miRNAs Regulate Cytokine Secretion Induced by Phosphorylated S100A8/A9 in Neutrophils
Abstract
:1. Introduction
2. Results
2.1. S100A9 Is Phosphorylated in Synovial Fluids from Rheumatoid Arthritis Patients
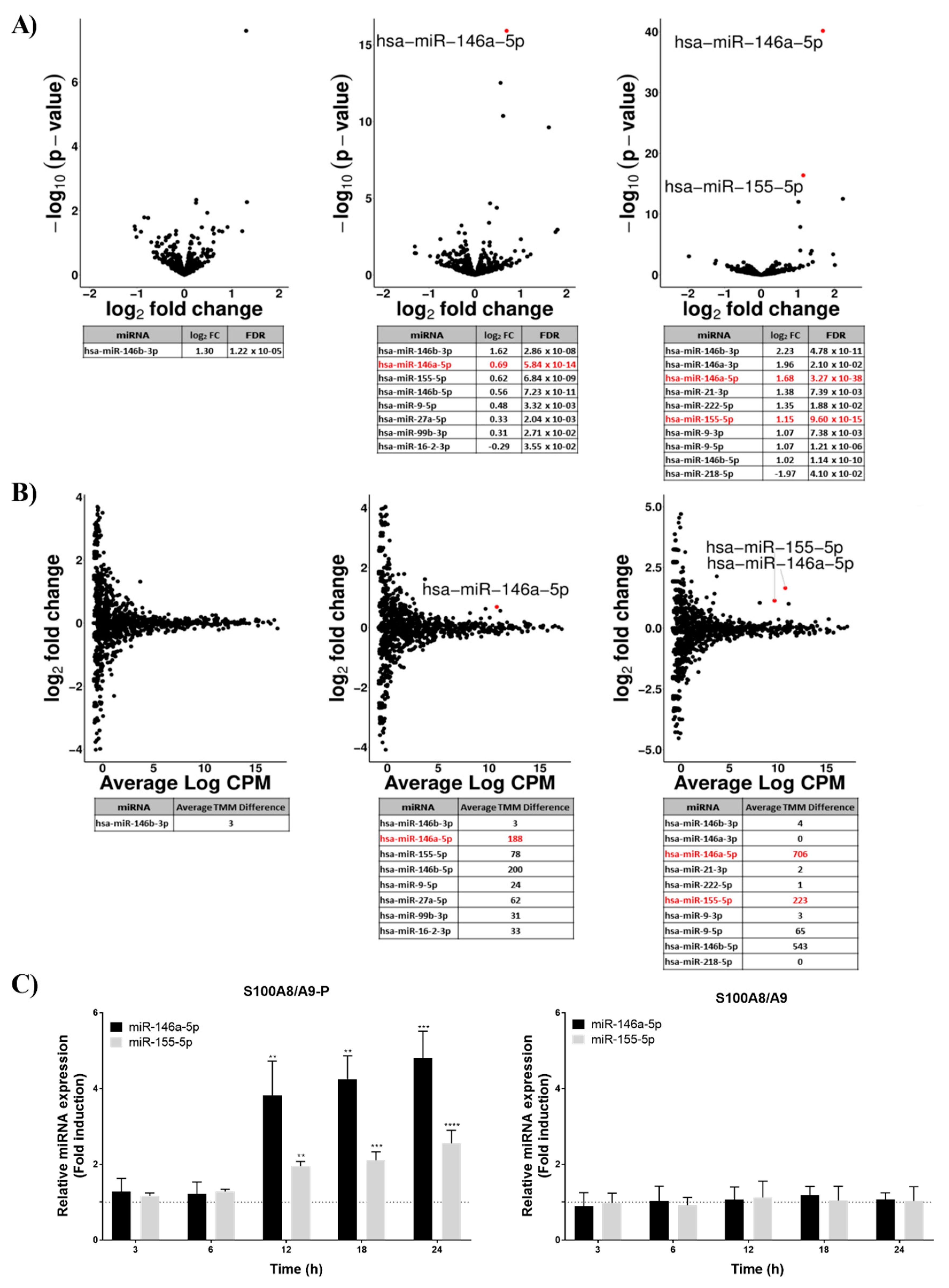
2.2. S100A8/A9-P Induces the Expression of miRNA in Neutrophil-Like Cells
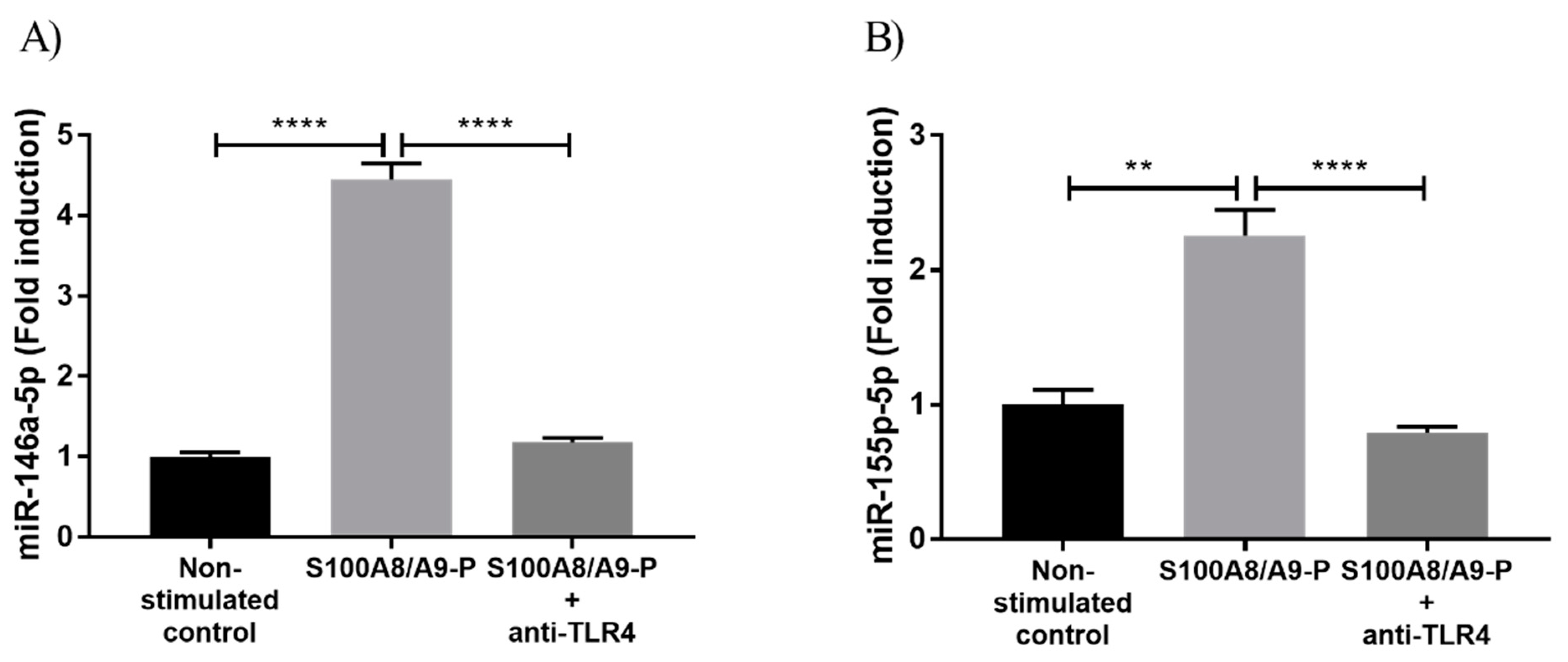
2.3. S100A8/A9-P Induces miR-146a and miR-155 Expression through TLR4 Signaling Pathways
2.4. miR-146a-5p and miR-155-5p Regulate Cytokine Secretion in dHL-60 Cells
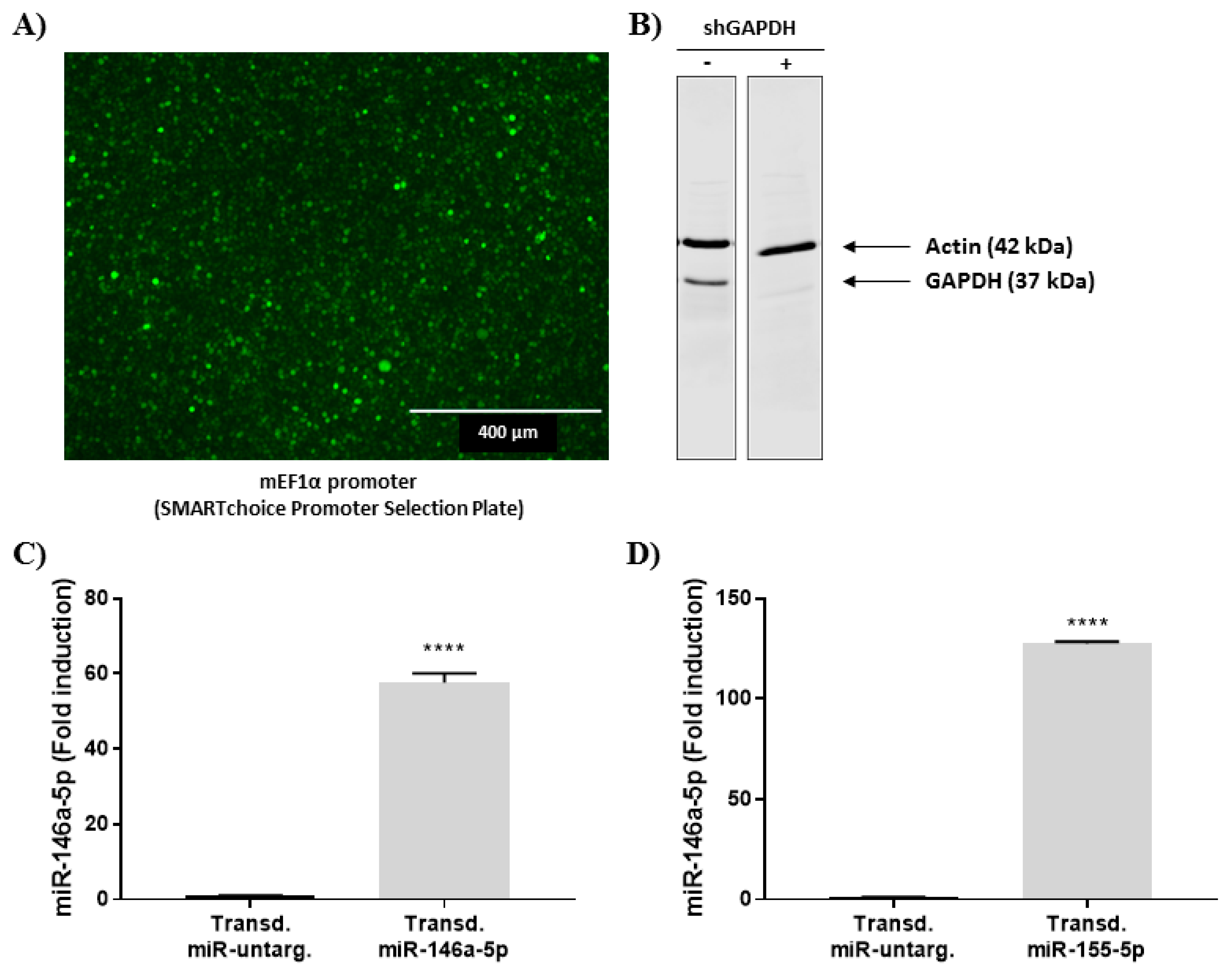
2.4.1. Stable Expression of miRNA Mimics
2.4.2. Effect of miR146a-5p and miR-155-5p Mimics on S100A8/A9-P-Induced Cytokine Secretion
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Cell Culture
4.3. Collection of Synovial Fluids
4.4. Western Blot of Synovial Fluids
4.5. Purification of S100A8/A9 and S100A8/A9-P
4.6. RNA Extraction and Reverse Transcription
4.7. miRNA Sequencing and Analysis
4.8. Quantitative Real-Time PCR
4.9. TLR4 Receptor Neutralization
4.10. Viral Transduction of miRNA Mimics in dHL-60
4.11. Measurement of Cytokine Secretion by Cytometric Bead Array (CBA) and ELISA
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| RA | Rheumatoid Arthritis |
| S100A8/A9-P | Phosphorylated form of S100A8/A9 |
| DAMP | Damage-Associated Molecular Patterns molecules |
| dHL-60 | DMSO-differentiated HL-60 cells |
| TLR4 | Toll-Like Receptor |
| OA | Osteoarthritis |
| CBA | Cytometric Bead Array |
| FC | Fold Change |
| FDR | False Discovery Rate |
| CPM | Count Per Million |
References
- Tecchio, C.; Micheletti, A.; Cassatella, M.A. Neutrophil-derived cytokines: Facts beyond expression. Front. Immunol. 2014, 5, 508. [Google Scholar] [CrossRef] [PubMed]
- Cassatella, M.A.; Östberg, N.K.; Tamassia, N.; Soehnlein, O. Biological roles of neutrophil-derived granule proteins and cytokines. Trends Immunol. 2019, 40, 648–664. [Google Scholar] [CrossRef] [PubMed]
- Wright, H.L.; Moots, R.J.; Bucknall, R.C.; Edwards, S.W. Neutrophil function in inflammation and inflammatory diseases. Rheumatology 2010, 49, 1618–1631. [Google Scholar] [CrossRef]
