Systematic Elucidation of the Mechanism of Genistein against Pulmonary Hypertension via Network Pharmacology Approach
Abstract
:1. Introduction
2. Results
2.1. Druglikeness Analysis of Genistein
2.2. Correlation Analysis between Targets of Genistein and PH
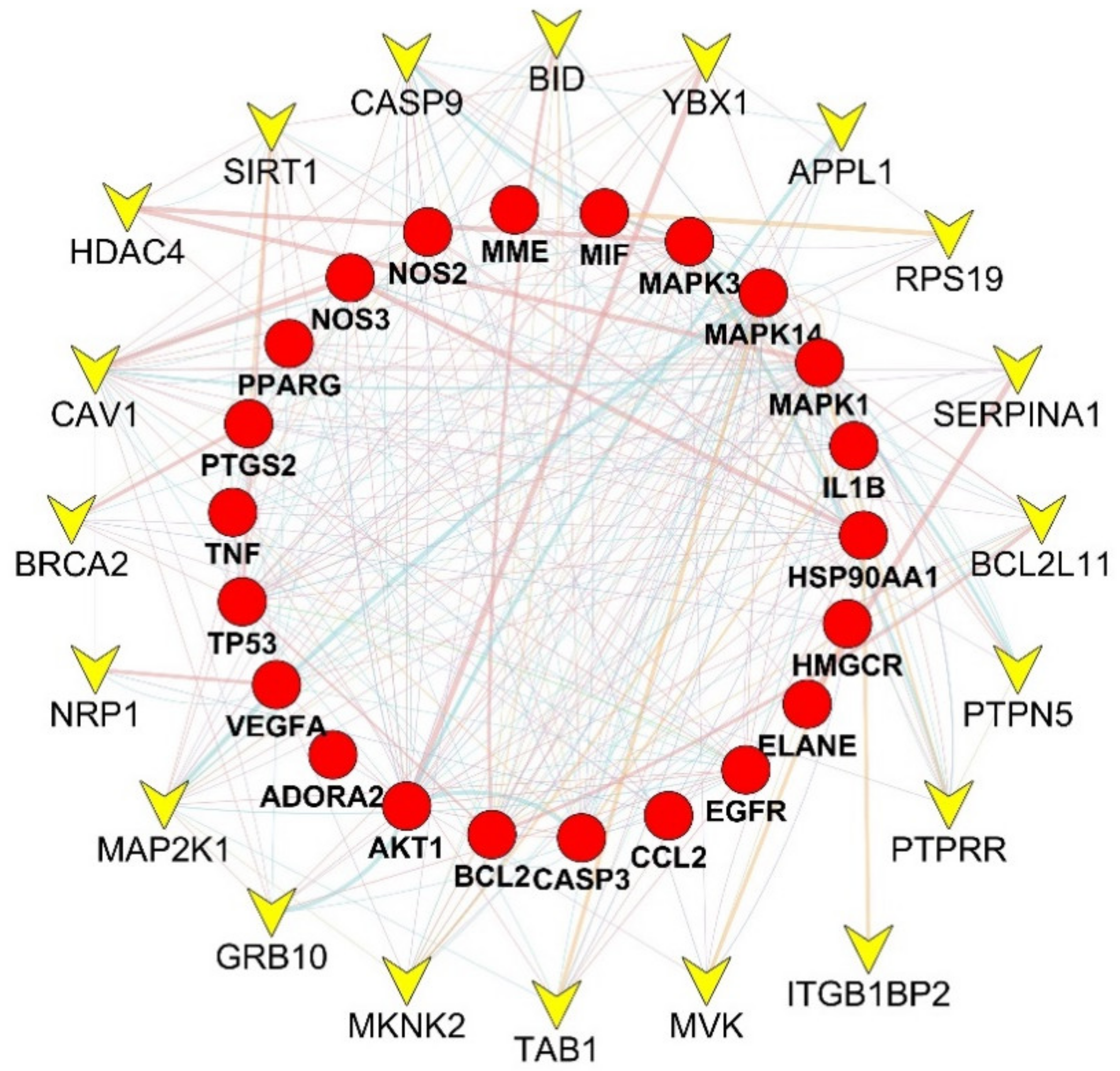
2.3. Protein–Protein Interaction Network
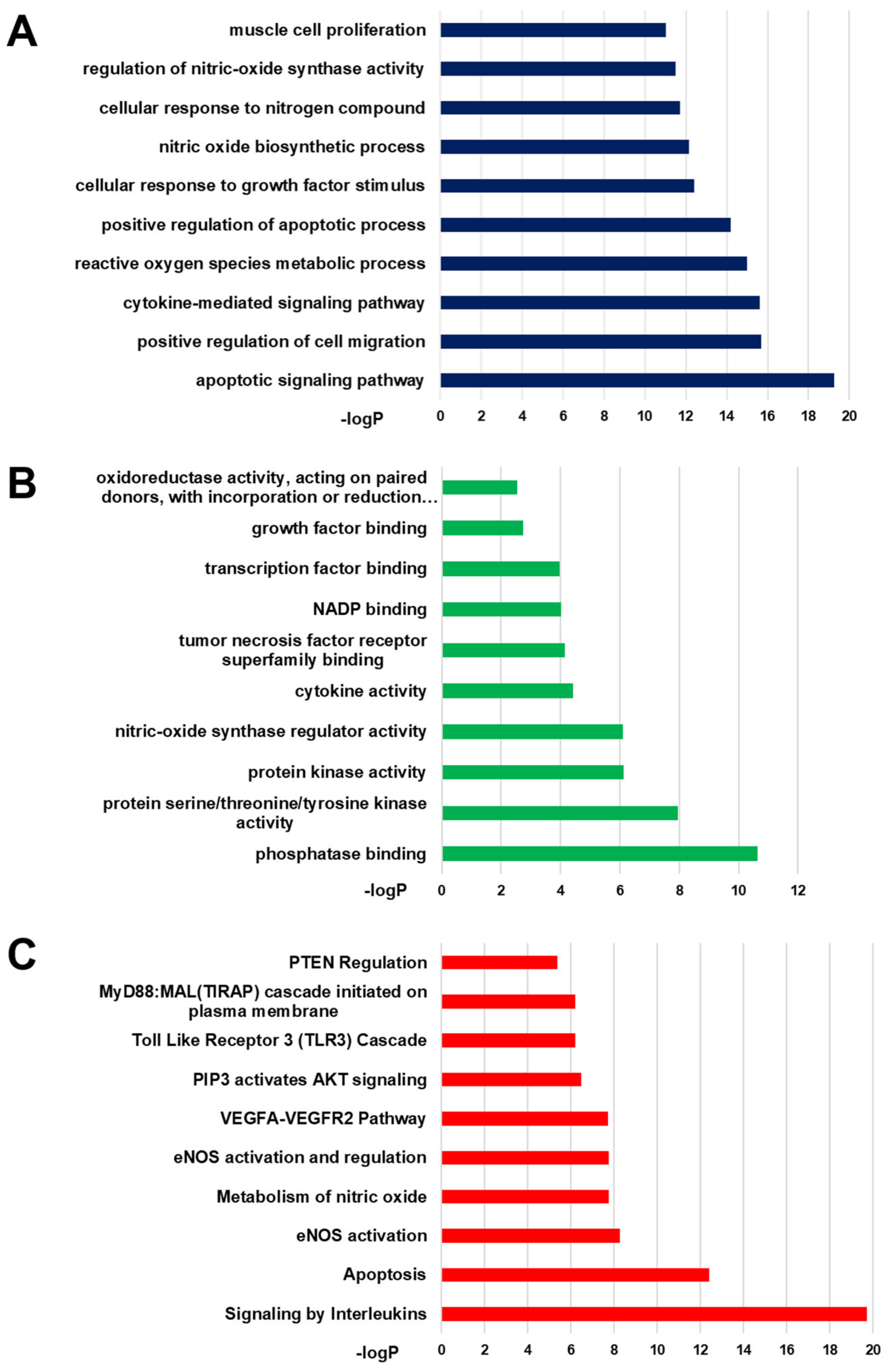
2.4. GO and Pathway Analysis
2.5. Target-Function Network (T-F Network)
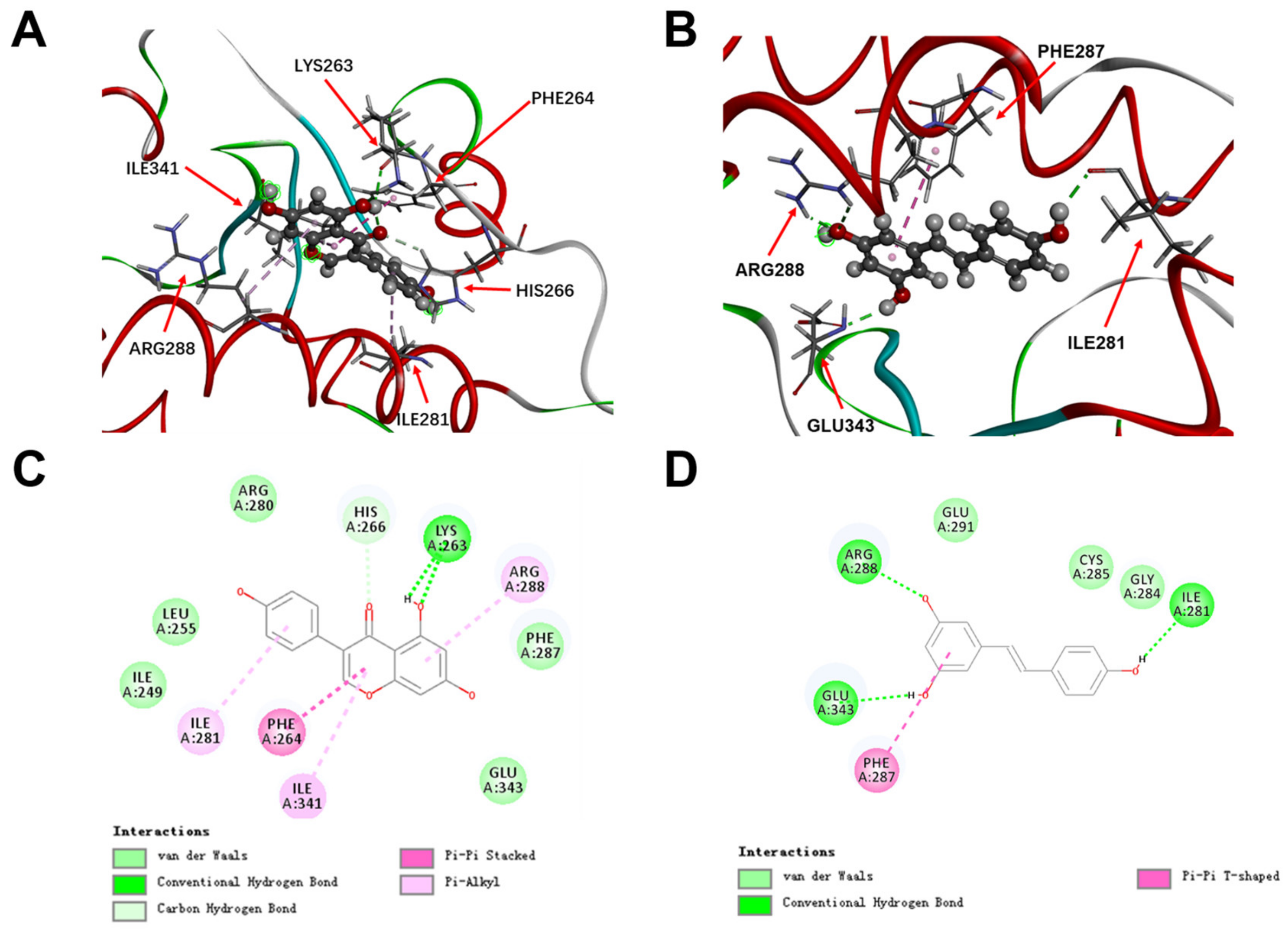
2.6. Molecular Docking
3. Discussion
4. Materials and Methods
4.1. Druglikeness Prediction
4.2. Predicting Targets of Genistein
4.3. Collecting Targets Related to Pulmonary Hypertension
4.4. Phenotypic Association Analysis of Genistein Targets and Pulmonary Hypertension
4.5. Protein-Protein Interaction Analysis
4.6. Gene Ontology Enrichment Analysis
4.7. Target-Pathway/Function Network Construction
4.8. Molecular Docking
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lau, E.M.T.; Giannoulatou, E.; Celermajer, D.S.; Humbert, M. Epidemiology and treatment of pulmonary arterial hypertension. Nat. Rev. Cardiol. 2017, 14, 603–614. [Google Scholar] [CrossRef]
- Satoh, K.; Kikuchi, N.; Satoh, T.; Kurosawa, R.; Sunamura, S.; Siddique, M.A.H.; Omura, J.; Yaoita, N.; Shimokawa, H. Identification of Novel Therapeutic Targets for Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2018, 19, 4801. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A.; Maron, B.A. Molecular Mechanisms of Pulmonary Vascular Remodeling in Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2016, 17, 761. [Google Scholar] [CrossRef] [PubMed]
- Schermuly, R.T.; Ghofrani, H.A.; Wilkins, M.R.; Grimminger, F. Mechanisms of disease: Pulmonary arterial hypertension. Nat. Rev. Cardiol. 2011, 8, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chen, J.L.; Xu, L.W.; Ji, G. Navigating traditional chinese medicine network pharmacology and computational tools. Evid. Based Complement. Alternat. Med. