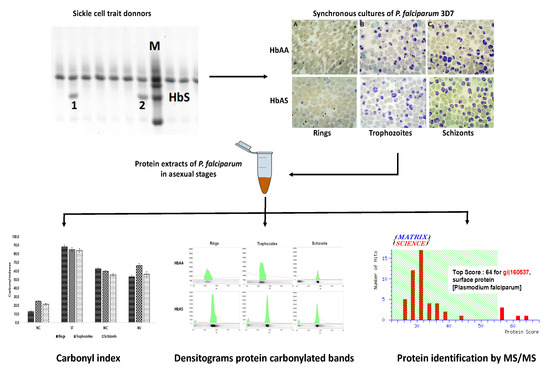
Sickle Cell Trait Induces Oxidative Damage on Plasmodium falciparum Proteome at Erythrocyte Stages
Abstract
:1. Introduction
2. Results
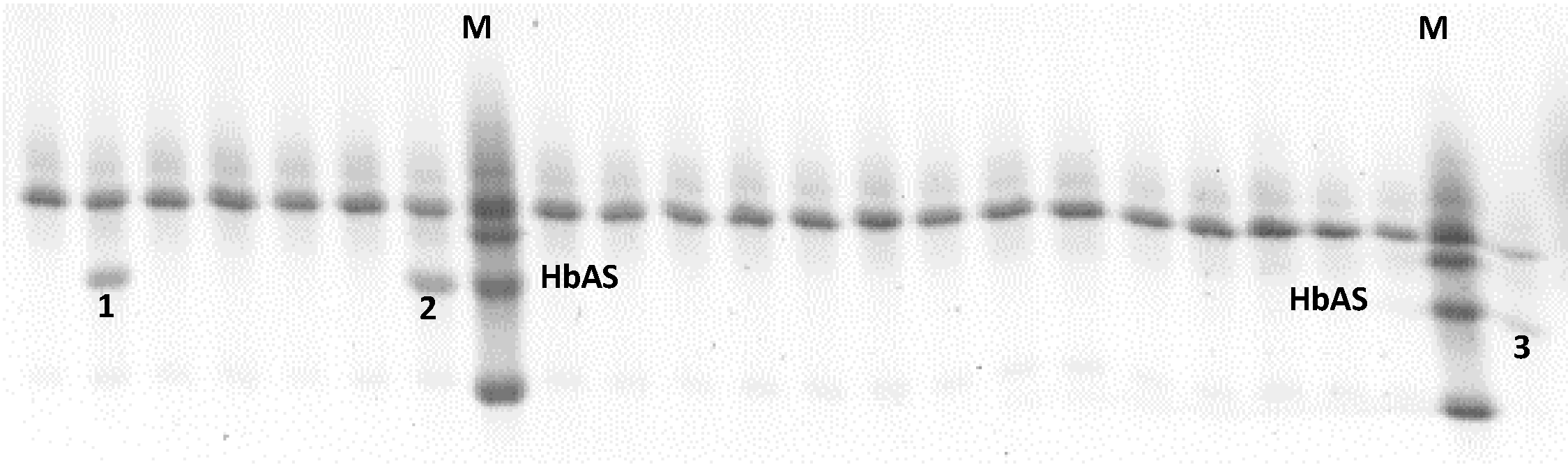
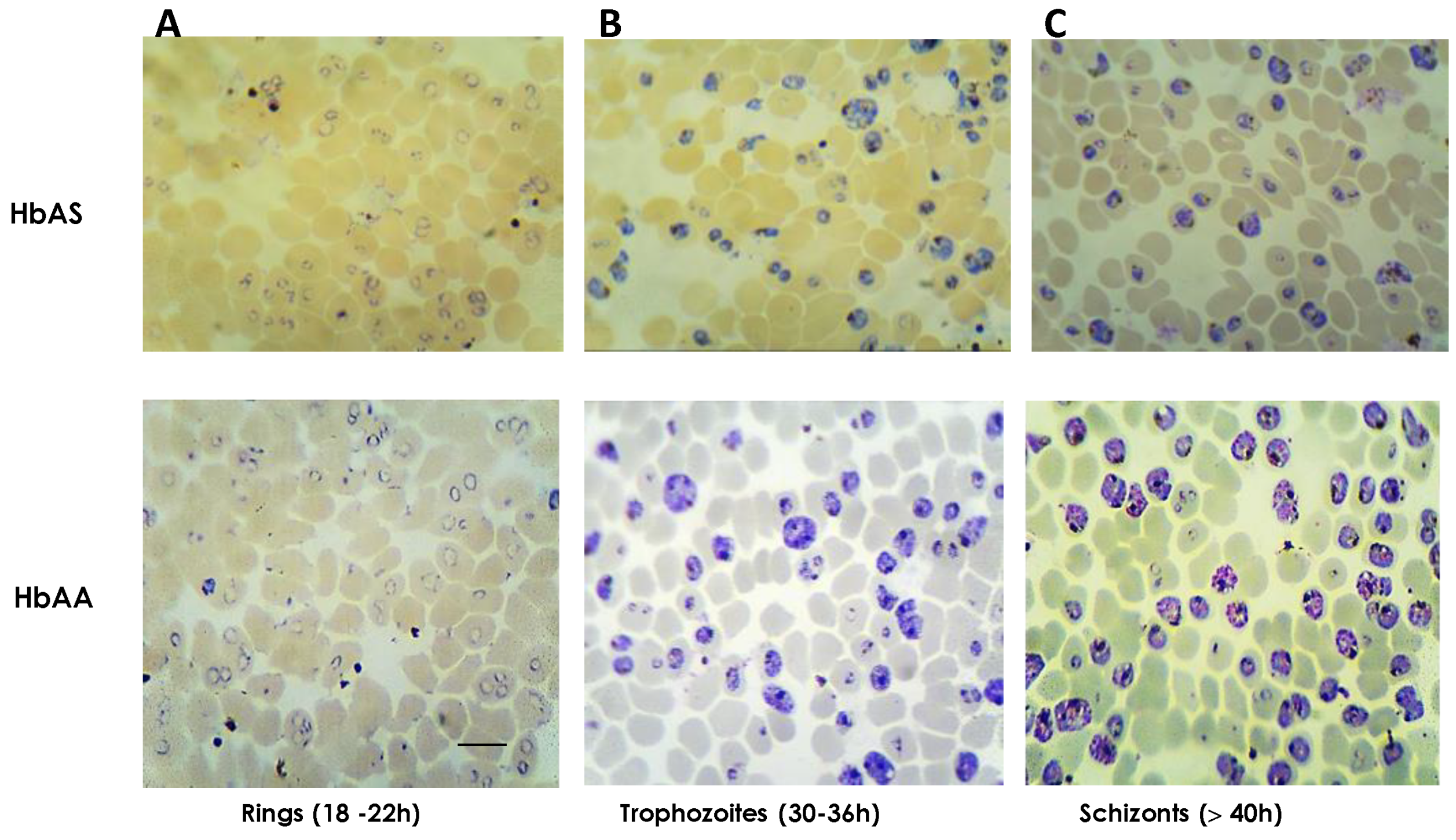
2.1. Cultures of P. falciparum 3D7 on HbAS Carriers and Control Donors
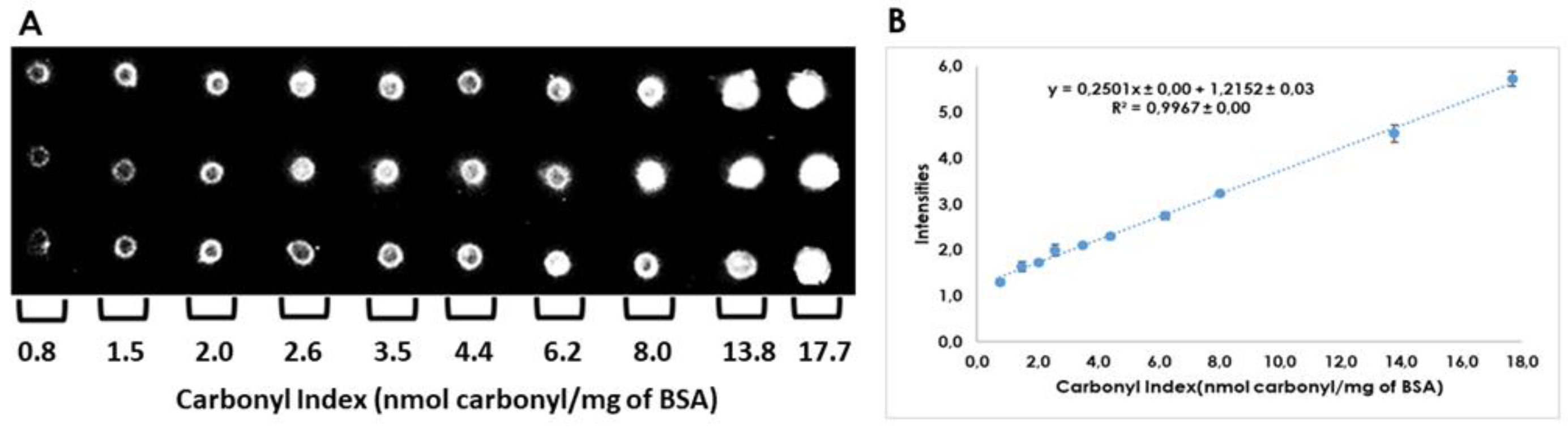
2.2. Quantitation of Carbonyl Index by Dot-Blot
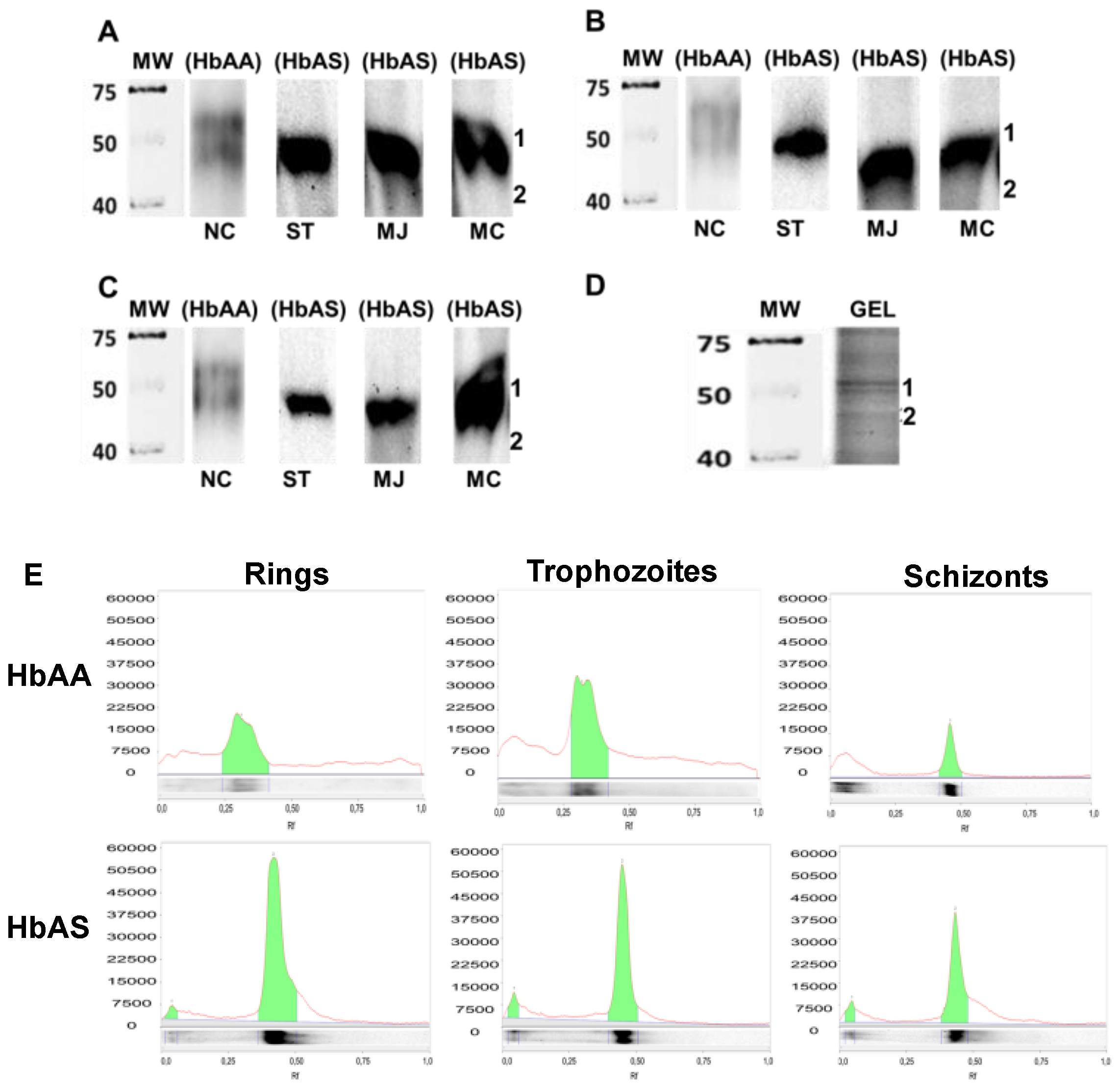
2.3. Determination of P. falciparum Protein Carbonylation Patterns on HbAA and HbAS Carriers
2.4. Identification of Proteins in Carbonylated Bands
3. Discussion
4. Materials and Methods
4.1. Identification of Donor’s Hemoglobin S Carriers
4.2. Plasmodium falciparum 3D7 Cultures and Parasite Protein Obtention
4.3. Carbonyl Index Quantitation of Parasite Protein Fractions
4.4. Determination of Carbonylation Patterns on P. falciparum 3D7 Proteins
4.5. Identification of P. falciparum 3D7 Proteins by Mass Spectrometry
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cyrklaff, M.; Sanchez, C.P.; Frischknecht, F.; Lanzer, M. Host actin remodeling and protection from malaria by hemoglobinopathies. Trends Parasitol. 2012, 28, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Modell, B.; Darlison, M. Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Org. 2008, 86, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Huttle, A.; Maestre, G.E.; Lantigua, R.; Green, N.S. Sickle cell in Latin America and the United States [corrected]. Pediatr. Blood Cancer 2015, 62, 1131–1136. [Google Scholar] [CrossRef] [PubMed]
- Piel, F.B.; Patil, A.P.; Howes, R.E.; Nyangiri, O.A.; Gething, P.; Dewi, M.; Temperley, W.H.; Williams, T.N.; Weatherall, D.J.; Hay, S.I. Global epidemiology of sickle haemoglobin in neonates: A contemporary geostatistical model-based map and population estimates. Lancet 2013, 381, 142–151. [Google Scholar] [CrossRef]
