Heat Shock Cognate 70 Functions as A Chaperone for the Stability of Kinetochore Protein CENP-N in Holocentric Insect Silkworms
Abstract
:1. Introduction
2. Results
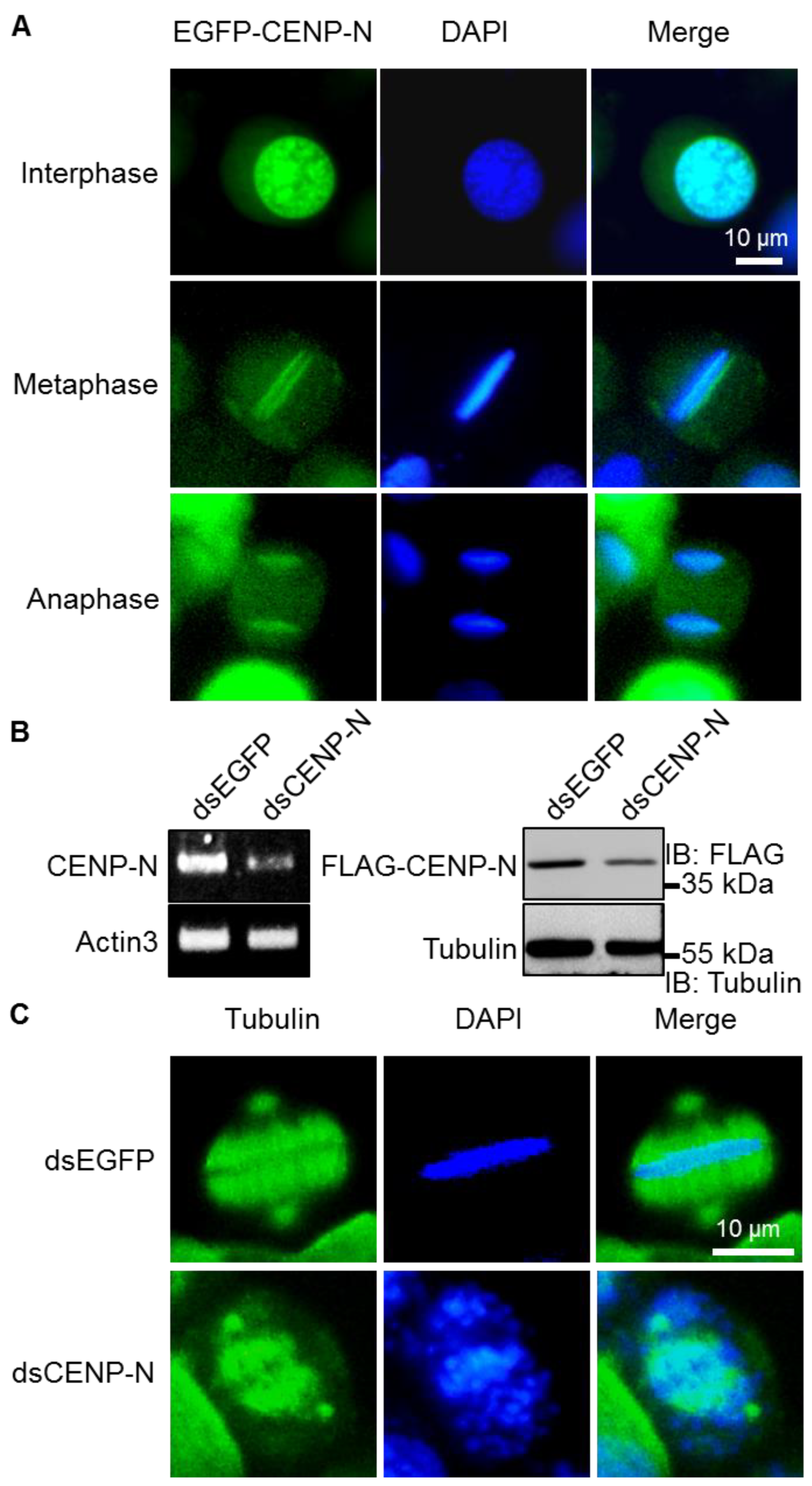
2.1. Kinetochore Function of CENP-N in Silkworms
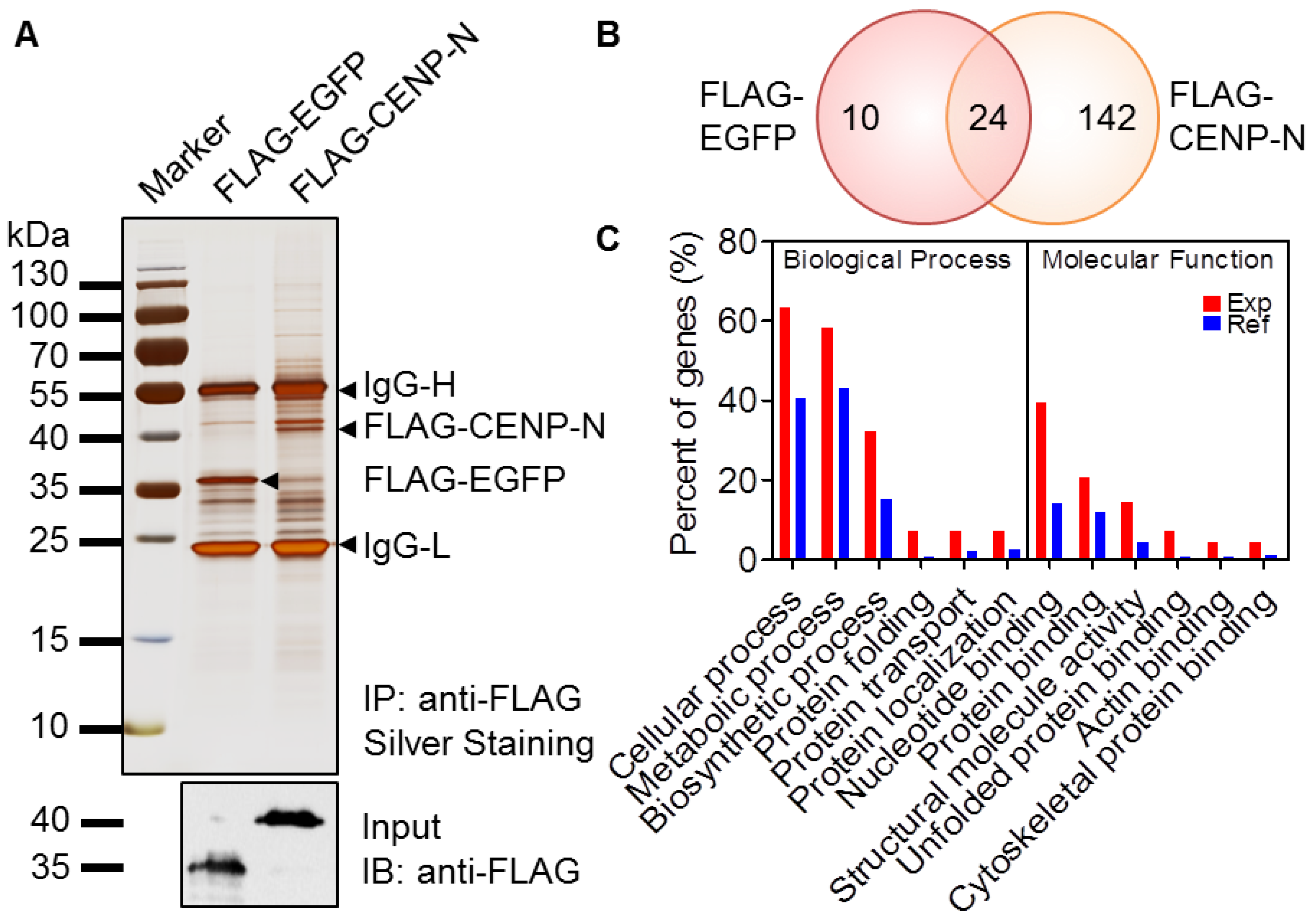
2.2. Identification of the CENP-N Complex
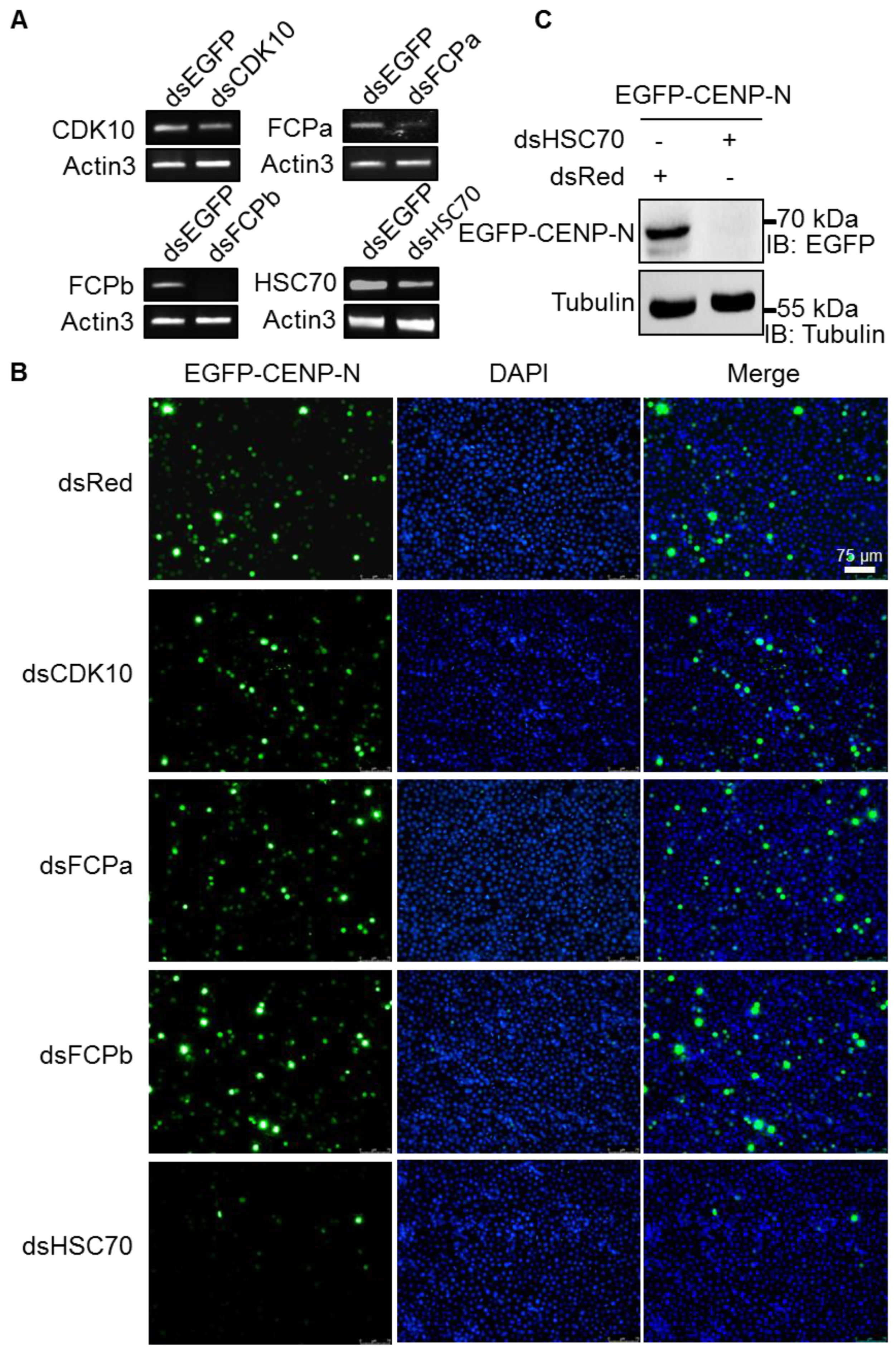
2.3. Reduced Expression of CENP-N by HSC70 Depletion
2.4. Stability of CENP-N via Interaction with HSC70 Chaperone
3. Discussion
4. Materials and Methods
4.1. Plasmids
4.2. Cell Culture and Transfection
4.3. RNA Interference
4.4. Immunoblotting
4.5. Immunofluorescence
4.6. Immunoprecipitation
4.7. Ubiquitin–Proteasome Inhibitor Assay
4.8. Silver Staining
4.9. LC-MS/MS Assay
4.10. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Allshire, R.C.; Karpen, G.H. Epigenetic regulation of centromeric chromatin: Old dogs, new tricks? Nat. Rev. Genet. 2008, 9, 923–937. [Google Scholar] [CrossRef] [PubMed]
- McKinley, K.L.; Cheeseman, I.M. The molecular basis for centromere identity and function. Nat. Rev. Mol. Cell Biol. 2016, 17, 16–29. [Google Scholar] [CrossRef] [PubMed]
