Novel Pituitary Actions of Epidermal Growth Factor: Receptor Specificity and Signal Transduction for UTS1, EGR1, and MMP13 Regulation by EGF
Abstract
1. Introduction
2. Results
2.1. Transcriptomic Analysis
2.2. EGF-Induced Critical DEGs in Grass Carp Pituitary Cells
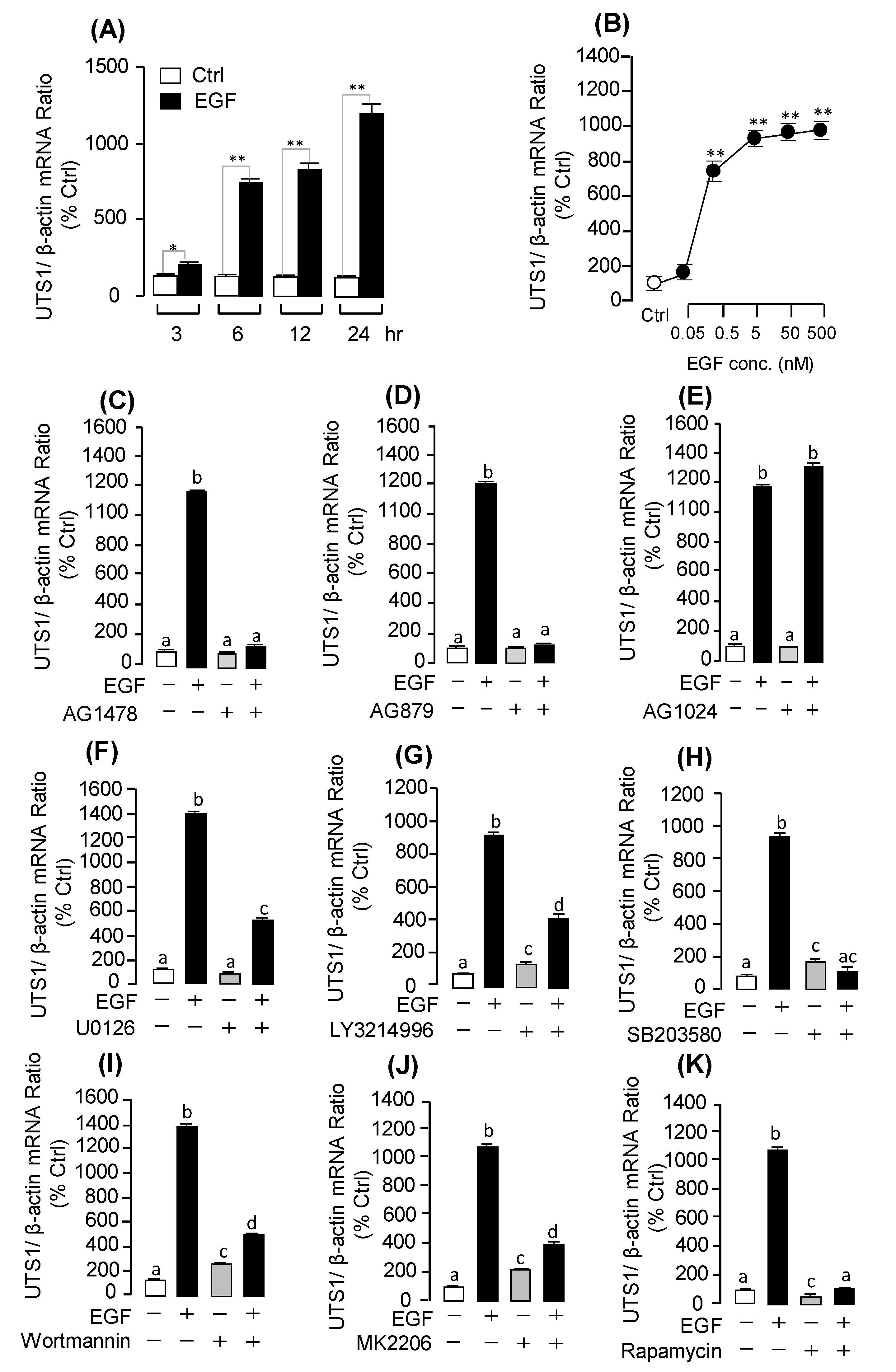
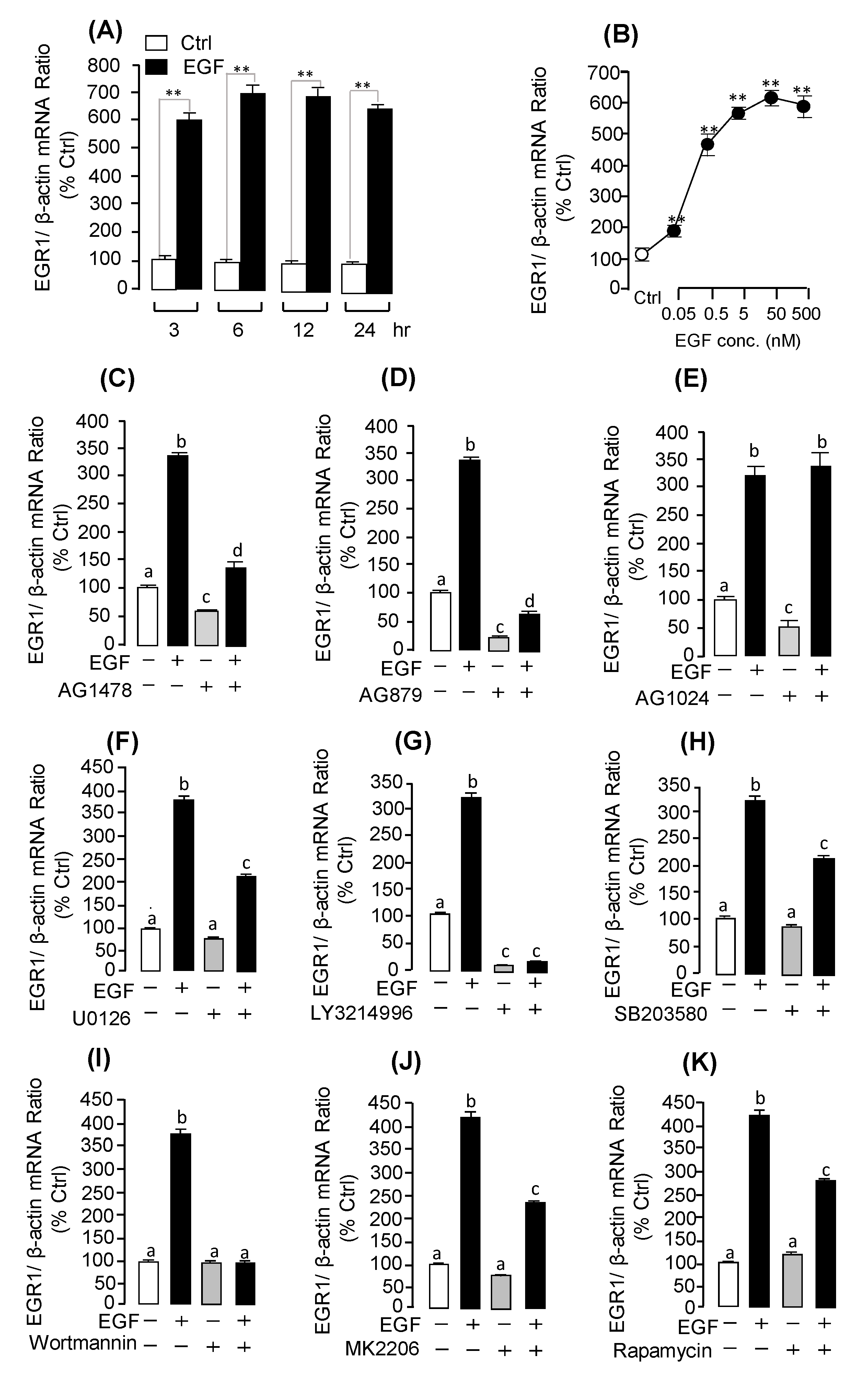
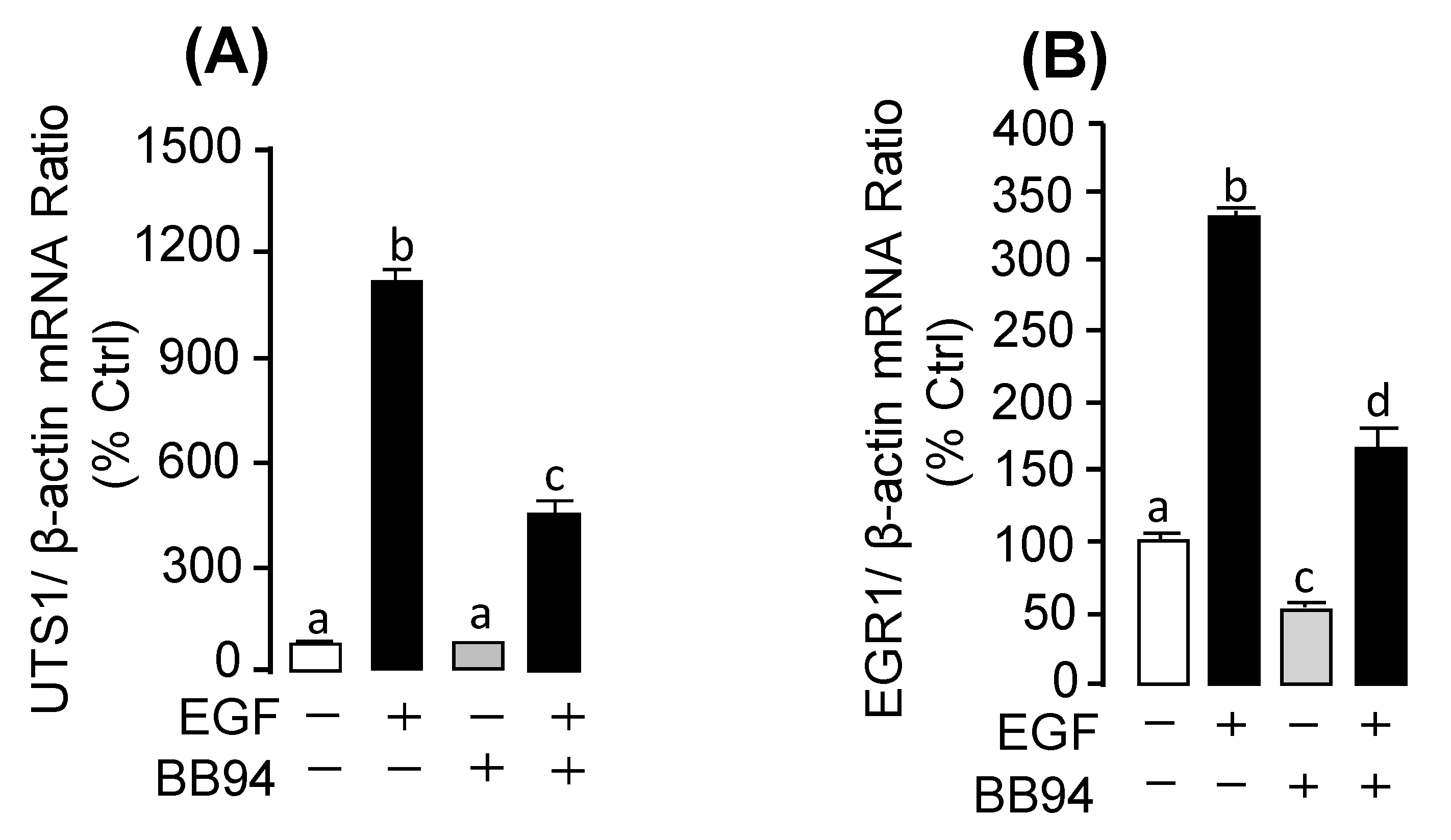
2.3. Receptor Specificity and Signal Transduction for EGF-Induced UTS1 and EGR1 Gene Expression
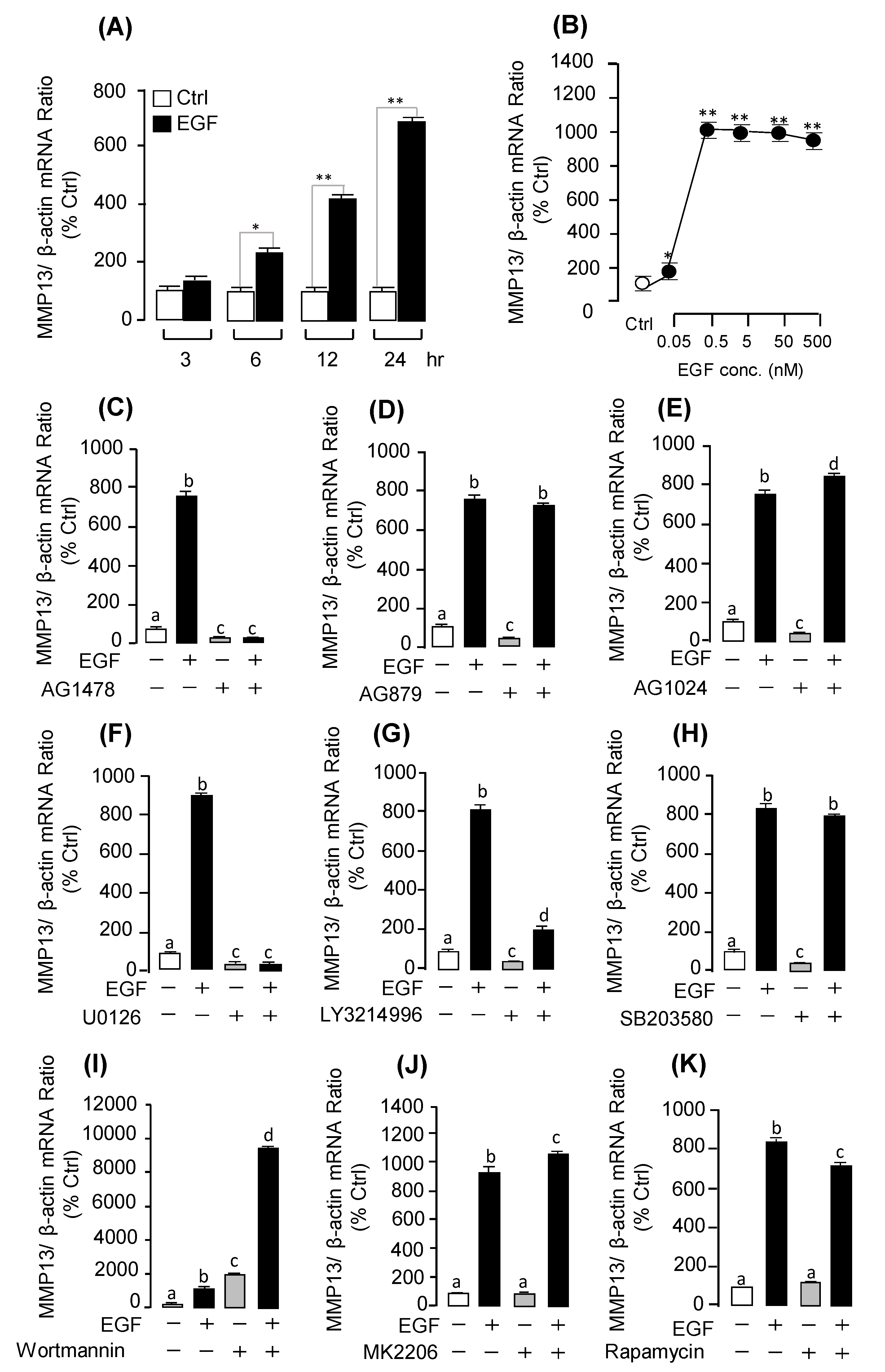
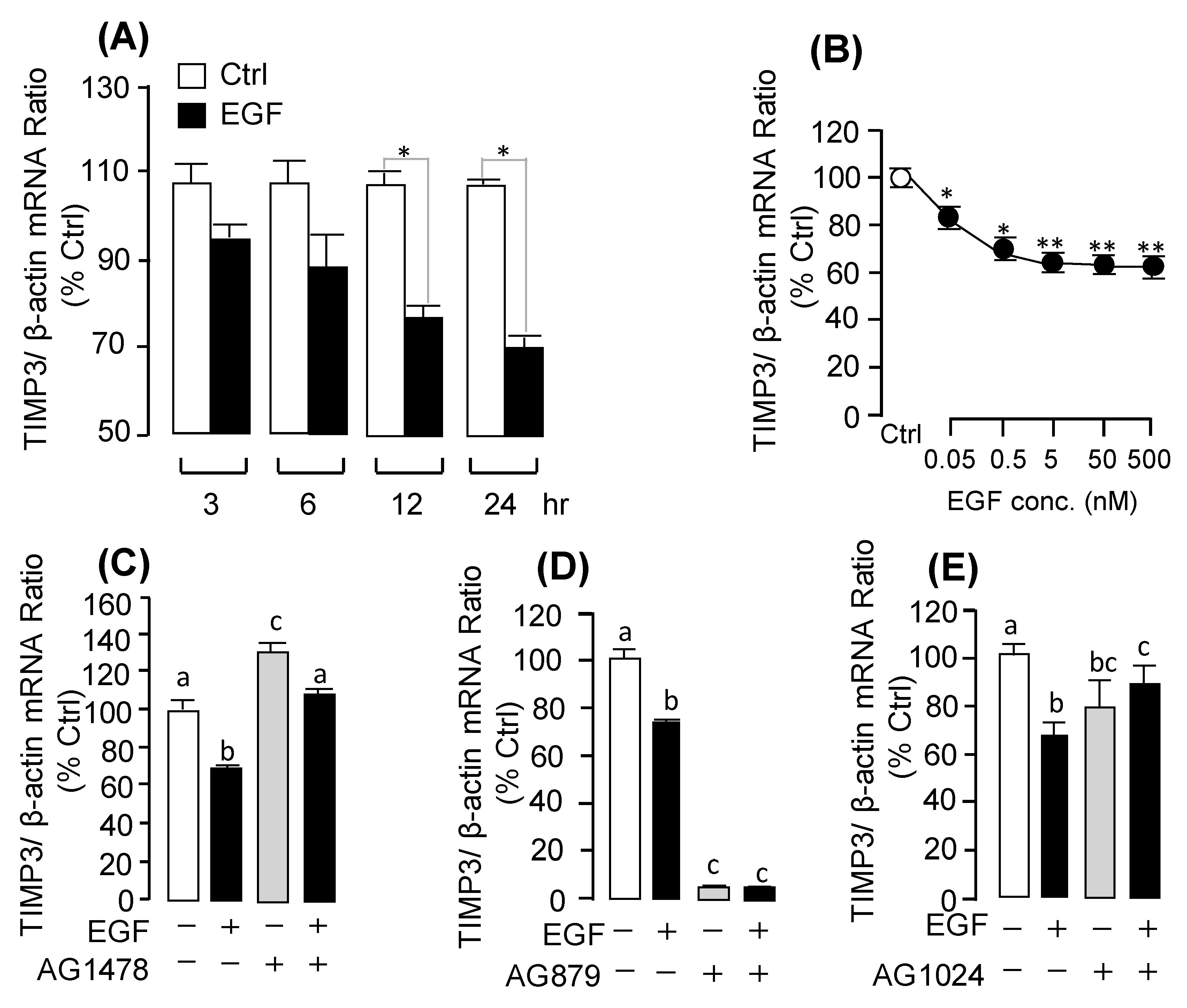
2.4. Receptor Specificity and Signal Transduction for EGF-Induced MMP13 mRNA Expression
2.5. Functional Role of MMP13 in EGF-Induced UTS1 and EGR1
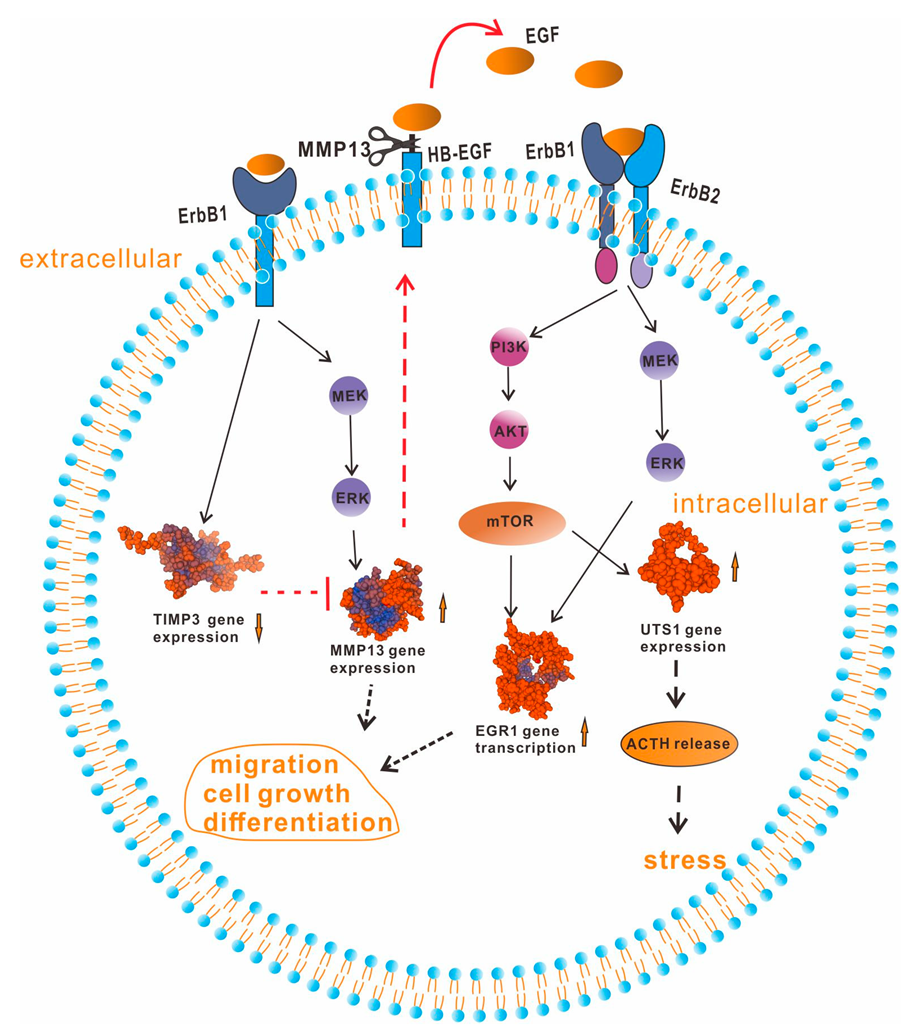
3. Discussion
4. Materials and Methods
4.1. Animals and Chemicals
4.2. Cell Culture, RNA Extraction and cDNA Library Construction
4.3. Differential Expression Genes (DEGs) Analysis and Functional Enrichment
4.4. Real-Time Quantitative PCR Validation
4.5. Data Transformation and Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ACTH | adrenocorticotropic hormone |
| Akt | protein kinase B |
| CRH | hypothalamic corticotropin releasing hormone |
| DEGs | differential expression genes |
| EGF | epidermal growth factor |
| EGFR | epidermal growth factor receptor |
| EGR1 | early growth response 1 |
| ERK | extracellular signal-regulated kinase |
