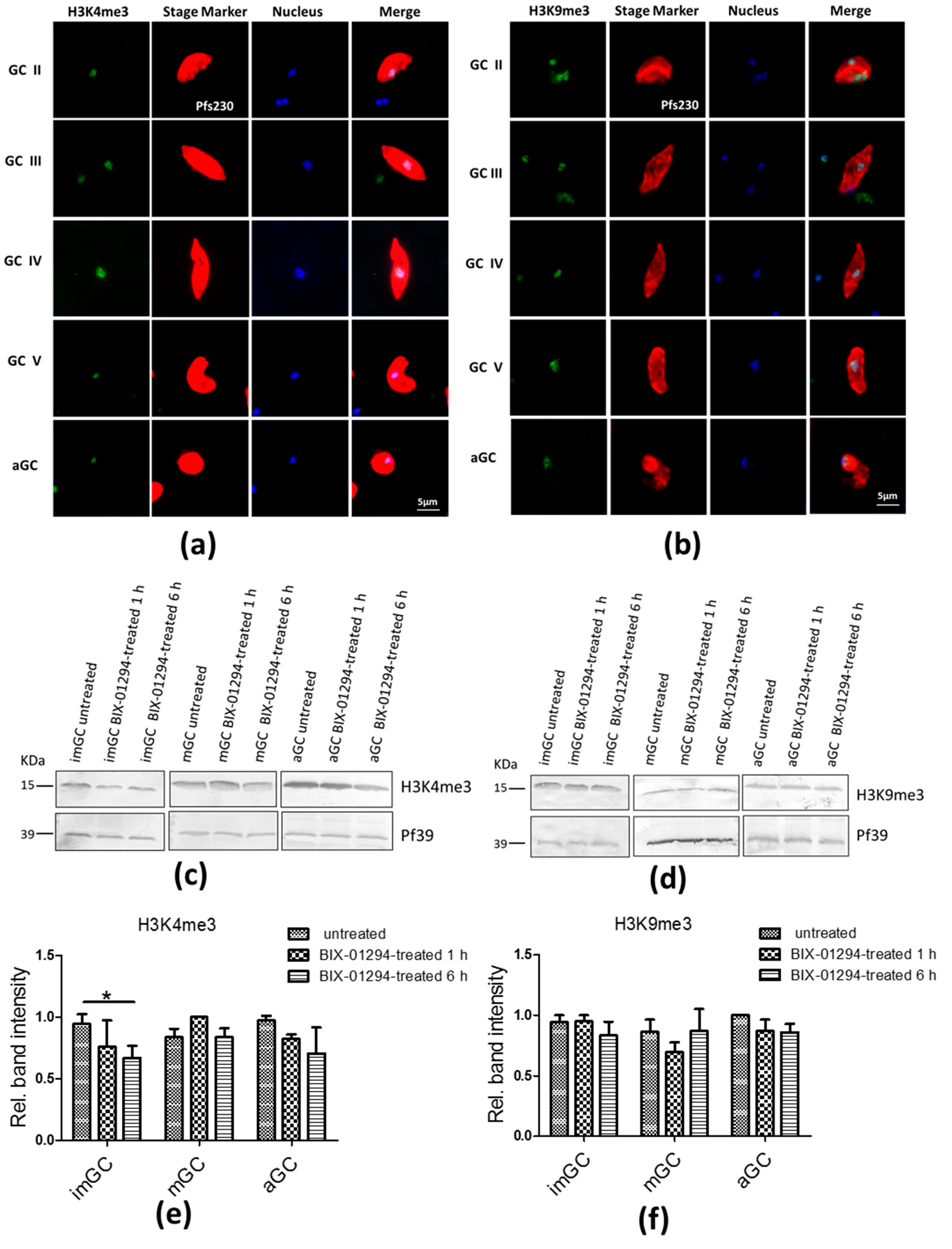
2.3. Treatment of Gametocytes with BIX-01294 Slightly Affects H3K4me3 and H3K9me3
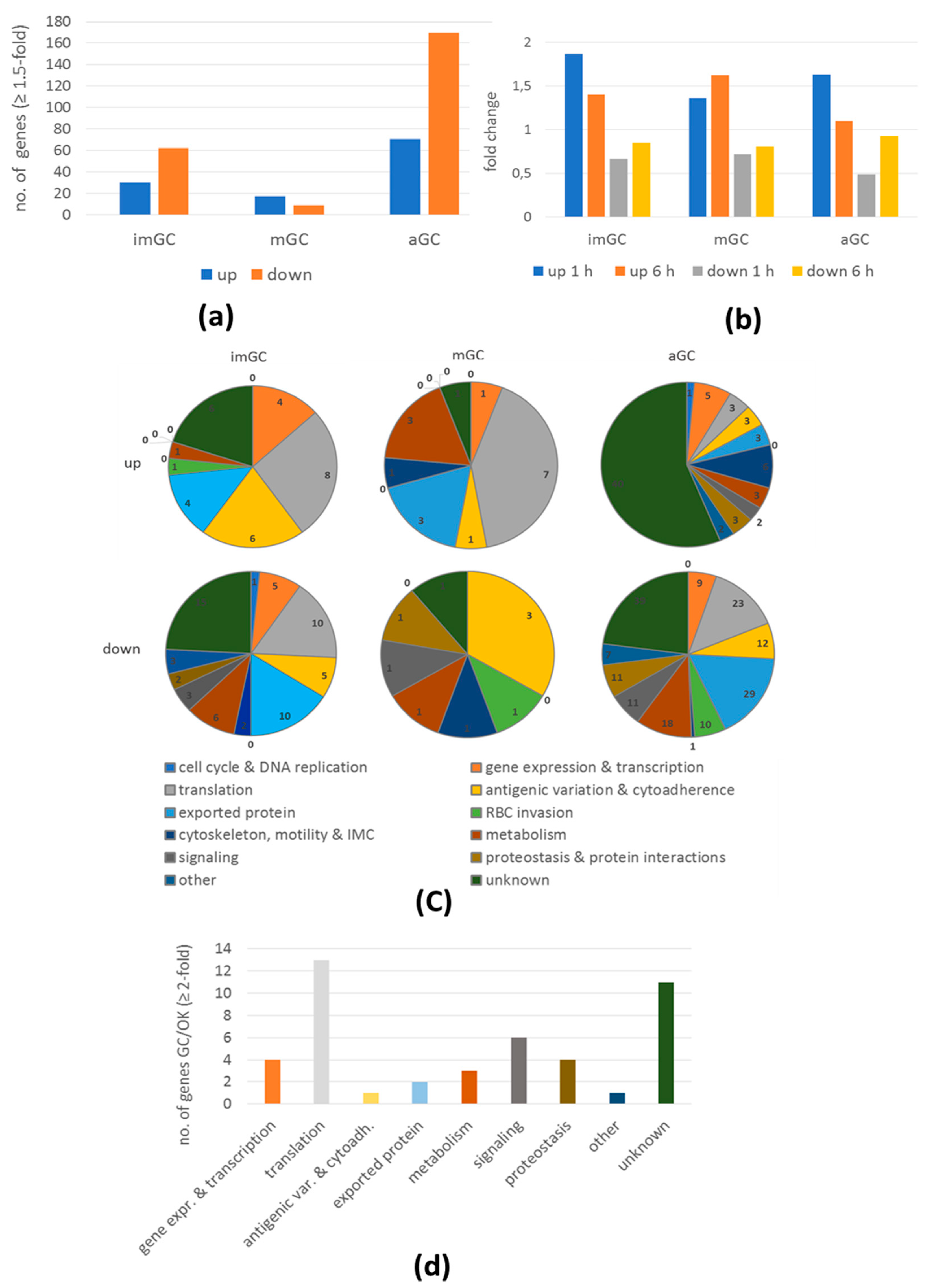
2.4. BIX-01294 Treatment Results in Modulation of Gene Expression During Gametocyte Development
3. Materials and Methods
3.1. Antibodies
3.2. Parasite Culture
3.3. Malstat Assay
3.4. Gametocyte Toxicity Test
3.5. Exflagellation Inhibition Assay
3.6. Macrogamete and Zygote Development Assays
3.7. Indirect Immunofluorescence Assay
3.8. Western Blot Analysis
3.9. Histone Methylation Detection
3.10. Microarray Analysis
3.11. Semi- Quantitative and Real Time RT-PCR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| aGC | Activated gametocytes |
| GC | Gametocytes |
| HAT | Histone acetyltransferase |
| LD | Linear dichroism |
| HDAC | Histone deacetylase |
| HDM | Histone demethylase |
| HMT | Histone methyl transferase |
| imGC | Immature gametocytes |
| mGC | Mature gametocytes |
| NRS | Normal rabbit serum |
| TSA | Trischostatin A |
| XA | Xanthurenic acid |
References
- World Health Organization. World Malaria Report 2018; WHO Press: Geneva, Switzerland, 2018. [Google Scholar]
- Kuehn, A.; Pradel, G. The coming-out of malaria gametocytes. J. Biomed. Biotechnol. 2010, 2010, 976827. [Google Scholar] [CrossRef] [PubMed]
- Bennink, S.; Kiesow, M.J.; Pradel, G. The development of malaria parasites in the mosquito midgut. Cell. Microbiol. 2016, 18, 905–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngwa, C.J.; Scheuermayer, M.; Mair, G.R.; Kern, S.; Brügl, T.; Wirth, C.C.; Aminake, M.N.; Wiesner, J.; Fischer, R.; Vilcinskas, A.; et al. Changes in the transcriptome of the malaria parasite Plasmodium falciparum during the initial phase of transmission from the human to the mosquito. BMC Genom. 2013, 14, 256. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, C.J.; Kiesow, M.J.; Papst, O.; Orchard, L.M.; Filarsky, M.; Rosinski, A.N.; Voss, T.S.; Llinás, M.; Pradel, G. Transcriptional Profiling Defines Histone Acetylation as a Regulator of Gene Expression during Human-to-Mosquito Transmission of the Malaria Parasite Plasmodium falciparum. Front. Cell. Infect. Microbiol. 2017, 7, 320. [Google Scholar] [CrossRef] [PubMed]