- Pellmé, S.; Mörgelin, M.; Tapper, H.; Mellqvist, U.H.; Dahlgren, C.; Karlsson, A. Localization of human neutrophil interleukin-8 (CXCL-8) to organelle(s) distinct from the classical granules and secretory vesicles. J. Leukoc. Biol. 2006, 79, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Stanley, A.C.; Lacy, P. Pathways for cytokine secretion. Physiology 2010, 25, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Tamassia, N.; Bianchetto-Aguilera, F.; Arruda-Silva, F.; Gardiman, E.; Gasperini, S.; Calzetti, F.; Cassatella, M.A. Cytokine production by human neutrophils: Revisiting the “dark side of the moon”. Eur. J. Clin. Investig. 2018, 48 (Suppl. 2), e12952. [Google Scholar] [CrossRef] [PubMed]
- Loser, K.; Vogl, T.; Voskort, M.; Lueken, A.; Kupas, V.; Nacken, W.; Klenner, L.; Kuhn, A.; Foell, D.; Sorokin, L.; et al. The Toll-like receptor 4 ligands Mrp8 and Mrp14 are crucial in the development of autoreactive CD8+ T cells. Nat. Med. 2010, 16, 713–717. [Google Scholar] [CrossRef]
- Foell, D.; Roth, J. Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum. 2004, 50, 3762–3771. [Google Scholar] [CrossRef]
- Kang, K.Y.; Woo, J.W.; Park, S.H. S100A8/A9 as a biomarker for synovial inflammation and joint damage in patients with rheumatoid arthritis. Korean J. Intern. Med. 2014, 29, 12–19. [Google Scholar] [CrossRef]
- Vogl, T.; Eisenblatter, M.; Voller, T.; Zenker, S.; Hermann, S.; van Lent, P.; Faust, A.; Geyer, C.; Petersen, B.; Roebrock, K.; et al. Alarmin S100A8/S100A9 as a biomarker for molecular imaging of local inflammatory activity. Nat. Commun. 2014, 5, 4593. [Google Scholar] [CrossRef]
- Austermann, J.; Spiekermann, C.; Roth, J. S100 proteins in rheumatic diseases. Nat. Rev. Rheumatol. 2018, 14, 528–541. [Google Scholar] [CrossRef] [PubMed]