| FSH | follicle-stimulating hormone |
| GH | growth hormone |
| GO | gene ontology |
| HB-EGF | Heparin-binding EGF-like growth factor |
| HPA | hypothalamus-pituitary-adrenal |
| IGF | insulin-like growth factor |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LH | luteinizing hormone |
| MEK | Methyl Ethyl Ketone |
| MAPK | mitogen-activated protein kinase |
| MMP13 | matrix metallopeptidase 13 |
| MMP9 | matrix metallopeptidase 9 |
| mTOR | mammalian target of rapamycin |
| PI3K | phosphatidylinositol-3-kinase |
| PRL | prolactin |
| SLα | somatolactin α |
| TACR3 | tachykinin receptor 3 |
| UTS1 | urotensin 1 |
References
- McGee, E.A. Initial and Cyclic Recruitment of Ovarian Follicles. Endocr. Rev. 2000, 21, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Wilson, K.J.; Gilmore, J.L.; Foley, J.; Lemmon, M.A.; Ii, D.J.R. Functional selectivity of egf family peptide growth factors: Implications for cancer. Pharmacol. Ther. 2009, 122, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Flores, C.; Kinkead, T.; Carboni, A.; Menon, M.; Seethalakshmi, L. Effects of Sialoadenectomy and Epidermal Growth Factor on Testicular Function of Sexually Mature Male Mice. J. Urol. 1994, 152, 554–561. [Google Scholar] [CrossRef]
- Normanno, N.; De Luca, A.; Bianco, C.; Strizzi, L.; Mancino, M.; Maiello, M.R.; Carotenuto, A.; De Feo, G.; Caponigro, F.; Salomon, D.S. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 2006, 366, 2–16. [Google Scholar] [CrossRef]