- Gong, L.; Parikh, S.; Rosenthal, P.J.; Greenhouse, B. Biochemical and immunological mechanisms by which sickle cell trait protects against malaria. Malar. J. 2013, 12, 317. [Google Scholar] [CrossRef]
- Cholera, R.; Brittain, N.J.; Gillrie, M.R.; Lopera-Mesa, T.M.; Diakité, S.A.; Arie, T.; Krause, M.A.; Guindo, A.; Tubman, A.; Fujioka, H.; et al. Impaired cytoadherence of plasmodium falciparum-infected erythrocytes containing sickle hemoglobin. Proc. Natl. Acad. Sci. USA 2008, 105, 991–996. [Google Scholar] [CrossRef]
- Lang, P.A.; Kasinathan, R.S.; Brand, V.B.; Duranton, C.; Lang, C.; Koka, S.; Shumilina, E.; Kempe, D.S.; Tanneur, V.; et al. Accelerated clearance of Plasmodium-infected erythrocytes in sickle cell trait and annexin-A7 deficiency. Cell. Physiol. Biochem. 2009, 24, 415–428. [Google Scholar] [CrossRef]
- LaMonte, G.; Philip, N.; Reardon, J.; Lacsina, J.R.; Majoros, W.; Chapman, L.; Thornburg, C.D.; Telen, M.J.; Ohler, U.; et al. Translocation of sickle cell erythrocyte microRNAs into Plasmodium falciparum inhibits parasite translation and contributes to malaria resistance. Cell Host Microbe 2012, 12, 187–199. [Google Scholar] [CrossRef]
- Ferreira, A.; Marguti, I.; Bechmann, I.; Jeney, V.; Chora, A.; Palha, N.R.; Rebelo, S.; Henri, A.; Beuzard, Y.; Soares, M.P. Sickle hemoglobin confers tolerance to Plasmodium infection. Cell 2011, 145, 398–409. [Google Scholar] [CrossRef]
- Becker, K.; Tilley, L.; Vennerstrom, J.L.; Roberts, D.; Rogerson, S.; Ginsburg, H. Oxidative stress in malaria parasite-infected erythrocytes: Host-parasite interactions. Int. J. Parasitol. 2004, 34, 163–189. [Google Scholar] [CrossRef]