- López-Barragán, M.J.; Lemieux, J.; Quiñones, M.; Williamson, K.C.; Molina-Cruz, A.; Cui, K.; Barillas-Mury, C.; Zhao, K.; Su, X. Directional gene expression and antisense transcripts in sexual and asexual stages of Plasmodium falciparum. BMC Genom. 2011, 12, 587. [Google Scholar] [CrossRef] [PubMed]
- Sautel, C.F.; Cannella, D.; Bastien, O.; Kieffer, S.; Aldebert, D.; Garin, J.; Tardieux, I.; Belrhali, H.; Hakimi, M.-A. SET8-Mediated Methylations of Histone H4 Lysine 20 Mark Silent Heterochromatic Domains in Apicomplexan Genomes. Mol. Cell. Biol. 2007, 27, 5711. [Google Scholar] [CrossRef]
- Greer, E.L.; Shi, Y. Histone methylation: A dynamic mark in health, disease and inheritance. Nat. Rev. Genet. 2012, 13, 343–357. [Google Scholar] [CrossRef]
- Abel, S.; Le Roch, K.G.; Roch, K. Le The role of epigenetics and chromatin structure in transcriptional regulation in malaria parasites. Brief. Funct. Genomics 2019, 1–12. [Google Scholar]
- Joshi, M.B.; Lin, D.T.; Chiang, P.H.; Goldman, N.D.; Fujioka, H.; Aikawa, M.; Syin, C. Molecular cloning and nuclear localization of a histone deacetylase homologue in Plasmodium falciparum. Mol. Biochem. Parasitol. 1999, 99, 11–19. [Google Scholar] [CrossRef]
- Gardner, M.; Hall, N.; Fung, E.; White, O.; Berriman, M.; Hyman, R.; Carlton, J.; Pain, A.; Nelson, K.; Bowman, S.; et al. Genome sequence of the human malaria parasite Plasmodium falciparum. Nature 2002, 419, 498–511. [Google Scholar] [CrossRef]
- Cui, L.; Miao, J. Chromatin-Mediated Epigenetic Regulation in the Malaria Parasite Plasmodium falciparum. Eukaryot. Cell 2010, 9, 1138–1149. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Miao, J.; Furuya, T.; Li, X.; Su, X.Z.; Cui, L. PfGCN5-mediated histone H3 acetylation plays a key role in gene expression in Plasmodium falciparum. Eukaryot. Cell 2007, 6, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Fan, Q.; Cui, L.; Li, X.; Wang, H.; Ning, G.; Reese, J.C.; Cui, L. The MYST family histone acetyltransferase regulates gene expression and cell cycle in malaria parasite Plasmodium falciparum. Mol. Microbiol. 2010, 78, 883–902. [Google Scholar] [CrossRef] [PubMed]