- Luger, A.; Calogero, A.E.; Kalogeras, K.; Gallucci, W.T.; Gold, P.W.; Loriaux, D.L.; Chrousos, G.P. Interaction of Epidermal Growth Factor with the Hypothalamic-Pituitary-Adrenal Axis: Potential Physiologic Relevance. J. Clin. Endocrinol. Metab. 1988, 66, 334–337. [Google Scholar] [CrossRef]
- Przylipiak, A.; Kiesel, L.; Rabe, T.; Helm, K.; Przylipiak, M.; Runnebaum, B. Epidermal growth factor stimulates luteinizing hormone and arachidonic acid release in rat pituitary cells. Mol. Cell. Endocrinol. 1988, 57, 157–162. [Google Scholar] [CrossRef]
- Childs, G.V.; Armstrong, J. Sites of Epidermal Growth Factor Synthesis and Action in the Pituitary: Paracrine and Autocrine Interactions. Clin. Exp. Pharmacol. Physiol. 2001, 28, 249–252. [Google Scholar] [CrossRef]
- Radford, H.M.; Panaretto, B.A.; Avenell, J.A.; Turnbull, K.E. Effect of mouse epidermal growth factor on plasma concentrations of FSH, LH and progesterone and on oestrus, ovulation and ovulation rate in Merino ewes. Reproduction 1987, 80, 383–393. [Google Scholar] [CrossRef]
- Johnson, L.K.; Baxter, J.D.; Vlodavsky, I.; Gospodarowicz, D. Epidermal growth factor and expression of specific genes: Effects on cultured rat pituitary cells are dissociable from the mitogenic response. Proc. Natl. Acad. Sci. USA 1980, 77, 394–398. [Google Scholar] [CrossRef]