- Contreras-Puentes, N.; Rodríguez-Cavallo, E.; Méndez-Cuadro, D. Membrane protein carbonylation of Plasmodium falciparum infected erythrocytes under conditions of sickle cell trait and G6PD deficiency. Mol. Biochem. Parasitol. 2019, 227, 5–14. [Google Scholar] [CrossRef] [PubMed]
- Mendez, D.; Linares, M.; Diez, A.; Puyet, A.; Bautista, J.M. Stress response and cytoskeletal proteins involved in erythrocyte membrane remodeling upon Plasmodium falciparum invasion are differentially carbonylated in G6PD A- deficiency. Free Radic. Biol. Med. 2011, 50, 1305–1313. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, M.; Bollineni, R.C.; Hoffmann, R. Protein carbonylation as a major hallmark of oxidative damage: Update of analytical strategies. Mass Spectrom. Rev. 2014, 33, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Silva, D.G.H.; Belini Junior, E.; de Almeida, E.A.; Bonini-Domingos, C.R. Oxidative stress in sickle cell disease: An overview of erythrocyte redox metabolism and current antioxidant therapeutic strategies. Free Radic. Biol. Med. 2013, 65, 1101–1109. [Google Scholar] [CrossRef] [PubMed]
- Archer, N.M.; Petersen, N.; Clark, M.A.; Buckee, C.O.; Childs, L.M.; Duraisingh, M.T. Resistance to Plasmodium falciparum in sickle cell trait erythrocytes is driven by oxygen-dependent growth inhibition. Proc. Natl. Acad. Sci. USA 2018, 115, 7350–7355. [Google Scholar] [CrossRef]
- Ueda, Y.; Bookchin, R.M. Effects of carbon dioxide and pH variations in vitro on blood respiratory functions, red blood cell volume, transmembrane pH gradients, and sickling in sickle cell anemia. J. Lab. Clin. Med. 1984, 104, 146–159. [Google Scholar]
- Radfar, A.; Méndez, D.; Moneriz, C.; Linares, M.; Marín-García, P.; Puyet, A.; Diez, A.; Bautista, J.M. Synchronous culture of Plasmodium falciparum at high parasitemia levels. Nat. Protoc. 2009, 4, 1899–1915. [Google Scholar] [CrossRef]