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, J.; Shen, F.; Qin, Z.; Jiang, M.; Zhu, J.; Wang, Z.; Zhou, J.; Fu, Y.; Chen, X.; et al. Systems pharmacology analysis of synergy of TCM: An example using saffron formula. Sci. Rep. 2018, 8, 380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.X.; Zhang, Y.Y.; Zhang, X.X.; Wang, P.Q.; Liu, J.; Liu, Q.; Wang, Z. Different network pharmacology mechanisms of Danshen-based Fangjis in the treatment of stable angina. Acta Pharmacol. Sin. 2018, 39, 952–960. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, C.; Marini, H.; Alibrandi, A.; Di Benedetto, A.; Bitto, A.; Adamo, E.B.; Altavilla, D.; Irace, C.; Di Vieste, G.; Pancaldo, D.; et al. Genistein Supplementation and Cardiac Function in Postmenopausal Women with Metabolic Syndrome: Results from a Pilot Strain-Echo Study. Nutrients 2017, 9, 584. [Google Scholar] [CrossRef]
- Qin, W.; Du, N.; Zhang, L.; Wu, X.; Hu, Y.; Li, X.; Shen, N.; Li, Y.; Yang, B.; Xu, C.; et al. Genistein alleviates pressure overload-induced cardiac dysfunction and interstitial fibrosis in mice. Br. J. Pharmacol. 2015, 172, 5559–5572. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Z.; Yu, S.; Zhang, W.; Peng, Y.; Pu, M.; Kang, T.; Zeng, J.; Yu, Y.; Li, G. Genistein attenuates monocrotaline-induced pulmonary arterial hypertension in rats by activating PI3K/Akt/eNOS signaling. Histol. Histopathol. 2017, 32, 35–41. [Google Scholar]
- Zhang, M.; Wu, Y.; Wang, M.; Wang, Y.; Tausif, R.; Yang, Y. Genistein rescues hypoxia-induced pulmonary arterial hypertension through estrogen receptor and beta-adrenoceptor signaling. J. Nutr. Biochem. 2018, 58, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Gao, M.; Wu, Z.; Guo, Y. Genistein attenuates low temperature induced pulmonary hypertension in broiler chicks by modulating endothelial function. Eur. J. Pharmacol. 2010, 649, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Sessa, W.C. Nitric oxide synthases: Regulation and function. Eur. Heart J. 2012, 33, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef]