- Santaguida, S.; Amon, A. Short- and long-term effects of chromosome mis-segregation and aneuploidy. Nat. Rev. Mol. Cell Biol. 2015, 16, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Mendiburo, M.J.; Padeken, J.; Fulop, S.; Schepers, A.; Heun, P. Drosophila CENH3 is sufficient for centromere formation. Science 2011, 334, 686–690. [Google Scholar] [CrossRef]
- Fukagawa, T.; Earnshaw, W.C. The centromere: Chromatin foundation for the kinetochore machinery. Dev. Cell 2014, 30, 496–508. [Google Scholar] [CrossRef]
- Musacchio, A.; Desai, A. A Molecular View of Kinetochore Assembly and Function. Biology (Basel) 2017, 6, 5. [Google Scholar] [CrossRef]
- Regnier, V.; Vagnarelli, P.; Fukagawa, T.; Zerjal, T.; Burns, E.; Trouche, D.; Earnshaw, W.; Brown, W. CENP-A is required for accurate chromosome segregation and sustained kinetochore association of BubR1. Mol. Cell. Biol. 2005, 25, 3967–3981. [Google Scholar] [CrossRef]
- Przewloka, M.R.; Glover, D.M. The kinetochore and the centromere: A working long distance relationship. Annu. Rev. Genet. 2009, 43, 439–465. [Google Scholar] [CrossRef]
- Westhorpe, F.G.; Straight, A.F. Functions of the centromere and kinetochore in chromosome segregation. Curr. Opin. Cell Biol. 2013, 25, 334–340. [Google Scholar] [CrossRef]
- Cheeseman, I.M.; Desai, A. Molecular architecture of the kinetochore-microtubule interface. Nat. Rev. Mol. Cell Biol. 2008, 9, 33–46. [Google Scholar] [CrossRef]