- Kavishe, R.A.; Koenderink, J.B.; Alifrangis, M. Oxidative stress in malaria and artemisinin combination therapy: Pros and Cons. FEBS J. 2017, 284, 2579–2591. [Google Scholar] [CrossRef]
- Radfar, A.; Diez, A.; Bautista, J.M. Chloroquine mediates specific proteome oxidative damage across the erythrocytic cycle of resistant Plasmodium falciparum. Free Radic. Biol. Med. 2008, 44, 2034–2042. [Google Scholar] [CrossRef]
- Alvear, C.C.; Barboza, M.; Viola-Rhenals, M.; Moneriz, C.; Araque, L.M. Pilot study of hemoglobinopathies in newborns of the Rafael Calvo maternity clinic of Cartagena, Colombia. Colomb. Med. 2012, 43, 196–199. [Google Scholar]
- Duran, C.L.; Morales, O.L.; Echeverri, S.J.; Isaza, M. Beta globin haplotypes in hemoglobin S carriers in Colombia. Biomedica 2012, 32, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Wehr, N.B.; Levine, R.L. Quantitation of protein carbonylation by dot blot. Anal. Biochem. 2012, 423, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Atamna, H.; Ginsburg, H. Origin of reactive oxygen species in erythrocytes infected with Plasmodium falciparum. Mol. Biochem. Parasitol. 1993, 61, 231–241. [Google Scholar] [CrossRef]
- Mohan, K.; Ganguly, N.K.; Dubey, M.L.; Mahajan, R.C. Oxidative damage of erythrocytes infected with Plasmodium falciparum. An in vitro study. Ann. Hematol. 1992, 65, 131–134. [Google Scholar] [CrossRef]
- Allen, S.M.; Lim, E.E.; Jortzik, E.; Preuss, J.; Chua, H.H.; MacRae, J.I.; Rahlfs, S.; Haeussler, K.; Downton, M.T.; et al. Plasmodium falciparum glucose-6-phosphate dehydrogenase 6-phosphogluconolactonase is a potential drug target. FEBS J. 2015, 282, 3808–3823. [Google Scholar] [CrossRef]