- Cui, L.; Fan, Q.; Cui, L.; Miao, J. Histone lysine methyltransferases and demethylases in Plasmodium falciparum. Int. J. Parasitol. 2008, 38, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Volz, J.; Carvalho, T.G.; Ralph, S.A.; Gilson, P.; Thompson, J.; Tonkin, C.J.; Langer, C.; Crabb, B.S.; Cowman, A.F. Potential epigenetic regulatory proteins localise to distinct nuclear sub-compartments in Plasmodium falciparum. Int. J. Parasitol. 2010, 40, 109–121. [Google Scholar] [CrossRef]
- Fan, Q.; Miao, J.; Cui, L.; Cui, L. Characterization of PRMT1 from Plasmodium falciparum. Biochem. J. 2009, 421, 107–118. [Google Scholar] [CrossRef]
- Jiang, L.; Mu, J.; Zhang, Q.; Ni, T.; Srinivasan, P.; Rayavara, K.; Yang, W.; Turner, L.; Lavstsen, T.; Theander, T.G.; et al. PfSETvs methylation of histone H3K36 represses virulence genes in Plasmodium falciparum. Nature 2013, 499, 223–227. [Google Scholar] [CrossRef]
- Duffy, M.F.; Selvarajah, S.A.; Josling, G.A.; Petter, M. Epigenetic regulation of the Plasmodium falciparum genome. Brief. Funct. Genom. 2014, 13, 203–216. [Google Scholar] [CrossRef]
- Duraisingh, M.T.; Horn, D. Epigenetic Regulation of Virulence Gene Expression in Parasitic Protozoa. Cell Host Microbe 2016, 19, 629–640. [Google Scholar] [CrossRef] [Green Version]
- Petter, M.; Lee, C.C.; Byrne, T.J.; Boysen, K.E.; Volz, J.; Ralph, S.A.; Cowman, A.F.; Brown, G.V.; Duffy, M.F. Expression of P. falciparum var genes involves exchange of the histone variant H2A.Z at the promoter. PLoS Pathog. 2011, 7, e1001292. [Google Scholar] [CrossRef]
- Llinás, M.; Deitsch, K.W.; Voss, T.S. Plasmodium gene regulation: Far more to factor in. Trends Parasitol. 2008, 24, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rubio, J.J.; Riviere, L.; Scherf, A. Shared epigenetic mechanisms control virulence factors in protozoan parasites. Curr. Opin. Microbiol. 2007, 10, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Rubio, J.J.; Gontijo, A.M.; Nunes, M.C.; Issar, N.; Hernandez Rivas, R.; Scherf, A. 5′ flanking region of var genes nucleate histone modification patterns linked to phenotypic inheritance of virulence traits in malaria parasites. Mol. Microbiol. 2007, 66, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Salcedo-Amaya, A.M.; van Driel, M.A.; Alako, B.T.; Trelle, M.B.; van den Elzen, A.M.G.; Cohen, A.M.; Janssen-Megens, E.M.; van de Vegte-Bolmer, M.; Selzer, R.R.; Iniguez, A.L.; et al. Dynamic histone H3 epigenome marking during the intraerythrocytic cycle of Plasmodium falciparum. Proc. Natl. Acad. Sci. 2009, 106, 9655–9660. [Google Scholar] [CrossRef] [PubMed]