- Hammer, H.B.; Haavardsholm, E.A.; Kvien, T.K. Calprotectin (a major leucocyte protein) is associated with the levels of anti-CCP and rheumatoid factor in a longitudinal study of patients with very early rheumatoid arthritis. Scand. J. Rheumatol. 2008, 37, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Hurnakova, J.; Hulejova, H.; Zavada, J.; Hanova, P.; Komarc, M.; Mann, H.; Klein, M.; Sleglova, O.; Olejarova, M.; Forejtova, S.; et al. Relationship between serum calprotectin (S100A8/9) and clinical, laboratory and ultrasound parameters of disease activity in rheumatoid arthritis: A large cohort study. PLoS ONE 2017, 12, e0183420. [Google Scholar] [CrossRef] [PubMed]
- Ryckman, C.; Vandal, K.; Rouleau, P.; Talbot, M.; Tessier, P.A. Proinflammatory activities of S100: Proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J. Immunol. 2003, 170, 3233–3242. [Google Scholar] [CrossRef]
- Ehrchen, J.M.; Sunderkötter, C.; Foell, D.; Vogl, T.; Roth, J. The endogenous Toll-like receptor 4 agonist S100A8/S100A9 (calprotectin) as innate amplifier of infection, autoimmunity, and cancer. J. Leukoc. Biol. 2009, 86, 557–566. [Google Scholar] [CrossRef]
- Vogl, T.; Roth, J.; Sorg, C.; Hillenkamp, F.; Strupat, K. Calcium-induced noncovalently linked tetramers of MRP8 and MRP14 detected by ultraviolet matrix-assisted laser desorption/ionization mass spectrometry. J. Am. Soc. Mass Spectrom. 1999, 10, 1124–1130. [Google Scholar]
- Schenten, V.; Plançon, S.; Jung, N.; Hann, J.; Bueb, J.L.; Bréchard, S.; Tschirhart, E.J.; Tolle, F. Secretion of the phosphorylated form of S100A9 from neutrophils is essential for the proinflammatory functions of extracellular S100A8/A9. Front. Immunol. 2018, 9, 447. [Google Scholar] [CrossRef]
- Murata, K.; Yoshitomi, H.; Furu, M.; Ishikawa, M.; Shibuya, H.; Ito, H.; Matsuda, S. MicroRNA-451 down-regulates neutrophil chemotaxis via p38 MAPK. Arthritis Rheumatol. 2014, 66, 549–559. [Google Scholar] [CrossRef]
- Pauley, K.M.; Satoh, M.; Chan, A.L.; Bubb, M.R.; Reeves, W.H.; Chan, E.K. Upregulated miR 146a expression in peripheral blood mononuclear cells from rheumatoid arthritis patients. Arthritis Res. Ther. 2008, 10, R101. [Google Scholar] [CrossRef]