- Scopes, D.A.; Bautista, J.M.; Vulliamy, T.J.; Mason, P.J. Plasmodium falciparum glucose-6-phosphate dehydrogenase (G6PD)-the N-terminal portion is homologous to a predicted protein encoded near to G6PD in Haemophilus influenzae. Mol. Microbiol. 1997, 23, 847–848. [Google Scholar] [CrossRef]
- Mendez, D.; Hernáez, M.L.; Kamali, A.N.; Diez, A.; Puyet, A.; Bautista, J.M. Differential carbonylation of cytoskeletal proteins in blood group O erythrocytes: Potential role in protection against severe malaria. Infect. Genet. Evol. 2012, 12, 1780–1787. [Google Scholar] [CrossRef]
- Akide-Ndunge, O.B.; Tambini, E.; Giribaldi, G.; McMillan, P.J.; Müller, S.; Arese, P.; Turrini, F. Co-ordinated stage-dependent enhancement of Plasmodium falciparum antioxidant enzymes and heat shock protein expression in parasites growing in oxidatively stressed or G6PD-deficient red blood cells. Malar. J. 2009, 8, 113. [Google Scholar] [CrossRef]
- Atamna, H.; Pascarmona, G.; Ginsburg, H. Hexose-monophosphate shunt activity in intact Plasmodium falciparum-infected erythrocytes and in free parasites. Mol. Biochem. Parasitol. 1994, 67, 79–89. [Google Scholar] [CrossRef]
- Linares, M.; Antonio, P.; Amalia, D.; José M., B. Oxidative Stress and Protein Carbonylation in Malaria. In Protein Carbonylation: Principles, Analysis, and Biological Implications; Ros, J., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2017; pp. 131–166. [Google Scholar]
- Grover, M.; Chaubey, S.; Ranade, S.; Tatu, U. Identification of an exported heat shock protein 70 in Plasmodium falciparum. Parasite 2013, 20, 2. [Google Scholar] [CrossRef]
- Baldi, D.L.; Andrews, K.T.; Waller, R.F.; Roos, D.S.; Howard, R.F.; Crabb, B.S.; Cowman, A.F. RAP1 controls rhoptry targeting of RAP2 in the malaria parasite Plasmodium falciparum. EMBO J. 2000, 19, 2435–2443. [Google Scholar] [CrossRef] [PubMed]