- Duraisingh, M.T.; Voss, T.S.; Marty, A.J.; Duffy, M.F.; Good, R.T.; Thompson, J.K.; Freitas-Junior, L.H.; Scherf, A.; Crabb, B.S.; Cowman, A.F. Heterochromatin Silencing and Locus Repositioning Linked to Regulation of Virulence Genes in Plasmodium falciparum. Cell 2005, 121, 13–24. [Google Scholar] [CrossRef]
- Freitas-Junior, L.H.; Hernandez-Rivas, R.; Ralph, S.A.; Montiel-Condado, D.; Ruvalcaba-Salazar, O.K.; Rojas-Meza, A.P.; Mâncio-Silva, L.; Leal-Silvestre, R.J.; Gontijo, A.M.; Shorte, S.; et al. Telomeric Heterochromatin Propagation and Histone Acetylation Control Mutually Exclusive Expression of Antigenic Variation Genes in Malaria Parasites. Cell 2005, 121, 25–36. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Rubio, J.-J.; Mancio-Silva, L.; Scherf, A. Genome-wide analysis of heterochromatin associates clonally variant gene regulation with perinuclear repressive centers in malaria parasites. Cell Host Microbe 2009, 5, 179–190. [Google Scholar] [CrossRef]
- Tonkin, C.J.; Carret, C.K.; Duraisingh, M.T.; Voss, T.S.; Ralph, S.A.; Hommel, M.; Duffy, M.F.; da Silva, L.M.; Scherf, A.; Ivens, A.; et al. Sir2 paralogues cooperate to regulate virulence genes and antigenic variation in Plasmodium falciparum. PLoS Biol. 2009, 7, e84. [Google Scholar] [CrossRef]
- Josling, G.A.; Llinás, M. Sexual development in Plasmodium parasites: Knowing when it’s time to commit. Nat. Rev. Microbiol. 2015, 13, 573–587. [Google Scholar] [CrossRef]
- Kafsack, B.F.C.; Rovira-Graells, N.; Clark, T.G.; Bancells, C.; Crowley, V.M.; Campino, S.G.; Williams, A.E.; Drought, L.G.; Kwiatkowski, D.P.; Baker, D.A.; et al. A transcriptional switch underlies commitment to sexual development in malaria parasites. Nature 2014, 507, 248–252. [Google Scholar] [CrossRef] [Green Version]
- Sinha, A.; Hughes, K.R.; Modrzynska, K.K.; Otto, T.D.; Pfander, C.; Dickens, N.J.; Religa, A.R.; Bushell, E.; Graham, A.L.; Cameron, R.; et al. A cascade of DNA-binding proteins for sexual commitment and development in Plasmodium. Nature 2014, 507, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Voss, T.S.; Bozdech, Z.; Bártfai, R. Epigenetic memory takes center stage in the survival strategy of malaria parasites. Curr. Opin. Microbiol. 2014, 20, 88–95. [Google Scholar] [CrossRef]