- Ogiyama, Y.; Ishii, K. The smooth and stable operation of centromeres. Genes Genet. Syst. 2012, 87, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Verdaasdonk, J.S.; Bloom, K. Centromeres: Unique chromatin structures that drive chromosome segregation. Nat. Rev. Mol. Cell Biol. 2011, 12, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Melters, D.P.; Paliulis, L.V.; Korf, I.F.; Chan, S.W. Holocentric chromosomes: Convergent evolution, meiotic adaptations, and genomic analysis. Chromosome Res. 2012, 20, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Wu, Y.; Koblizkova, A.; Torres, G.A.; Wang, K.; Iovene, M.; Neumann, P.; Zhang, W.; Novak, P.; Buell, C.R.; et al. Repeatless and repeat-based centromeres in potato: Implications for centromere evolution. Plant. Cell 2012, 24, 3559–3574. [Google Scholar] [CrossRef]
- Gent, J.I.; Wang, N.; Dawe, R.K. Stable centromere positioning in diverse sequence contexts of complex and satellite centromeres of maize and wild relatives. Genome Biol. 2017, 18, 121. [Google Scholar] [CrossRef]
- Ma, J.; Wing, R.A.; Bennetzen, J.L.; Jackson, S.A. Plant centromere organization: A dynamic structure with conserved functions. Trends Genet. 2007, 23, 134–139. [Google Scholar] [CrossRef]