- Nakasa, T.; Miyaki, S.; Okubo, A.; Hashimoto, M.; Nishida, K.; Ochi, M.; Asahara, H. Expression of microRNA-146 in rheumatoid arthritis synovial tissue. Arthritis Rheum. 2008, 58, 1284–1292. [Google Scholar] [CrossRef]
- Tsitsiou, E.; Lindsay, M.A. microRNAs and the immune response. Curr. Opin. Pharmacol. 2009, 9, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wan, Y.; Guo, Q.; Zou, L.; Zhang, J.; Fang, Y.; Zhang, J.; Zhang, J.; Fu, X.; Liu, H.; et al. Altered microRNA expression profile with miR-146a upregulation in CD4+ T cells from patients with rheumatoid arthritis. Arthritis Res. Ther. 2010, 12, R81. [Google Scholar] [CrossRef] [PubMed]
- Blüml, S.; Bonelli, M.; Niederreiter, B.; Puchner, A.; Mayr, G.; Hayer, S.; Koenders, M.I.; van den Berg, W.B.; Smolen, J.; Redlich, K. Essential role of microRNA-155 in the pathogenesis of autoimmune arthritis in mice. Arthritis Rheum. 2011, 63, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Kurowska-Stolarska, M.; Alivernini, S.; Ballantine, L.E.; Asquith, D.L.; Millar, N.L.; Gilchrist, D.S.; Reilly, J.; Ierna, M.; Fraser, A.R.; Stolarski, B.; et al. MicroRNA-155 as a proinflammatory regulator in clinical and experimental arthritis. Proc. Natl. Acad. Sci. USA 2011, 108, 11193–11198. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Jing, Z.; Cheng, G. MicroRNAs: new regulators of Toll-like receptor signalling pathways. Biomed. Res. Intern. 2014, 2014, 945169. [Google Scholar] [CrossRef] [PubMed]
- Ryckman, C.; McColl, S.R.; Vandal, K.; de Médicis, R.; Lussier, A.; Poubelle, P.E.; Tessier, P.A. Role of S100A8 and S100A9 in neutrophil recruitment in response to monosodium urate monohydrate crystals in the air-pouch model of acute gouty arthritis. Arthritis Rheum. 2003, 48, 2310–2320. [Google Scholar] [CrossRef]
- Baillet, A.; Trocmé, C.; Berthier, S.; Arlotto, M.; Grange, L.; Chenau, J.; Quétant, S.; Sève, M.; Berger, F.; Juvin, R.; et al. Synovial fluid proteomic fingerprint: S100A8, S100A9 and S100A12 proteins discriminate rheumatoid arthritis from other inflammatory joint diseases. Rheumatology 2010, 49, 671–682. [Google Scholar] [CrossRef]