- Brancucci, N.M.B.; Bertschi, N.L.; Zhu, L.; Niederwieser, I.; Chin, W.H.; Wampfler, R.; Rottmann, M.; Felger, I.; Bozdech, Z.; Voss, T.S. Heterochromatin Protein 1 Secures Survival and Transmission of Malaria Parasites. Cell Host Microbe 2014, 16, 165–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malmquist, N.A.; Moss, T.A.; Mecheri, S.; Scherf, A.; Fuchter, M.J. Small-molecule histone methyltransferase inhibitors display rapid antimalarial activity against all blood stage forms in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2012, 109, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Malmquist, N.A.; Sundriyal, S.; Caron, J.; Chen, P.; Witkowski, B.; Menard, D.; Suwanarusk, R.; Renia, L.; Nosten, F.; Jiménez-Díaz, M.B.; et al. Histone methyltransferase inhibitors are orally bioavailable, fast-acting molecules with activity against different species causing malaria in humans. Antimicrob. Agents Chemother. 2015, 59, 950–959. [Google Scholar] [CrossRef]
- Tachibana, M.; Sugimoto, K.; Nozaki, M.; Ueda, J.; Ohta, T.; Ohki, M.; Fukuda, M.; Takeda, N.; Niida, H.; Kato, H.; et al. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002, 16, 1779–1791. [Google Scholar] [CrossRef] [Green Version]
- Volz, J.C.; Bártfai, R.; Petter, M.; Langer, C.; Josling, G.A.; Tsuboi, T.; Schwach, F.; Baum, J.; Rayner, J.C.; Stunnenberg, H.G.; et al. PfSET10, a Plasmodium falciparum Methyltransferase, Maintains the Active var Gene in a Poised State during Parasite Division. Cell Host Microbe 2012, 11, 7–18. [Google Scholar] [CrossRef]
- Coetzee, N.; Sidoli, S.; van Biljon, R.; Painter, H.; Llinás, M.; Garcia, B.A.; Birkholtz, L.-M. Quantitative chromatin proteomics reveals a dynamic histone post-translational modification landscape that defines asexual and sexual Plasmodium falciparum parasites. Sci. Rep. 2017, 7, 607. [Google Scholar] [CrossRef]
- Bozdech, Z.; Llinás, M.; Pulliam, B.L.; Wong, E.D.; Zhu, J.; DeRisi, J.L. The transcriptome of the intraerythrocytic developmental cycle of Plasmodium falciparum. PLoS Biol. 2003, 1, 85–100. [Google Scholar] [CrossRef]