- Patrono, C. Cardiovascular effects of cyclooxygenase-2 inhibitors: A mechanistic and clinical perspective. Br. J. Clin. Pharmacol. 2016, 82, 957–964. [Google Scholar] [CrossRef]
- Chagas, C.M.; Moss, S.; Alisaraie, L. Drug metabolites and their effects on the development of adverse reactions: Revisiting Lipinski’s Rule of Five. Int. J. Pharm. 2018, 549, 133–149. [Google Scholar] [CrossRef]
- Watson, G.; Oliver, E.; Zhao, L.; Wilkins, M.R. Pulmonary hypertension: Old targets revisited (statins, PPARs, beta-blockers). Pharmacother. Pulm. Hypertens. 2013, 218, 531–548. [Google Scholar]
- Voelkel, N.F.; Gomez-Arroyo, J. The role of vascular endothelial growth factor in pulmonary arterial hypertension. The angiogenesis paradox. Am. J. Respir. Cell Mol. Biol. 2014, 51, 474–484. [Google Scholar] [CrossRef]
- Fredenburgh, L.E.; Ma, J.; Perrella, M.A. Cyclooxygenase-2 inhibition and hypoxia-induced pulmonary hypertension: Effects on pulmonary vascular remodeling and contractility. Trends Cardiovasc. Med. 2009, 19, 31–37. [Google Scholar] [CrossRef]
- Varshney, R.; Ali, Q.; Wu, C.; Sun, Z. Monocrotaline-Induced Pulmonary Hypertension Involves Downregulation of Antiaging Protein Klotho and eNOS Activity. Hypertension 2016, 68, 1255–1263. [Google Scholar] [CrossRef] [Green Version]
- Afdal, P.; AbdelMassih, A.F. Is pulmonary vascular disease reversible with PPAR agonists? Microcirculation 2018, 25, e12444. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, L.A.; Obaid, A.A.; Zaki, H.F.; Agha, A.M. Role of oxidative stress, inflammation, nitric oxide and transforming growth factor-beta in the protective effect of diosgenin in monocrotaline-induced pulmonary hypertension in rats. Eur. J. Pharmacol. 2014, 740, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Sahara, M.; Sata, M.; Morita, T.; Hirata, Y.; Nagai, R. Nicorandil attenuates monocrotaline-induced vascular endothelial damage and pulmonary arterial hypertension. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Montani, D.; Chaumais, M.C.; Guignabert, C.; Gunther, S.; Girerd, B.; Jais, X.; Algalarrondo, V.; Price, L.C.; Savale, L.; Sitbon, O.; et al. Targeted therapies in pulmonary arterial hypertension. Pharmacol. Ther. 2014, 141, 172–191. [Google Scholar] [CrossRef]