- Pang, Y. Epidermal Growth Factor and TGF Promote Zebrafish Oocyte Maturation in Vitro: Potential Role of the Ovarian Activin Regulatory System. Endocrinology 2002, 143, 47–54. [Google Scholar] [CrossRef]
- Wang, Y.; Ge, W. Cloning of Epidermal Growth Factor (EGF) and EGF Receptor from the Zebrafish Ovary: Evidence for EGF as a Potential Paracrine Factor from the Oocyte to Regulate Activin/Follistatin System in the Follicle Cells1. Boil. Reprod. 2004, 71, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-W.; Ge, W. Differential regulation of gonadotropins (FSH and LH) and growth hormone (GH) by neuroendocrine, endocrine, and paracrine factors in the zebrafish—An in vitro approach. Gen. Comp. Endocrinol. 2009, 160, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Ye, C.; Zhou, X.; Jia, J.; Xu, S.; Hu, Q.; Hu, G. NK3R Mediates the EGF-Induced SLα Secretion and mRNA Expression in Grass Carp Pituitary. Int. J. Mol. Sci. 2019, 20, 91. [Google Scholar] [CrossRef] [PubMed]
- Birman, P.; Michard, M.; Li, J.Y.; Peillon, F.; Bression, D. Epidermal Growth Factor-Binding Sites, Present in Normal Human and Rat Pituitaries, Are Absent in Human Pituitary Adenomas. J. Clin. Endocrinol. Metab. 1987, 65, 275–281. [Google Scholar] [CrossRef]
- Fisheries Bureau of Agriculture Ministry of China. China Fisheries Yearbook; China Agriculture Press: Beijing, China, 2019.
