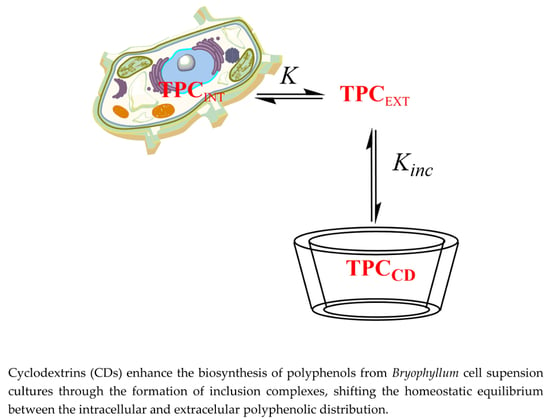
Cyclodextrin-Elicited Bryophyllum Suspension Cultured Cells: Enhancement of the Production of Bioactive Compounds
Abstract
:1. Introduction
- (1)
- CDs can be easily incorporated in PSCC, acting as “hosts” that favor the accumulation of bioactive compounds in the culture medium, from where they can be easily recovered avoiding biomass destruction [6]. Since CDs are natural compounds, the use of aqueous solutions of CDs can be considered as an ecological and environmentally friendly alternative for the extraction of potential bioactive compounds.
- (2)
- CDs can improve the availability of hydrophobic (e.g., poorly water soluble) bioactive compounds, substantially improving their ability to cross hydrophobic cell membranes.
- (3)
- CDs can act as efficient elicitors in PSCC due to their structural similarity (Scheme 1) to pectic oligosaccharides released from cell walls as a result of fungal infection, promoting the biosynthesis of a variety of bioactive compounds, including polyphenols [6,7], as a natural response to induced stress.
2. Results and Discussion
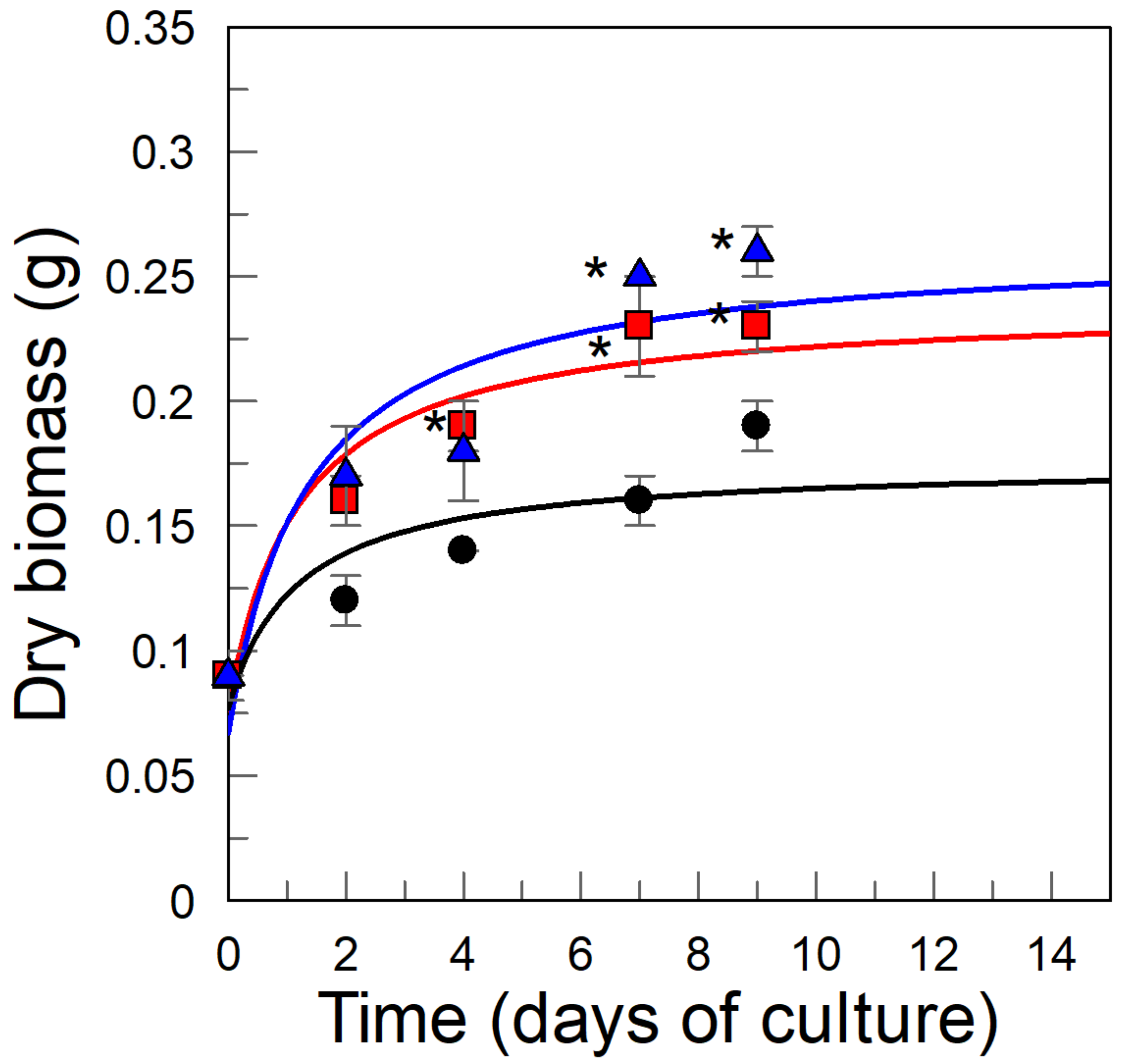
2.1. Kinetics of the B. × houghtonii Suspension Cultured Cells (BHSCC) Growth: Effects of Cyclodextrins (CDs)
2.2. Intracellular and Extracellular Production of Total Phenolic Compounds in BHSCC: Effects of Added CDs
2.3. Cyclodextrins (CDs) as Elicitors: Effects on the Intracellular and Extracellular Production of Flavonoids from B. x houghtonii Suspension Cultured Cells (BHSCC)
2.4. Radical Scavenging Activity of Intracellular and Extracellular Phenolics and Flavonoids: Effects of Cyclodextrins (CDs)
2.5. Determination of the Inclusion Constants of Representative Polyphenols with Cyclodextrins (CDs) (Kinc)
2.6. Effect of Cyclodextrins (CDs) on the Radical Scavenging Activity of Gallic Acid Derivatives
3. Materials and Methods
3.1. Materials
3.2. Plant Material
3.3. Callus Induction
3.4. Establishment of Cell Suspension Culture
3.5. Elicitation Experiments and Growth Kinetics Determination
3.6. Phenolic Extraction
3.7. Total Phenolic Content (TPC) Determination
3.8. Flavonoid Content (FC) Determination
3.9. Radical Scavenging Activity (RSA) Determination
3.10. Determination of Inclusion Constants (Kinc) of Gallates with Cyclodextrins (CDs): Spectral Shift and Phase–Solubility Methods
- Spectral UV–Vis shift method
- Phase solubility method
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Eibl, R.; Meier, P.; Stutz, I.; Schildberger, D.; Hühn, T.; Eibl, D. Plant cell culture technology in the cosmetics and food industries: Current state and future trends. Appl. Microbiol. Biotechnol. 2018, 102, 8661–8675. [Google Scholar] [CrossRef] [PubMed]