- Yang, Y.; Nie, W.; Yuan, J.; Zhang, B.; Wang, Z.; Wu, Z.; Guo, Y. Genistein activates endothelial nitric oxide synthase in broiler pulmonary arterial endothelial cells by an Akt-dependent mechanism. Exp. Mol. Med. 2010, 42, 768–776. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.P.; Zhao, J.H.; Yu, H.X.; Guo, D.X. Genistein ameliorated endothelial nitric oxidase synthase uncoupling by stimulating sirtuin-1 pathway in ox-LDL-injured HUVECs. Environ. Toxicol. Pharmacol. 2016, 42, 118–124. [Google Scholar] [CrossRef]
- Fox, C.J.; Cornett, E.M.; Hart, B.M.; Kaye, A.J.; Patil, S.S.; Turpin, M.C.; Valdez, A.; Urman, R.D.; Kaye, A.D. Pulmonary vasodilators: Latest evidence and outcomes in the perioperative setting. Best. Pract. Res. Clin. Anaesthesiol. 2018, 32, 237–250. [Google Scholar] [CrossRef]
- Akagi, S.; Matsubara, H.; Nakamura, K.; Ito, H. Modern treatment to reduce pulmonary arterial pressure in pulmonary arterial hypertension. J. Cardiol. 2018, 72, 466–472. [Google Scholar] [CrossRef] [Green Version]
- Barnes, H.; Yeoh, H.L.; Fothergill, T.; Burns, A.; Humbert, M.; Williams, T. Prostacyclin for pulmonary arterial hypertension. Cochrane Database Syst. Rev. 2019, 5. [Google Scholar] [CrossRef]
- Zhang, Y.F.; Xu, H.M.; Yu, F.; Wang, M.; Li, M.Y.; Xu, T.; Gao, Y.Y.; Wang, J.X.; Li, P.F. Crosstalk between MicroRNAs and Peroxisome Proliferator-Activated Receptors and Their Emerging Regulatory Roles in Cardiovascular Pathophysiology. PPAR Res. 2018, 2018, 8530371. [Google Scholar] [CrossRef]
- Idris-Khodja, N.; Ouerd, S.; Trindade, M.; Gornitsky, J.; Rehman, A.; Barhoumi, T.; Offermanns, S.; Gonzalez, F.J.; Neves, M.F.; Paradis, P.; et al. Vascular smooth muscle cell peroxisome proliferator-activated receptor gamma protects against endothelin-1-induced oxidative stress and inflammation. J. Hypertens. 2017, 35, 1390–1401. [Google Scholar] [CrossRef] [PubMed]