- Minematsu, T.; Miyai, S.; Kajiya, H.; Suzuki, M.; Sanno, N.; Takekoshi, S.; Teramoto, A.; Osamura, R.Y. Recent Progress in Studies of Pituitary Tumor Pathogenesis. Endocrine 2005, 28, 37–42. [Google Scholar] [CrossRef]
- Holsboer, F.; Spengler, D.; Heuser, I. The role of corticotropin-releasing hormone in the pathogenesis of Cushing’s disease, anorexia nervosa, alcoholism, affective disorders and dementia. Prog. Brain Res. 1992, 93, 385–417. [Google Scholar]
- Horne, B.L.; Lederis, K. Separation of urotensins I and II by density gradient centrifugation. Can. Fed. Biol. Soc. 1973, 16, 105. [Google Scholar]
- Lederis, K.; Letter, A.; McMaster, D.; Moore, G.; Schlesinger, D. Complete amino acid sequence of urotensin I, a hypotensive and corticotropin-releasing neuropeptide from Catostomus. Science 1982, 218, 162–165. [Google Scholar] [CrossRef]
- Lederis, K.; Letter, A.; McMaster, D.; Ichikawa, T.; MacCannell, K.L.; Kobayashi, Y.; Rivier, J.; Rivier, C.; Vale, W.; Fryer, J. Isolation, analysis of structure, synthesis, and biological actions of urotensin I neuropeptides. Can. J. Biochem. Cell Boil. 1983, 61, 602–614. [Google Scholar] [CrossRef]
- Gashler, A. Early growth response protein 1(Egr-1): Prototype of z zinc-finger family of transcription factors. Prog. Nucleic Acid Res. Mol. Biol. 1995, 50, 191–224. [Google Scholar]
- Thiel, G.; Cibelli, G. Regulation of life and death by the zinc finger transcription factor Egr-1. J. Cell. Physiol. 2002, 193, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, F.; Bélanger, S.D.; Biron-Pain, K.; St-Pierre, Y. EGR-1 activation by EGF inhibits MMP-9 expression and lymphoma growth. Blood 2010, 116, 759–766. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, H.; Kimura, T.; Dalal, S.; Okada, Y.; D’Armiento, J. Joint diseases and matrix metalloproteinases: A role for MMP-13. Curr. Pharm. Biotechnol. 2008, 9, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Vincenti, M.P.; Brinckerhoff, C.E. Transcriptional regulation of collagenase (mmp-1, mmp-13) genes in arthritis: Integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002, 4, 157–164. [Google Scholar] [CrossRef]
- Balbin, M.; Pendas, A.M.; Uría, J.A.; Jiménez, M.G.; Freije, J.P.; Freije, J.M.; López-Otín, C.; Lopez-Otin, C. Expression and regulation of collagenase-3 (MMP-13) in human malignant tumors. APMIS 1999, 107, 45–53. [Google Scholar] [CrossRef]
- Murphy, G. Tissue inhibitors of metalloproteinases. Genome Biol. 2012, 12, 233. [Google Scholar] [CrossRef]
- Köse, M. GPCRs and EGFR—Cross-talk of membrane receptors in cancer. Bioorganic Med. Chem. Lett. 2017, 27, 3611–3620. [Google Scholar]
- Ferguson, K.M.; Berger, M.B.; Mendrola, J.M.; Cho, H.-S.; Leahy, D.J.; Lemmon, M.; Lemmon, M. EGF Activates Its Receptor by Removing Interactions that Autoinhibit Ectodomain Dimerization. Mol. Cell 2003, 11, 507–517. [Google Scholar] [CrossRef]
- Cooper, O.; Vlotides, G.; Fukuoka, H.; Greene, M.I.; Melmed, S. Expression and function of ErbB receptors and ligands in the pituitary. Endocr. Relat. Cancer 2011, 18, R197–R211. [Google Scholar] [CrossRef]
- Olayioye, M.A.; Neve, R.M.; Lane, H.A.; Hynes, N.E. The ErbB signaling network: Receptor heterodimerization in development and cancer. EMBO J. 2000, 19, 3159–3167. [Google Scholar] [CrossRef]
- Cho, H.-S.; Mason, K.; Ramyar, K.X.; Stanley, A.M.; Gabelli, S.B.; Denney, D.W.; Leahy, D.J. Structure of the extracellular region of HER2 alone and in complex with the Herceptin Fab. Nature 2003, 421, 756. [Google Scholar] [CrossRef] [PubMed]
- Graus-Porta, D.; Beerli, R.R.; Daly, J.M.; Hynes, N.E. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. EMBO J. 1997, 16, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Serova, O.V.; Chachina, N.A.; Gantsova, E.A.; Popova, N.V.; Petrenko, A.G.; Deyev, I.E. Autophosphorylation of Orphan Receptor ERBB2 Can Be Induced by Extracellular Treatment with Mildly Alkaline Media. Int. J. Mol. Sci. 2019, 20, 1515. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zhou, X.; Li, X.; Tang, Y.; Sun, Y.; Fang, J. Inhibition of epidermal growth factor receptor signaling prohibits metastasis of gastric cancer via downregulation of MMP7 and MMP13. Tumor Boil. 2014, 35, 10891–10896. [Google Scholar] [CrossRef] [PubMed]