- Beeson, J.G.; Drew, D.R.; Boyle, M.J.; Feng, G.; Fowkes, F.J.; Richards, J.S. Merozoite surface proteins in red blood cell invasion, immunity and vaccines against malaria. FEMS Microbiol. Rev. 2016, 40, 343–372. [Google Scholar] [CrossRef] [PubMed]
- Ardeshir, F.; Flint, J.E.; Richman, S.J.; Reese, R.T. A 75 kd merozoite surface protein of Plasmodium falciparum which is related to the 70 kd heat-shock proteins. EMBO J. 1987, 6, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Reithmeier, R.A.; Casey, J.R.; Kalli, A.C.; Sansom, M.S.; Alguel, Y.; Iwata, S. Band 3, the human red cell chloride/bicarbonate anion exchanger (AE1, SLC4A1), in a structural context. Biochim. Biophys. Acta 2016, 1858, 1507–1532. [Google Scholar] [CrossRef] [PubMed]
- Pantaleo, A.; Ferru, E.; Giribaldi, G.; Mannu, F.; Carta, F.; Matte, A.; de Franceschi, L.; Turrini, F. Oxidized and poorly glycosylated band 3 is selectively phosphorylated by Syk kinase to form large membrane clusters in normal and G6PD-deficient red blood cells. Biochem. J. 2009, 418, 359–367. [Google Scholar] [CrossRef] [Green Version]
- Voskou, S.; Aslan, M.; Fanis, P.; Phylactides, M.; Kleanthous, M. Oxidative stress in beta-thalassaemia and sickle cell disease. Redox. Biol. 2015, 6, 226–239. [Google Scholar] [CrossRef] [Green Version]
- Petrov, D.; Zagrovic, B. Microscopic analysis of protein oxidative damage: Effect of carbonylation on structure, dynamics, and aggregability of villin headpiece. J. Am. Chem. Soc. 2011, 133, 7016–7024. [Google Scholar] [CrossRef]