- Georgiev, V.; Slavov, A.; Vasileva, I.; Pavlov, A. Plant cell culture as emerging technology for production of active cosmetic ingredients. Eng. Life Sci. 2018, 18, 779–798. [Google Scholar] [CrossRef]
- Sharma, P.; Sharma, S.; Yadav, S.; Srivastava, A.; Purohit, I.; Shrivastava, N. Plant Derived Bioactive Molecules: Culture Vessels to Bioreactors. In Production of Biomass and Bioactive Compounds Using Bioreactor Technology; Paek, K.Y., Murthy, H.N., Zhong, J.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Karla Ramirez-Estrada, K.; Vidal-Limon, H.; Hidalgo, D.; Moyano, E.; Golenioswki, M.; Cusidó, R.M.; Palazon, J. Elicitation, an Effective Strategy for the Biotechnological Production of Bioactive High-Added Value Compounds in Plant Cell Factories. Molecules 2016, 21, 182. [Google Scholar] [CrossRef] [PubMed]
- Bru, R.; Sellés, S.; Casado-Vela, J.; Belchí-Navarro, S.; Pedreño, M.A. Modified cyclodextrins are chemically defined glucan inducers of defense responses in grapevine cell cultures. J. Agric. Food Chem. 2006, 11, 65–71. [Google Scholar] [CrossRef]
- Almagro, L.; Belchí-Navarro, S.; Martínez-Márquez, A.; Bru, R.; Pedreño, M.A. Enhanced extracellular production of trans-resveratrol in Vitis vinifera suspension cultured cells by using cyclodextrins and coronatine. Plant Physiol. Biochem. 2015, 97, 361–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zamboni, A.; Gatto, P.; Cestaro, A.; Pilati, S.; Viola, R.; Mattivi, F.; Moser, C.; Velasco, R. Grapevine cell early activation of specific responses to DIMEB, a resveratrol elicitor. BMC Genom. 2009, 10, 363. [Google Scholar] [CrossRef] [PubMed]
- Martínez Alonso, A.; Losada-Barreiro, S.; Bravo-Díaz, C. Encapsulation and solubilization of antioxidants gallic acid and ethyl, propyl and butyl gallate with B-cyclodextrin. J. Mol. Liquids 2015, 210, 143–150. [Google Scholar] [CrossRef]
- Gidwani, B.; Vyas, A. A comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. BioMed Res. Int. 2015, 2015, 1–15. [Google Scholar] [CrossRef]
- Carvotto, G.; Binello, A.; Baranelli, E.; Carraro, P.; Trotta, F. Cyclodextrins as food additives and in food processing. Curr. Nutr. Food Sci. 2006, 2, 334–350. [Google Scholar] [CrossRef]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- García-Pérez, P.; Barreal, M.; Rojo-de Dios, L.; Cameselle-Teijeiro, J.F.; Gallego, P.P. Bioactive natural products from the genus Kalanchoe as cancer chemopreventive agents: A review. In Studies in Natural Products Chemistry; ur Rahman, A., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; Volume 61, pp. 49–84. [Google Scholar]