- Toral, M.; Romero, M.; Perez-Vizcaino, F.; Duarte, J.; Jimenez, R. Antihypertensive effects of peroxisome proliferator-activated receptor-beta/delta activation. Am. J. Physiol. Heart Circ. Physiol. 2017, 312, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Maccallini, C.; Mollica, A.; Amoroso, R. The Positive Regulation of eNOS Signaling by PPAR Agonists in Cardiovascular Diseases. Am. J. Cardiovasc. Drugs 2017, 17, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Pluchart, H.; Khouri, C.; Blaise, S.; Roustit, M.; Cracowski, J.L. Targeting the Prostacyclin Pathway: Beyond Pulmonary Arterial Hypertension. Trends Pharmacol. Sci. 2017, 38, 512–523. [Google Scholar] [CrossRef]
- Rabinovitch, M.; Guignabert, C.; Humbert, M.; Nicolls, M.R. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ. Res. 2014, 115, 165–175. [Google Scholar] [CrossRef]
- Bello-Klein, A.; Mancardi, D.; da Rosa Araujo, A.S.; Schenkel, P.C.; de Lima Seolin, B.G.; Turck, P. Role of Redox Homeostasis and Inflammation in the Pathogenesis of Pulmonary Arterial Hypertension. Curr. Med. Chem. 2018, 25, 1340–1351. [Google Scholar] [CrossRef]
- Farkas, D.; Thompson, A.A.R.; Bhagwani, A.R.; Hultman, S.; Ji, H.; Kotha, N.; Farr, G.; Arnold, N.D.; Braithwaite, A.; Casbolt, H.; et al. Toll-like Receptor 3 Is a Therapeutic Target for Pulmonary Hypertension. Am. J. Respir. Crit. Care Med. 2019, 199, 199–210. [Google Scholar] [CrossRef]
- Chen, C.Y.; Chen, J.; He, L.; Stiles, B.L. PTEN: Tumor Suppressor and Metabolic Regulator. Front. Endocrinol. 2018, 9, 338. [Google Scholar] [CrossRef] [Green Version]
- Ravi, Y.; Selvendiran, K.; Meduru, S.; Citro, L.; Naidu, S.; Khan, M.; Rivera, B.K.; Sai-Sudhakar, C.B.; Kuppusamy, P. Dysregulation of PTEN in cardiopulmonary vascular remodeling induced by pulmonary hypertension. Cell Biochem. Biophys. 2013, 67, 363–732. [Google Scholar] [CrossRef]
- Tajsic, T.; Morrell, N.W. Smooth muscle cell hypertrophy, proliferation, migration and apoptosis in pulmonary hypertension. Compr. Physiol. 2011, 1, 295–317. [Google Scholar]