- Kafsack, B.F.C.; Painter, H.J.; Llinás, M. New Agilent platform DNA microarrays for transcriptome analysis of Plasmodium falciparum and Plasmodium berghei for the malaria research community. Malar. J. 2012, 11, 187. [Google Scholar] [CrossRef]
- Ciechomska, I.A.; Przanowski, P.; Jackl, J.; Wojtas, B.; Kaminska, B. BIX01294, an inhibitor of histone methyltransferase, induces autophagy-dependent differentiation of glioma stem-like cells. Nat. Publ. Gr. 2016, 6, 38723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maleszewska, M.; Steranka, A.; Kaminska, B. The effects of selected inhibitors of histone modifying enzyme on C6 glioma cells. Pharmcol. Rep. 2014, 66, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Völkel, P.; Angrand, P.-O. The control of histone lysine methylation in epigenetic regulation. Biochimie 2007, 89, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Mair, G.R. Regulation of Sexual Development of Plasmodium by Translational Repression. Science (80-. ). 2006, 313, 667–669. [Google Scholar] [CrossRef]
- Mair, G.R.; Lasonder, E.; Garver, L.S.; Franke-Fayard, B.M.D.; Carret, C.K.; Wiegant, J.C.A.G.; Dirks, R.W.; Dimopoulos, G.; Janse, C.J.; Waters, A.P. Universal features of post-transcriptional gene regulation are critical for Plasmodium zygote development. PLoS Pathog. 2010, 6, e1000767. [Google Scholar] [CrossRef]
- Miao, J.; Fan, Q.; Parker, D.; Li, X.; Li, J.; Cui, L. Puf mediates translation repression of transmission-blocking vaccine candidates in malaria parasites. PLoS Pathog. 2013, 9, e1003268. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Scholz, S.M.; Simon, N.; Lavazec, C.; Dude, M.A.; Templeton, T.J.; Pradel, G. PfCCp proteins of Plasmodium falciparum: Gametocyte-specific expression and role in complement-mediated inhibition of exflagellation. Int. J. Parasitol. 2008, 38, 327–340. [Google Scholar] [CrossRef]
- Ifediba, T.; Vanderberg, J.P. Complete in vitro maturation of Plasmodium falciparum gametocytes. Nature 1981, 294, 364–366. [Google Scholar] [CrossRef]
- Lambros, C.; Vanderberg, J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979, 65, 418–420. [Google Scholar] [CrossRef]