- Lee, D.G.; Woo, J.W.; Kwok, S.K.; Cho, M.L.; Park, S.H. MRP8 promotes Th17 differentiation via upregulation of IL-6 production by fibroblast-like synoviocytes in rheumatoid arthritis. Exp. Mol. Med. 2013, 45, e20. [Google Scholar] [CrossRef]
- Holzinger, D.; Nippe, N.; Vogl, T.; Marketon, K.; Mysore, V.; Weinhage, T.; Dalbeth, N.; Pool, B.; Merriman, T.; Baeten, D.; et al. Myeloid-related proteins 8 and 14 contribute to monosodium urate monohydrate crystal-induced inflammation in gout. Arthritis Rheumatol. 2014, 66, 1327–1339. [Google Scholar] [CrossRef]
- Sunahori, K.; Yamamura, M.; Yamana, J.; Takasugi, K.; Kawashima, M.; Yamamoto, H.; Chazin, W.J.; Nakatani, Y.; Yui, S.; Makino, H. The S100A8/A9 heterodimer amplifies proinflammatory cytokine production by macrophages via activation of nuclear factor kappa B and p38 mitogen-activated protein kinase in rheumatoid arthritis. Arthritis Res. Ther. 2006, 8, R69. [Google Scholar] [CrossRef]
- Cesaro, A.; Anceriz, N.; Plante, A.; Page, N.; Tardif, M.R.; Tessier, P.A. An inflammation loop orchestrated by S100A9 and calprotectin is critical for development of arthritis. PLoS ONE 2012, 7, e45478. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Song, R.; Wang, Z.; Jing, Z.; Wang, S.; Ma, J. S100A8/A9 in Inflammation. Front. Immunol. 2018, 9, 1298. [Google Scholar] [CrossRef] [PubMed]
- Simard, J.C.; Noel, C.; Tessier, P.A.; Girard, D. Human S100A9 potentiates IL-8 production in response to GM-CSF or fMLP via activation of a different set of transcription factors in neutrophils. FEBS Lett. 2014, 588, 2141–2146. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.; Stratis, A.; Wixler, V.; Voller, T.; Thurainayagam, S.; Jorch, S.K.; Zenker, S.; Dreiling, A.; Chakraborty, D.; Fröhling, M.; et al. Autoinhibitory regulation of S100A8/S100A9 alarmin activity locally restricts sterile inflammation. J. Clin. Investig. 2018, 128, 1852–1866. [Google Scholar] [CrossRef]