- Meierjohann, S.; Hufnagel, A.; Wende, E.; Kleinschmidt, M.; Wolf, K.; Friedl, P.; Gaubatz, S.; Schartl, M. MMP13 mediates cell cycle progression in melanocytes and melanoma cells: In vitro studies of migration and proliferation. Mol. Cancer 2010, 9, 201. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.; Ng, S.; Lee, E.; Leung, R.; Ho, W. Somatostatin Inhibits (d-Arg6, Pro9-NEt) Salmon Gonadotropin-Releasing Hormone- and Dopamine D1-Stimulated Growth Hormone Release from Perifused Pituitary Cells of Chinese Grass Carp,Ctenopharyngodon idellus. Gen. Comp. Endocrinol. 1998, 110, 29–45. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Benjamini, Y.; Drai, D.; Elmer, G.; Kafkafi, N.; Golani, I. Controlling the false discovery rate in behavior genetics research. Behav. Brain Res. 2001, 125, 279–284. [Google Scholar] [CrossRef]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef]
- Ye, J.; Fang, L.; Zheng, H.; Zhang, Y.; Chen, J.; Zhang, Z.; Wang, J.; Li, S.; Li, R.; Bolund, L.; et al. WEGO: A web tool for plotting GO annotations. Nucleic Acids Res. 2006, 34, W293–W297. [Google Scholar] [CrossRef]
- Xie, C.; Mao, X.; Huang, J.; Ding, Y.; Wu, J.; Dong, S.; Kong, L.; Gao, G.; Li, C.-Y.; Wei, L. KOBAS 2.0: A web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011, 39, W316–W322. [Google Scholar] [CrossRef] [PubMed]
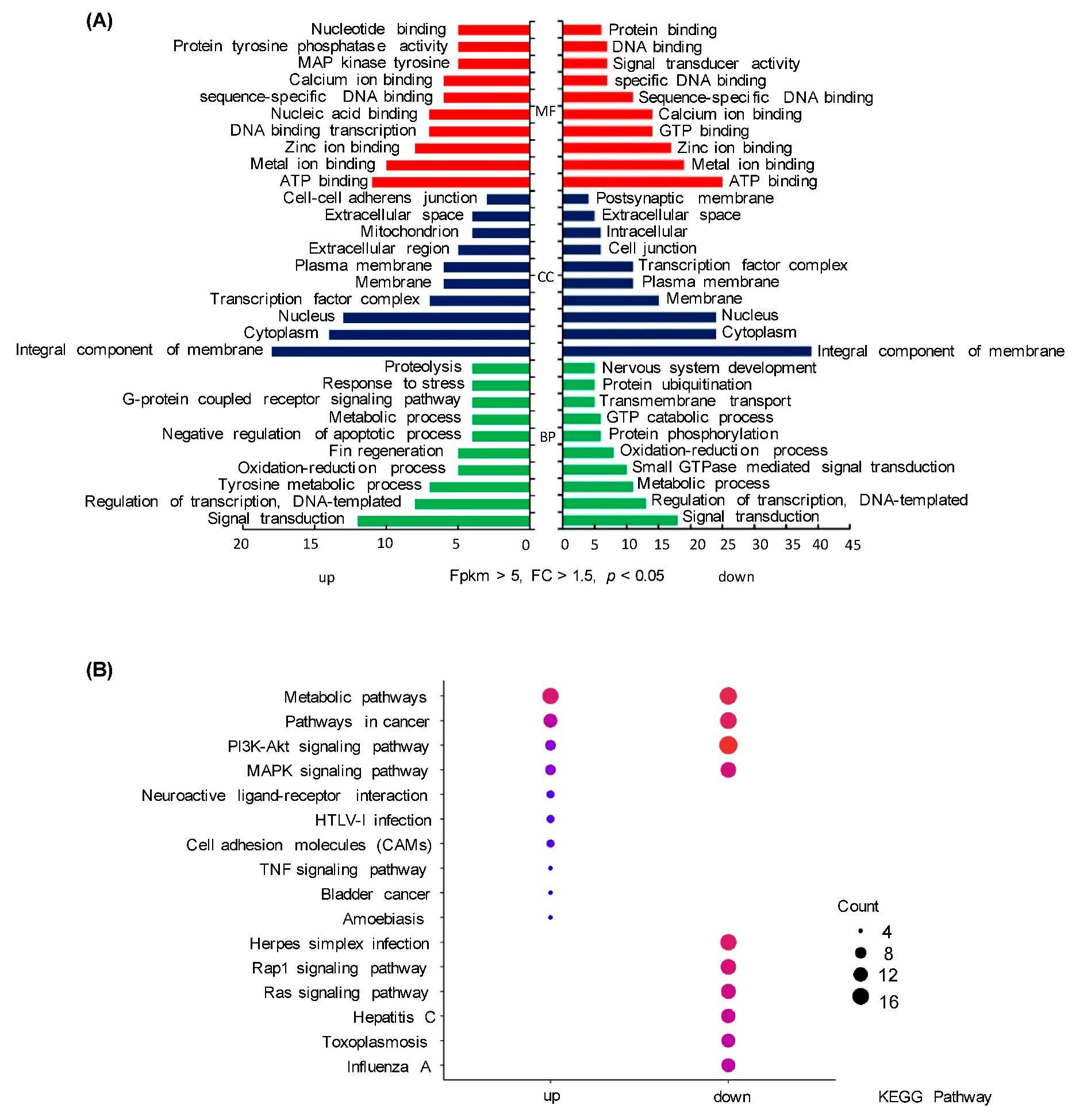
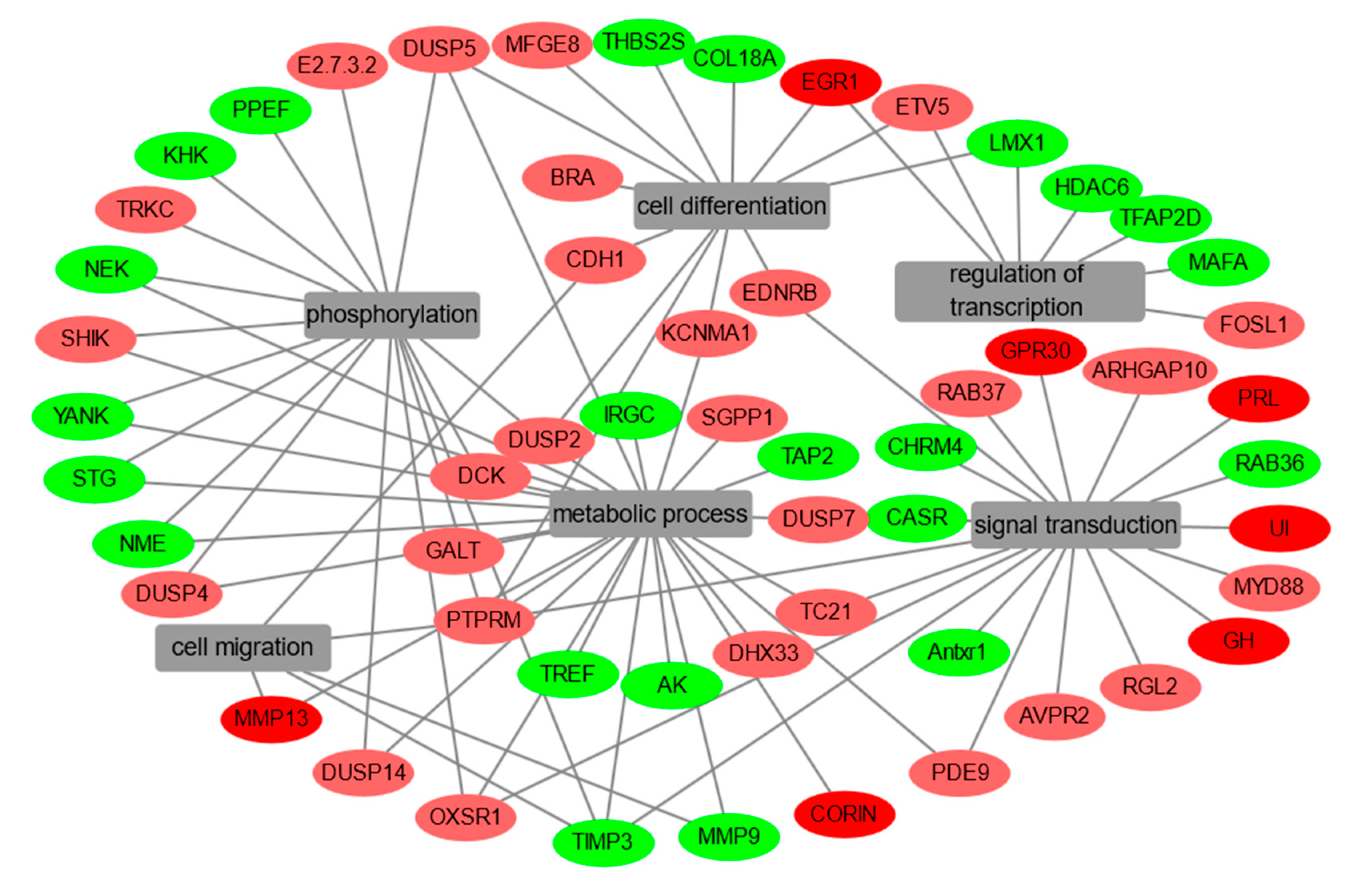
| Gene | FC | p-Value | Description | Molecular Function |
|---|---|---|---|---|
| DHX33 | 1.81 | 1.39 × 10−3 | DEAH-box helicase 33 | ATP binding, helicase activity |
| E2.7.3.2 | 2.04 | 4.99 × 10−8 | Creatine kinase M-type | ATP binding, kinase activity |
| DCK | 1.74 | 8.70 × 10−4 | Deoxycytidine kinase | ATP binding, nucleoside kinase activity |
| MFGE8 | 2.11 | 1.05 × 10−38 | Rho GTPase-activating protein 10 | Calcium ion binding |
| KCNMA1 | 1.7 | 3.82 × 10−11 | Calcium-activated potassium channel subunit alpha | Calcium-activated potassium channel activity |
| SGPP1 | 1.85 | 5.09 × 10−14 | Sphingosine-1-phosphate phosphatase 1 | Catalytic activity |
| COX6B | 1.9 | 1.39 × 10−5 | Cytochrome c oxidase subunit 6B1 | Cytochrome-c oxidase activity |
| EGR1 | 3.75 | 2.20 × 10−151 | Early growth response protein 1 | DNA binding,metal ion binding |
| EDNRB | 1.74 | 1.01 × 10−10 | Endothelin B receptor | Endothelin receptor activity |
| ETV5 | 1.7 | 7.60 × 10−16 | ETS translocation variant 5 | Equence-specific DNA binding |
| FABP7 | 2.93 | 9.81 × 10−15 | Fatty acid-binding protein, brain | Fatty acid binding,transporter activity |
| CDH1 | 3.53 | 6.37 × 10−7 | Cadherin-1 | G-protein alpha-subunit binding |
| RAB37 | 2.12 | 5.92 × 10−9 | Ras-related protein Rab-37 | GTP binding |
| RRAS2 | 1.99 | 2.19 × 10−9 | Ras-related protein R-Ras2 | GTP binding |
| ARHGAP10 | 1.93 | 8.35 × 10−11 | Rho GTPase-activating protein 10 | Gtpase activator activity |
| RGL2 | 1.75 | 9.95 × 10−28 | Ral guanine nucleotide dissociation stimulator | Guanyl-nucleotide exchange factor activity |
| UTS1 | 24.99 | 7.16 × 10−149 | Urotensin1 | Hormone activity |
| Slα | 1.83 | 2.80 × 10−49 | Somatolactin | Hormone activity |
| PRL | 1.86 | 1.09 × 10−51 | Prolactin | Hormone activity |
| HasA | 1.79 | 7.90 × 10−10 | Hyaluronan synthase 2 | Hyaluronan synthase activity |
| CDKAL1 | 1.7 | 3.56 × 10−2 | CDK5 regulatory subunit-associated protein 1-like 1 | Kdo transferase activity |
| PDE9 | 2.32 | 9.56 × 10−59 | High affinity cGMP-specific 3 | Metal ion binding |
| GALNT12 | 1.79 | 2.06 × 10−19 | Polypeptide N-acetylgalactosaminyltransferase 12 | Metal ion binding, transferase activity |
| MMP13 | 289.6 | 0.00 | Collagenase 3 | Metalloendopeptidase activity |