- Alviz-Amador, A.; Galindo-Murillo, R.; Pineda-Alemán, R.; Pérez-González, H.; Rodríguez-Cavallo, E.; Vivas-Reyes, R.; Méndez-Cuadro, D. 4-HNE carbonylation induces local conformational changes on bovine serum albumin and thioredoxin. A molecular dynamics study. J. Mol. Graph. Model. 2019, 86, 298–307. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Mesquita, C.S.; Oliveira, R.; Bento, F.; Geraldo, D.; Rodrigues, J.V.; Marcos, J.C. Simplified 2,4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal. Biochem. 2014, 458, 69–71. [Google Scholar] [CrossRef]
- Levine, R.L.; Garland, D.; Oliver, C.N.; Amici, A.; Climent, I.; Lenz, A.G.; Ahn, B.W.; Shaltiel, S.; Stadtman, E.R. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990, 186, 464–478. [Google Scholar] [PubMed]
- Sechi, S.; Chait, B.T. Modification of cysteine residues by alkylation. A tool in peptide mapping and protein identification. Anal. Chem. 1998, 70, 5150–5158. [Google Scholar] [CrossRef] [PubMed]
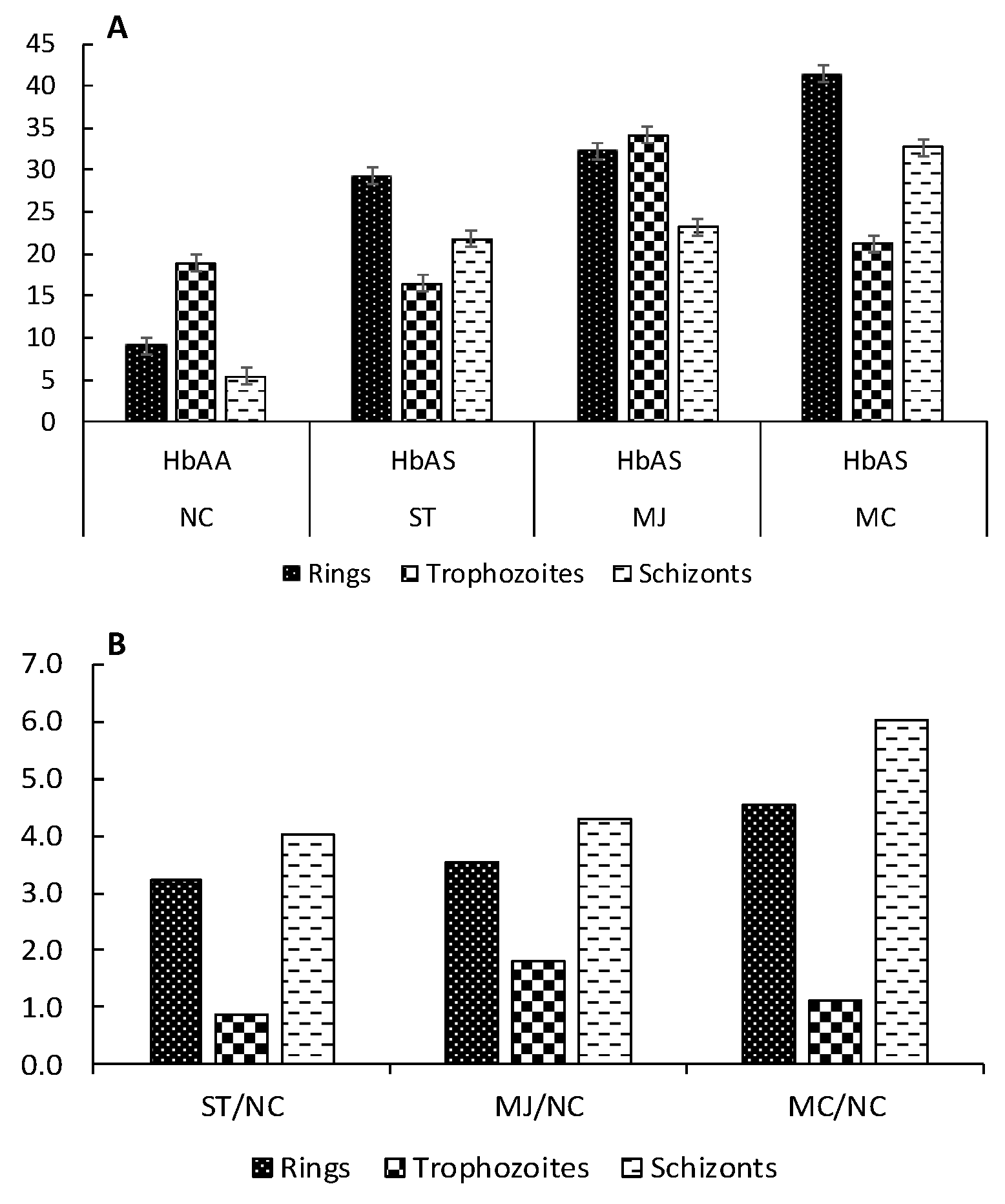
| Condition | Patients | C.I. (nmol of Carbonyls/mg of Protein) | ||
|---|---|---|---|---|
| Rings | Trophozoites | Schizonts | ||
| HbAA | NC | 12.7 ± 1.07 | 24.9 ± 0.83 | 21.4 ± 0.95 |
| HbAS | ST | 88.0 ± 2.10 | 84.9 ± 2.15 | 83.9 ± 2.23 |
| MC | 62.6 ± 1.04 | 59.7 ± 0.92 | 55.6 ± 1.29 | |
| MJ | 53.1 ± 1.99 | 66.3 ± 2.56 | 56.3 ± 3.00 | |
| Condition | HbAS/HbAA | C.I. HbAS/CI HbAA | ||
|---|---|---|---|---|
| Rings | Trophozoites | Schizonts | ||
| HbAS | ST/NC | 6.92 | 3.38 | 3.93 |
| MC/NC | 4.92 | 2.38 | 2.60 | |
| MJ/NC | 4.18 | 2.64 | 2.64 | |
| Means ± SD | 5.34 ± 1.42 | 2.80 ± 0.52 | 3.05 ± 0.75 | |
| Band | Identification | Mass (Da) | Score | Name Submission | Biology Process |
|---|---|---|---|---|---|
| 1.1 | B3AT_HUMAN | 102,013 | 130 | Anion transport protein, band 3 | Anion and ion transport |
| BB_HUMAN | 16,102 | 82 | Hemoglobin subunit beta | Oxygen transport | |
| PF3D7_0501600 | 43,672 | 112 | Rhoptry-associated protein 2 | Entrance to the host cell | |