- Lim, S.Y.; Raftery, M.; Cai, H.; Hsu, K.; Yan, W.X.; Hseih, H.L.; Watts, R.N.; Richardson, D.; Thomas, S.; Perry, M.; et al. S-nitrosylated S100A8: Novel anti-inflammatory properties. J. Immunol. 2008, 181, 5627–5636. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.Y.; Raftery, M.J.; Goyette, J.; Geczy, C.L. S-glutathionylation regulates inflammatory activities of S100A9. J. Biol. Chem. 2010, 285, 14377–14388. [Google Scholar] [CrossRef] [Green Version]
- Gomes, L.H.; Raftery, M.J.; Yan, W.X.; Goyette, J.D.; Thomas, P.S.; Geczy, C.L. S100A8 and S100A9-oxidant scavengers in inflammation. Free Radic. Biol. Med. 2013, 58, 170–186. [Google Scholar] [CrossRef]
- Lim, S.Y.; Raftery, M.J.; Geczy, C.L. Oxidative modifications of DAMPs suppress inflammation: The case for S100A8 and S100A9. Antioxid. Redox Signal. 2011, 15, 2235–2248. [Google Scholar] [CrossRef]
- Mestas, J.; Hughes, C.C.W. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef] [Green Version]
- Edgeworth, J.; Freemont, P.; Hogg, N. Ionomycin-regulated phosphorylation of the myeloid calcium-binding protein p14. Nature 1989, 342, 189–192. [Google Scholar] [CrossRef]
- Naegelen, I.; Plançon, S.; Nicot, N.; Kaoma, T.; Muller, A.; Vallar, L.; Tschirhart, E.J.; Bréchard, S. An essential role of syntaxin 3 protein for granule exocytosis and secretion of IL-1α, IL-1β, IL-12b, and CCL4 from differentiated HL-60 cells. J. Leukoc. Biol. 2005, 97, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Niimoto, T.; Nakasa, T.; Ishikawa, M.; Okuhara, A.; Izumi, B.; Deie, M.; Suzuki, O.; Adachi, N.; Ochi, M. MicroRNA-146a expresses in interleukin-17 producing T cells in rheumatoid arthritis patients. BMC Musculoskelet. Disord. 2010, 11, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moran-Moguel, M.C.; Petarra-del Rio, S.; Mayorquin-Galvan, E.E.M.; Zavala-Cerna, M.G. Rheumatoid arthritis and miRNAs: A critical review through a functional view. J. Immunol. Res. 2018, 2018, 2474529. [Google Scholar] [CrossRef] [PubMed]
- Su, L.C.; Huang, A.F.; Jia, H.; Liu, Y.; Xu, W.D. Role of microRNA-155 in rheumatoid arthritis. Int. J. Rheum. Dis. 2017, 20, 1631–1637. [Google Scholar] [CrossRef]