- Kocka, A.B.; Zidorn, C.; Kasprzycka, M.; Szymczak, G.; Szewczyk, K. Phenolic acid content, antioxidant and cytotoxic activities of four Kalanchoe species. Saudi J. Biol. Sci. 2018, 25, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Perassolo, M.; Smith, S.M.; Giulietti, A.M.; Rodríguez Talou, J. Synergistic effect of methyl jasmonate and cyclodextrins on anthraquinone accumulation in cell suspension cultures of Morinda citrifolia and Rubia tinctorum. Plant Cell Tissue Organ Cult. 2016, 124, 319–330. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, J.; Zhu, J.; He, S.; Zhang, W.; Yu, R.; Zi, J.; Song, L.; Huang, X. Effects of β-cyclodextrin and methyl jasmonate on the production of vindoline, catharanthine, and ajmalicine in Catharanthus roseus cambial meristematic cell cultures. Appl. Microbiol. Biotechnol. 2015, 99, 7035–7045. [Google Scholar] [CrossRef] [PubMed]
- Durante, M.; Caretto, S.; Quarta, A.; De Paolis, A.; Nisi, R.; Mita, G. β-Cyclodextrins enhance artemisinin production in Artemisia annua suspension cell cultures. Appl. Microbiol. Biotechnol. 2011, 90, 1905–1913. [Google Scholar] [CrossRef] [PubMed]
- Miras-Moreno, B.; Almagro, L.; Pedreno, M.A.; Sabater-Jara, A. Enhanced accumulation of phytosterols and phenolic compounds in cyclodextrin-elicited cell suspension culture of Daucus carota. Plant Sci. 2016, 250, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Belchí-Navarro, S.; Almagro, L.; Lijavetzky, D.; Bru, R.; Pedreño, M.A. Enhanced extracellular production of trans-resveratrol in Vitis vinifera suspension cultured cells by using cyclodextrins and methyljasmonate. Plant Cell Rep. 2012, 31, 81–89. [Google Scholar] [CrossRef]
- Belchí-Navarro, S.; Pedreño, M.A.; Almagro, L. Critical parameters on which the production of trans-resveratrol in Vitis vinifera cv Monastrell cell cultures depends. Plant Cell Tissue Organ Cult. 2019. [Google Scholar] [CrossRef]
- Morandi Vuolo, M.; Lima, S.; Maróstica Junior, M.R. Phenolic Compounds: Structure, Classification, and Antioxidant Power. In Bioactive Compounds: Health Benefits and Potential Applications; Campos, M.R.S., Ed.; Woodhead Publishing: Sawston, UK, 2019; pp. 33–50. [Google Scholar]
- Lijavetzky, D.; Almagro, L.; Belchi-Navarro, S.; Martínez-Zapater, J.M.; Bru, R.; Pedreño, M.A. Synergistic effect of methyljasmonate and cyclodextrin on stilbene biosynthesis pathway gene expression and resveratrol production in Monastrell grapevine cell cultures. BMC Res. Notes 2008, 1, 132. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant phenolics: Recent advances on their biosynthesis, genetics, and ecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Losada-Barreiro, S.; Bravo-Díaz, C. Free radicals and polyphenols: The redox chemistry of neurodegenerative diseases. Eur. J. Med. Chem. 2017, 133, 379–402. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25. [Google Scholar] [CrossRef]