| NTRK3 | 2.09 | 2.62 × 10−6 | _ | Neurotrophin receptor activity |
| CADM4 | 1.86 | 9.31 × 10−32 | Cell adhesion molecule 4 | Protein binding |
| STK40 | 1.97 | 2.60 × 10−27 | threonine-protein kinase 40 | Protein serine/threonine kinase activity |
| Dusp14 | 1.82 | 6.61 × 10−18 | Dual specificity protein phosphatase 14 | Protein tyrosine phosphatase activity |
| Dusp2 | 2.28 | 9.33 × 10−18 | Dual specificity protein phosphatase 2 | Protein tyrosine phosphatase activity |
| Dusp4 | 2.46 | 4.73 × 10−16 | Dual specificity protein phosphatase 4 | Protein tyrosine phosphatase activity |
| Dusp5 | 1.83 | 7.81 × 10−6 | Dual specificity protein phosphatase 5 | Protein tyrosine phosphatase activity |
| Dusp7 | 2.08 | 7.58 × 10−31 | Dual specificity protein phosphatase 7 | Protein tyrosine phosphatase activity |
| OXSR1 | 2.85 | 1.93 × 10−14 | Serine-proteinkinase OSR1 | Receptor signaling protein kinase activity |
| CORIN | 5.22 | 4.15 × 10−47 | Corin, serine peptidase | Serine-type endopeptidase activity |
| SERPINB | 2.05 | 1.85 × 10−3 | Leukocyte elastase inhibitor | Serine-type endopeptidase inhibitor activity |
| KRT2 | 2.14 | 5.43 × 10−17 | Keratin, type II cytoskeletal 8 | Structural molecule activity |
| CPLX3_4 | 1.7 | 1.02 × 10−4 | Complexin-3 | Syntaxin binding |
| TPMT | 1.68 | 2.34 × 10−3 | Thiopurine S-methyltransferase | Thiopurine S-methyltransferase activity |
| FOSL1 | 2.46 | 5.59 × 10−47 | Fos-related antigen 1 | Transcription factor activity |
| BRA, T | 2 | 1.12 × 10−6 | Brachyury protein homolog A | Transcription regulatory region DNA binding |
| PTPRM | 1.78 | 3.30 × 10−12 | Receptor-type tyrosine-protein phosphatase mu | Transmembrane receptor activity |
| RNF144 | 1.72 | 1.73 × 10−10 | Probable E3 ubiquitin-protein ligase RNF144A-A | Tubulin-glycine ligase activity |
| MYD88 | 1.68 | 3.04 × 10−13 | Myeloid differentiation primary response protein MyD88 | Tyrosine kinase activity |
| AVPR2 | 1.94 | 6.54 × 10−10 | Vasopressin V2 receptor | Vasopressin receptor activity |
| GALT | 2.39 | 1.18 × 10−3 | Galactose-1-phosphate uridylyltransferase | Zinc ion binding |
| ZCCHC9 | 1.74 | 1.44 × 10−2 | Zinc finger CCHC domain-containing protein 9 | Zinc ion binding, nucleic acid binding |
| Gene | FC | p-Value | Description | Molecular Function |
|---|---|---|---|---|
| TER | 0.55 | 1.69 × 10−5 | Very-long-chain enoyl-CoA reductase | Acting on the CH-CH group of donors |
| ADCY6 | 0.49 | 1.5 × 10−20 | Adenylate cyclase type 6 | Adenylate cyclase activity |
| NRIP2 | 0.50 | 8.4×10−15 | Nuclear receptor-interacting protein 2 | Aspartic-type endopeptidase activity |
| SEK | 0.36 | 7.2 × 10−35 | Ephrin type-A receptor 3 | ATP binding |
| Hsc70 | 0.52 | 1.1 × 10−21 | Heat shock cognate 70 | ATP binding |
| Hsp70 | 0.55 | 6.03 × 10−8 | Heat shock protein70 | ATP binding |
| CDH11 | 0.38 | 2.2 × 10−40 | Cadherin-11 | Calcium ion binding |
| CHP2 | 0.51 | 0.000023 | Calcineurin B homologous protein 1 | Calcium ion binding |
| PH-4 | 0.50 | 6.3 × 10−9 | Transmembrane prolyl 4-hydroxylase | Calcium ion binding |
| E4.2.1.1 | 0.53 | 2.3 × 10−11 | Carbonic anhydrase 2 | Carbonate dehydratase activity, zinc ion binding |
| KCNC1 | 0.51 | 4.1 × 10−8 | _ | Delayed rectifier potassium channel activity |
| RYBP | 0.58 | 1.84 × 10−13 | RING1 and YY1-binding protein A | DNA binding |
| EIF2AK2 | 0.57 | 1.5 × 10−6 | Eukaryotic translation initiation factor 2-alpha kinase 2 | Double-stranded RNA adenosine deaminase activity |
| THBS1 | 0.56 | 5.83 × 10−23 | Thrombospondin-1 | Extracellular matrix binding |
| NTSR1 | 0.50 | 7.1 × 10−8 | Neurotensin receptor type 1 | G-protein coupled neurotensin receptor activity |
| RAB39B | 0.57 | 0.00158 | Ras-related protein Rab-39B | GTP binding |
| REM2 | 0.44 | 6.6 × 10−20 | GTP-binding protein REM 2 | GTP binding |
| RND3 | 0.56 | 9.36 × 10−7 | Rho-related GTP-binding protein RhoE | GTP binding |
| RIG-I | 0.57 | 5.4 × 10−8 | Probable ATP-dependent RNA helicase DDX58 | Helicase activity, nucleic acid binding |