- Collins, S.J.; Gallo, R.C.; Gallagher, R.E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature 1997, 270, 347–349. [Google Scholar] [CrossRef]
- van den Bos, C.; Rammes, A.; Vogl, T.; Boynton, R.; Zaia, J.; Sorg, C.; Roth, J. Copurification of P6, MRP8, and MRP14 from human granulocytes and separation of individual proteins. Protein Expr. Purif. 1998, 13, 313–318. [Google Scholar] [CrossRef]
- Jung, N.; Schenten, V.; Bueb, J.L.; Tolle, F.; Bréchard, S. Immune Cells and Inflammatory Diseases group, Life Sciences Research Unit, University of Luxembourg, L-4367, Belvaux. Western blot analysis of S100A9-P and S100A12 contaminant in the different fractions eluted by HPLC.
- Jiang, W.; Hua, R.; Wei, M.; Li, C.; Qiu, Z.; Yang, X.; Zhang, C. An optimized method for high-titer lentivirus preparations without ultracentrifugation. Sci. Rep. 2015, 5, 13875. [Google Scholar] [CrossRef] [Green Version]
- Dodo, K.; Chono, H.; Saito, N.; Tanaka, Y.; Tahara, K.; Nukaya, I.; Mineno, J. An efficient large-scale retroviral transduction method involving preloading the vector into a RetroNectin-coated bag with low-temperature shaking. PLoS ONE 2014, 9, e86275. [Google Scholar] [CrossRef] [Green Version]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, N.; Schenten, V.; Bueb, J.-L.; Tolle, F.; Bréchard, S. miRNAs Regulate Cytokine Secretion Induced by Phosphorylated S100A8/A9 in Neutrophils. Int. J. Mol. Sci. 2019, 20, 5699. https://doi.org/10.3390/ijms20225699
Jung N, Schenten V, Bueb J-L, Tolle F, Bréchard S. miRNAs Regulate Cytokine Secretion Induced by Phosphorylated S100A8/A9 in Neutrophils. International Journal of Molecular Sciences. 2019; 20(22):5699. https://doi.org/10.3390/ijms20225699
Chicago/Turabian StyleJung, Nicolas, Véronique Schenten, Jean-Luc Bueb, Fabrice Tolle, and Sabrina Bréchard. 2019. "miRNAs Regulate Cytokine Secretion Induced by Phosphorylated S100A8/A9 in Neutrophils" International Journal of Molecular Sciences 20, no. 22: 5699. https://doi.org/10.3390/ijms20225699