- Foti, M. Use and Abuse of the DPPH radical. J. Agric. Food Chem. 2015, 14, 8765–8776. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Pu, H.; Tang, P.; Tang, B.; Sun, Q.; Li, H. Propyl gallate/cyclodextrin supramolecular complexes with enhanced solubility and radical scavenging capacity. Food Chem. 2018, 245, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Lucas-Abellán, C.; Mercader-Ros, M.T.; Zafrilla, M.P.; Gabaldón, J.A.; Núñez-Delicado, E. Comparative study of different methods to measure antioxidant activity of resveratrol in the presence of cyclodextrins. Food Chem. Toxicol. 2011, 49, 1255–1260. [Google Scholar] [CrossRef]
- Soares, D.G.; Andreazza, A.C.; Salvador, M. Sequestering ability of butylated hydroxytoluene, propyl gallate, resveratrol, and vitamins C and E against ABTS, DPPH, and hydroxyl free radicals in chemical and biological systems. J. Agric. Food Chem. 2003, 12, 1077–1080. [Google Scholar] [CrossRef]
- Pal, K.; Chandra, F.; Mallick, S.; Koner, A.L. pH dependent supramolecular recognition of dapoxyl sodium sulfonate with 2-hydroxypropyl b-cyclodextrin: An application towards foodadditive formulation. New J. Chem. 2016, 40, 6093–6100. [Google Scholar] [CrossRef]
- Mercader-Ros, M.T.; Lucas-Abellán, C.; Fortea, M.I.; Gabaldón, J.A.; Núñez-Delicado, E. Effect of HP-b-cyclodextrins complexation on the antioxidant activity of flavonols. Food Chem. 2010, 118, 769–773. [Google Scholar] [CrossRef]
- Kríz, Z.; Koc, J.; Imberty, A.; Charlot, A.; Auzély-Velty, R. Investigation of the complexation of ()-catechin by -cyclodextrin by a combination of NMR, microcalorimetry and molecular modeling techniques. Org. Biomol. Chem. 2003, 1, 2590–2595. [Google Scholar] [CrossRef]
- Jullian, C.; Brossard, V.; Gonzalez, I.; Alfaro, M.; Olea-Azar, C. Cyclodextrins-Kaempferol Inclusion Complexes: Spectroscopic and Reactivity Studies. J. Solut. Chem. 2011, 40, 727–739. [Google Scholar] [CrossRef]
- Górnaś, P.; Neunert, G.; Baczyński, K.; Polewski, K. Beta-cyclodextrin complexes with chlorogenic and caffeic acids from coffee brew: Spectroscopic, thermodynamic and molecular modelling study. Food Chem. 2009, 114, 190–196. [Google Scholar] [CrossRef]
- Zhang, M.; Li, J.; Zhanga, L.; Chaob, J. Preparation and spectral investigation of inclusion complex of caffeic acid with hydroxypropyl-β-cyclodextrin. Spectrochim. Acta Part A 2009, 71, 1891–1895. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, P.; Losada-Barreiro, S.; Gallego, P.P.; Bravo-Díaz, C. Adsorption of gallic acid, propyl gallate and polyphenols from Bryophyllum extracts on activated carbon. Sci. Rep. 2019. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–483. [Google Scholar] [CrossRef]
- Pan, Z.W.; Wang, H.Q.; Zhong, J.J. Scale-up on suspension cultured of Taxus chinensis cells for production of taxanediterpene. Enzyme Microb. Technol. 2000, 27, 714–723. [Google Scholar] [CrossRef]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin-Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef] [PubMed]