| gi|11125364 | 55,782 | 56 | Protein disulfide isomerase (Plasmodium falciparum) | Protein folding, Cell redox homeostasis, and response to endoplasmic reticulum stress | |
| 1.2 | B3AT_HUMAN | 102,013 | 274 | Anion transport protein, Band 3 | Anion and ion transport |
| 2 | HBB_HUMAN | 16,102 | 201 | Hemoglobin subunit beta | Oxygen transport |
| gi|160112 | 33,774 | 62 | β-galactosidase, partial (P. falciparum) | Metabolic process of cellular carbohydrates Catabolic galactose process | |
| gi|160537 | 27,813 | 64 | Surface protein (P. falciparum) | Pathogenesis | |
| gi|124512406 | 74,382 | 57 | Heat shock 70 kDa protein (P. falciparum 3D7) | Stress response, ATP metabolic process, cellular response to oxidative stress |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Castillo, A.; Contreras-Puentes, N.; Alvear-Sedán, C.; Moneriz-Pretell, C.; Rodríguez-Cavallo, E.; Mendez-Cuadro, D. Sickle Cell Trait Induces Oxidative Damage on Plasmodium falciparum Proteome at Erythrocyte Stages. Int. J. Mol. Sci. 2019, 20, 5769. https://doi.org/10.3390/ijms20225769
Díaz-Castillo A, Contreras-Puentes N, Alvear-Sedán C, Moneriz-Pretell C, Rodríguez-Cavallo E, Mendez-Cuadro D. Sickle Cell Trait Induces Oxidative Damage on Plasmodium falciparum Proteome at Erythrocyte Stages. International Journal of Molecular Sciences. 2019; 20(22):5769. https://doi.org/10.3390/ijms20225769
Chicago/Turabian StyleDíaz-Castillo, Alber, Neyder Contreras-Puentes, Ciro Alvear-Sedán, Carlos Moneriz-Pretell, Erika Rodríguez-Cavallo, and Darío Mendez-Cuadro. 2019. "Sickle Cell Trait Induces Oxidative Damage on Plasmodium falciparum Proteome at Erythrocyte Stages" International Journal of Molecular Sciences 20, no. 22: 5769. https://doi.org/10.3390/ijms20225769