- Lyle, M.A.; Davis, J.P.; Brozovich, F.V. Regulation of Pulmonary Vascular Smooth Muscle Contractility in Pulmonary Arterial Hypertension: Implications for Therapy. Front. Physiol. 2017, 8, 614. [Google Scholar] [CrossRef] [PubMed]
- Matori, H.; Umar, S.; Nadadur, R.D.; Sharma, S.; Partow-Navid, R.; Afkhami, M.; Amjedi, M.; Eghbali, M. Genistein, a soy phytoestrogen, reverses severe pulmonary hypertension and prevents right heart failure in rats. Hypertension 2012, 60, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.; Ikeda, K.; Takebe, M.; Yamori, Y. Genistein, daidzein and glycitein inhibit growth and DNA synthesis of aortic smooth muscle cells from stroke-prone spontaneously hypertensive rats. J. Nutr. 2001, 131, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Chirumbolo, S. Genistein as a nature-derived PPAR agonist in adipogenesis and weight gain. Eur. J. Nutr. 2015, 54, 489–491. [Google Scholar] [CrossRef]
- Wang, L.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Blunder, M.; Liu, X.; Malainer, C.; Blazevic, T.; Schwaiger, S.; Rollinger, J.M.; Heiss, E.H.; et al. Natural product agonists of peroxisome proliferator-activated receptor gamma (PPARgamma): A review. Biochem. Pharmacol. 2014, 92, 73–89. [Google Scholar] [CrossRef]
- Vora, J.; Patel, S.; Sinha, S.; Sharma, S.; Srivastava, A.; Chhabria, M.; Shrivastava, N. Molecular docking, QSAR and ADMET based mining of natural compounds against prime targets of HIV. J. Biomol. Struct. Dyn. 2019, 37, 131–146. [Google Scholar] [CrossRef]
- Kuhn, M.; Szklarczyk, D.; Franceschini, A.; von Mering, C.; Jensen, L.J.; Bork, P. STITCH 3: Zooming in on protein-chemical interactions. Nucleic Acids Res. 2012, 40, 876–880. [Google Scholar] [CrossRef]
- Ru, J.; Li, P.; Wang, J.; Zhou, W.; Li, B.; Huang, C.; Li, P.; Guo, Z.; Tao, W.; Yang, Y.; et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J. Cheminform. 2014, 6, 13. [Google Scholar] [CrossRef]
- Gilson, M.K.; Liu, T.; Baitaluk, M.; Nicola, G.; Hwang, L.; Chong, J. BindingDB in 2015: A public database for medicinal chemistry, computational chemistry and systems pharmacology. Nucleic Acids Res. 2016, 44, 1045–1053. [Google Scholar] [CrossRef]