- PękalKrystyna, A.; Pyrzynska, K. Evaluation of aluminium complexation reaction for flavonoid content assay. Food Anal. Methods 2014, 7, 1776–1782. [Google Scholar]
- Waghmare, S.; Lokhande, V.; Penna, S.; Bapat, V.A. Preparation and evaluation of antioxidant capacity of Jackfruit (Artocarpus heterophyllus Lam.) wine and its protective role against radiation induced DNA damage. Ind. Crops. Prod. 2011, 34, 1595–1601. [Google Scholar]
- Gallego, P.P. Designing new culture media for plant using artificial intelligence tools. In Vitro Cell. Dev. Biol. Plant 2018, 54, S60–S72. [Google Scholar]
- Gallego, P.P.; Gago, J.; Landín, M. Artificial Neural Networks Technology to Model and Predict Plant Biology Process. In Artificial Neural Networks Technology to Model and Predict Plant. Biology Process; Suzuki, K., Ed.; Intech: London, UK, 2011; pp. 197–216. [Google Scholar]









| Antioxidant (AO) | Cyclodextrin (CD) | Kinc (M−1) | AO | CD | Kinc (M−1) |
|---|---|---|---|---|---|
| Gallic acid | HP–β–CD | 551 [29] | Coumaric acid | HP–β–CD | 320 [29] |
| Methyl gallate | HP–β–CD | 595 [29] | Quercetin | HP–β–CD | 900 [30] (pH 7.4) |
| Ethyl gallate | HP–β–CD | 380 [29] | Catechin | HP–β–CD | 8860 ± 270 [31] |
| Propyl gallate (PG) | β–CD | 125 ± 11(a) [8] 105 ± 2 (b) [8] | Kaempferol | β–CD | 580 [32] |
| HP–β–CD | 343 ± 9 (a) 328 ± 7 (b) 250 [29] | M–β–CD | 5432 [32] | ||
| Butyl gallate (BG) | β–CD | 198 ± 13 (a) [8] 200 ± 15 (b) [8] | HP–β–CD | 6175 [32] | |
| HP–β–CD | 706 ± 38 (a) | Caffeic acid | β–CD | 278 [33] (T= 20 °C) | |
| Octyl gallate (OG) | HP–β–CD | 4810 ± 98 (b) | HP–β–CD | 580 ± 56 [34] (pH 3) 279 ± 11 [34] (pH 6.5) | |
| Ferulic acid | HP–β–CD | 555 [29] | Chlorogenic acid | β–CD | 424 [33] (T= 20 °C) |
| 105 EC50 (M) | ||
|---|---|---|
| Antioxidants (AOs) | 0 M β–CD | 11 mM β–CD |
| GA | 6.5 ± 0.1 | 5.8 ± 0.1 |
| PG | 5.7 ± 0.1 | 3.9 ± 0.1 |
| BG | 5.2 ± 0.3 | 4.1 ± 0.2 |
| OG | 5.7 ± 0.1 | 4.1 ± 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Pérez, P.; Losada-Barreiro, S.; Gallego, P.P.; Bravo-Díaz, C. Cyclodextrin-Elicited Bryophyllum Suspension Cultured Cells: Enhancement of the Production of Bioactive Compounds. Int. J. Mol. Sci. 2019, 20, 5180. https://doi.org/10.3390/ijms20205180
García-Pérez P, Losada-Barreiro S, Gallego PP, Bravo-Díaz C. Cyclodextrin-Elicited Bryophyllum Suspension Cultured Cells: Enhancement of the Production of Bioactive Compounds. International Journal of Molecular Sciences. 2019; 20(20):5180. https://doi.org/10.3390/ijms20205180
Chicago/Turabian StyleGarcía-Pérez, Pascual, Sonia Losada-Barreiro, Pedro P. Gallego, and Carlos Bravo-Díaz. 2019. "Cyclodextrin-Elicited Bryophyllum Suspension Cultured Cells: Enhancement of the Production of Bioactive Compounds" International Journal of Molecular Sciences 20, no. 20: 5180. https://doi.org/10.3390/ijms20205180