- Fivelman, Q.L.; McRobert, L.; Sharp, S.; Taylor, C.J.; Saeed, M.; Swales, C.A.; Sutherland, C.J.; Baker, D.A. Improved synchronous production of Plasmodium falciparum gametocytes in vitro. Mol. Biochem. Parasitol. 2007, 154, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Kariuki, M.M.; Kiaira, J.K.; Mulaa, F.K.; Mwangi, J.K.; Wasunna, M.K.; Martin, S.K. Plasmodium falciparum: Purification of the various gametocyte developmental stages from in vitro-cultivated parasites. Am. J. Trop. Med. Hyg. 1998, 59, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Aminake, M.N.; Schoof, S.; Sologub, L.; Leubner, M.; Kirschner, M.; Arndt, H.-D.; Pradel, G. Thiostrepton and Derivatives Exhibit Antimalarial and Gametocytocidal Activity by Dually Targeting Parasite Proteasome and Apicoplast. Antimicrob. Agents Chemother. 2011, 55, 1338–1348. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohrich, C.R.; Ngwa, C.J.; Wiesner, J.; Schmidtberg, H.; Degenkolb, T.; Kollewe, C.; Fischer, R.; Pradel, G.; Vilcinskas, A. Harmonine, a defence compound from the harlequin ladybird, inhibits mycobacterial growth and demonstrates multi-stage antimalarial activity. Biol. Lett. 2012, 8, 308–311. [Google Scholar] [CrossRef] [PubMed]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ngwa, C.J.; Kiesow, M.J.; Orchard, L.M.; Farrukh, A.; Llinás, M.; Pradel, G. The G9a Histone Methyltransferase Inhibitor BIX-01294 Modulates Gene Expression during Plasmodium falciparum Gametocyte Development and Transmission. Int. J. Mol. Sci. 2019, 20, 5087. https://doi.org/10.3390/ijms20205087
Ngwa CJ, Kiesow MJ, Orchard LM, Farrukh A, Llinás M, Pradel G. The G9a Histone Methyltransferase Inhibitor BIX-01294 Modulates Gene Expression during Plasmodium falciparum Gametocyte Development and Transmission. International Journal of Molecular Sciences. 2019; 20(20):5087. https://doi.org/10.3390/ijms20205087
Chicago/Turabian StyleNgwa, Che Julius, Meike Jutta Kiesow, Lindsey Marie Orchard, Afia Farrukh, Manuel Llinás, and Gabriele Pradel. 2019. "The G9a Histone Methyltransferase Inhibitor BIX-01294 Modulates Gene Expression during Plasmodium falciparum Gametocyte Development and Transmission" International Journal of Molecular Sciences 20, no. 20: 5087. https://doi.org/10.3390/ijms20205087