- Liu, T.; Lin, Y.; Wen, X.; Jorissen, R.N.; Gilson, M.K. BindingDB: A web-accessible database of experimentally determined protein-ligand binding affinities. Nucleic Acids Res. 2007, 35, 198–201. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissTargetPrediction: Updated data and new features for efficient prediction of protein targets of small molecules. Nucleic Acids Res. 2019, 47, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017, 45, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Stelzer, G.; Plaschkes, I.; Oz-Levi, D.; Alkelai, A.; Olender, T.; Zimmerman, S.; Twik, M.; Belinky, F.; Fishilevich, S.; Nudel, R.; et al. VarElect: The phenotype-based variation prioritizer of the GeneCards Suite. BMC Genom. 2016, 17, 444. [Google Scholar] [CrossRef] [PubMed]
- Franz, M.; Rodriguez, H.; Lopes, C.; Zuberi, K.; Montojo, J.; Bader, G.D.; Morris, Q. GeneMANIA update 2018. Nucleic Acids Res. 2018, 46, 60–64. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, B.; Pache, L.; Chang, M.; Khodabakhshi, A.H.; Tanaseichuk, O.; Benner, C.; Chanda, S.K. Metascape provides a biologist-oriented resource for the analysis of systems-level datasets. Nat. Commun. 2019, 10, 1523. [Google Scholar] [CrossRef] [PubMed]


| Property | Parameter |
|---|---|
| MW | 270.24 g/mol |
| PSA | 87 ^A |
| XLogP3-AA | 2.7 |
| H-bond donor | 3 |
| H-bond acceptor | 5 |
| Rotatable bond count | 1 |
| ADMET Absorption Level | 0 |
| BBB Level | 3 |
| NO. | Symbol | Description | Phenotypic Correlation | Score |
|---|---|---|---|---|
| 1 | NOS3 | Nitric Oxide Synthase 3 | Directly | 20.12 |
| 2 | NOS2 | Nitric Oxide Synthase 2 | Directly | 16.1 |
| 3 | VEGFA | Vascular Endothelial Growth Factor A | Directly | 11.77 |
| 4 | TNF | Tumor Necrosis Factor | Directly | 10.34 |
| 5 | PTGS2 | Prostaglandin-Endoperoxide Synthase 2 | Directly | 8.69 |
| 6 | ELANE | Elastase, Neutrophil Expressed | Directly | 8.53 |
| 7 | CCL2 | C-C Motif Chemokine Ligand 2 | Directly | 7.54 |