| CYP4V | 0.29 | 6.2 × 10−36 | Cytochrome P450 4V2 | Heme binding, iron ion binding |
| RAC3 | 0.42 | 2 × 10−7 | p21-Rac3; Flags: Precursor | Hydrolase activity |
| LPL | 0.31 | 2.7×10−55 | Lipoprotein lipase | Lipoprotein lipase activity |
| GALNT13 | 0.46 | 0.000016 | Polypeptide GalNAc transferase 13 | Metal ion binding |
| DNAJA4 | 0.54 | 1.1 × 10−7 | DNA J homolog subfamily A member 4 | Metal ion binding, heat shock protein binding |
| GTF3A | 0.54 | 2.17 × 10−10 | General transcription factor IIIA | Metal ion binding,nucleic acid binding |
| PARP7S | 0.57 | 2.26 × 10−6 | Poly (ADP-ribose) polymerase family, member 12b | Metal ion binding |
| IFT54 | 0.58 | 4.74 × 10−7 | TRAF3-interacting protein 1 | Microtubule binding |
| TIMP3 | 0.58 | 1.43 × 10−13 | Tissue inhibitor of metalloproteinase 3 | _ |
| ACTC1 | 0.52 | 1.5 × 10−10 | Actin, alpha cardiac muscle 1 | Myosin binding |
| DDX58 | 0.54 | 0.00001 | DEAD box protein 58 | Nucleic acid binding |
| NKTR | 0.53 | 4.2 × 10−17 | NK-tumor recognition protein | Peptidyl-prolyl cis-trans isomerase activity |
| MOX44 | 0.53 | 2.8 × 10−10 | CD53 molecule | Protein binding |
| CCK4 | 0.52 | 1.8 × 10−13 | Protein tyrosine kinase 7 | Protein tyrosine kinase activity |
| SIAH1 | 0.47 | 1.6 × 10−11 | Siah E3 ubiquitin protein ligase 1 | Protein-glycine ligase activity |
| ITGB2 | 0.41 | 1.9 × 10−34 | Integrin beta-2 | Receptor activity |
| NOTCH | 0.53 | 7.4 × 10−12 | Notch 1 extracellular truncation | Receptor activity, calcium ion binding |
| NRP2 | 0.36 | 3.3 × 10−46 | Neuropilin-2 | Semaphorin receptor activity |
| NFKB1 | 0.56 | 0.00635 | Nuclear factor NF-kappa-B p105 subunit | DNA binding transcription factor activity |
| IAT7E | 0.57 | 0.003 | GalNAc alpha-2,6-sialyltransferase III | Sialyltransferase activity |
| SLC1A3 | 0.46 | 6 × 10−43 | Excitatory amino acid transporter 1 | Sodium:dicarboxylate symporter activity |
| UGT | 0.47 | 4.9×10−17 | UDP-glucuronosyltransferase 1-1 | Transferase activity |
| B4GALT3 | 0.55 | 4.51 × 10−7 | Beta-1,4-galactosyltransferase 3 | Transferase activity, transferring glycosyl groups |
| NTRK2 | 0.52 | 7.5 × 10−12 | NT-3 growth factors receptor | Transmembrane receptor protein tyrosine kinase activity |
| SLC16A7 | 0.36 | 7.2 × 10−63 | Monocarboxylate transporter 2 | Transmembrane transporter activity |
| SV2 | 0.43 | 6.1 × 10−17 | Synaptic vesicle glycoprotein 2B | Transmembrane transporter activity |
| RNF41 | 0.33 | 3.2 × 10−30 | Ligand of Numb protein X 4 | Ubiquitin-protein transferase activity |
| SFRP2 | 0.43 | 9.4 × 10−30 | Secreted frizzled-related protein 2 | Wnt-protein binding |
| AMZ2 | 0.54 | 0.002 | Archaemetzincin-2 | Zinc ion binding |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Q.; Xu, S.; Ye, C.; Jia, J.; Zhou, L.; Hu, G. Novel Pituitary Actions of Epidermal Growth Factor: Receptor Specificity and Signal Transduction for UTS1, EGR1, and MMP13 Regulation by EGF. Int. J. Mol. Sci. 2019, 20, 5172. https://doi.org/10.3390/ijms20205172
Hu Q, Xu S, Ye C, Jia J, Zhou L, Hu G. Novel Pituitary Actions of Epidermal Growth Factor: Receptor Specificity and Signal Transduction for UTS1, EGR1, and MMP13 Regulation by EGF. International Journal of Molecular Sciences. 2019; 20(20):5172. https://doi.org/10.3390/ijms20205172
Chicago/Turabian StyleHu, Qiongyao, Shaohua Xu, Cheng Ye, Jingyi Jia, Lingling Zhou, and Guangfu Hu. 2019. "Novel Pituitary Actions of Epidermal Growth Factor: Receptor Specificity and Signal Transduction for UTS1, EGR1, and MMP13 Regulation by EGF" International Journal of Molecular Sciences 20, no. 20: 5172. https://doi.org/10.3390/ijms20205172
APA StyleHu, Q., Xu, S., Ye, C., Jia, J., Zhou, L., & Hu, G. (2019). Novel Pituitary Actions of Epidermal Growth Factor: Receptor Specificity and Signal Transduction for UTS1, EGR1, and MMP13 Regulation by EGF. International Journal of Molecular Sciences, 20(20), 5172. https://doi.org/10.3390/ijms20205172