| 8 | PPARG | Peroxisome Proliferator Activated Receptor Gamma | Directly | 7.45 |
| 9 | IL1B | Interleukin 1 Beta | Directly | 6.44 |
| 10 | EGFR | Epidermal Growth Factor Receptor | Directly | 3.69 |
| 11 | MIF | Macrophage Migration Inhibitory Factor | Directly | 3.57 |
| 12 | TP53 | Tumor Protein P53 | Directly | 3.16 |
| 13 | AKT1 | AKT Serine/Threonine Kinase 1 | Directly | 2.53 |
| 14 | CASP3 | Caspase 3 | Directly | 2.38 |
| 15 | MAPK1 | Mitogen-Activated Protein Kinase 1 | Directly | 1.98 |
| 16 | HSP90AA1 | Heat Shock Protein 90 Alpha Family Class A Member 1 | Directly | 1.91 |
| 17 | HMGCR | 3-Hydroxy-3-Methylglutaryl-CoA Reductase | Directly | 1.83 |
| 18 | MME | Membrane Metalloendopeptidase | Directly | 0.64 |
| 19 | MAPK3 | Mitogen-Activated Protein Kinase 3 | Directly | 0.58 |
| 20 | BCL2 | BCL2, Apoptosis Regulator | Directly | 0.29 |
| 21 | MAPK14 | Mitogen-Activated Protein Kinase 14 | InDirectly | 8.24 |
| 22 | ADORA2A | Adenosine A2a Receptor | InDirectly | 3.27 |
| Compound | -CDCOKER Energy (kcal/mol) | -CDCOKER Interaction_Energy (kcal/mol) |
|---|---|---|
| Genistein | 29.4126 | 37.9578 |
| Trans-resveratrol | 25.1709 | 33.4104 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Chen, D.; Liu, S.; Yuan, T.; Guo, J.; Fang, L.; Du, G. Systematic Elucidation of the Mechanism of Genistein against Pulmonary Hypertension via Network Pharmacology Approach. Int. J. Mol. Sci. 2019, 20, 5569. https://doi.org/10.3390/ijms20225569
Chen Y, Chen D, Liu S, Yuan T, Guo J, Fang L, Du G. Systematic Elucidation of the Mechanism of Genistein against Pulmonary Hypertension via Network Pharmacology Approach. International Journal of Molecular Sciences. 2019; 20(22):5569. https://doi.org/10.3390/ijms20225569
Chicago/Turabian StyleChen, Yucai, Di Chen, Sijia Liu, Tianyi Yuan, Jian Guo, Lianhua Fang, and Guanhua Du. 2019. "Systematic Elucidation of the Mechanism of Genistein against Pulmonary Hypertension via Network Pharmacology Approach" International Journal of Molecular Sciences 20, no. 22: 5569. https://doi.org/10.3390/ijms20225569