- Panchenko, T.; Black, B.E. The epigenetic basis for centromere identity. Prog. Mol. Subcell Biol. 2009, 48, 1–32. [Google Scholar]
- Drinnenberg, I.A.; deYoung, D.; Henikoff, S.; Malik, H.S. Recurrent loss of CenH3 is associated with independent transitions to holocentricity in insects. Elife 2014, 3, e03676. [Google Scholar] [CrossRef]
- Biggins, S.; Walczak, C.E. Captivating capture: How microtubules attach to kinetochores. Curr. Biol. 2003, 13, 449–460. [Google Scholar] [CrossRef]
- Perpelescu, M.; Fukagawa, T. The ABCs of CENPs. Chromosoma 2011, 120, 425–446. [Google Scholar] [CrossRef]
- Westermann, S.; Schleiffer, A. Family matters: Structural and functional conservation of centromere-associated proteins from yeast to humans. Trends Cell Biol. 2013, 23, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Heeger, S.; Leismann, O.; Schittenhelm, R.; Schraidt, O.; Heidmann, S.; Lehner, C.F. Genetic interactions of separase regulatory subunits reveal the diverged Drosophila Cenp-C homolog. Genes Dev. 2005, 19, 2041–2053. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Li, S.; Feng, Q. Advances in silkworm studies accelerated by the genome sequencing of Bombyx mori. Annu Rev. Entomol. 2014, 59, 513–536. [Google Scholar] [CrossRef] [PubMed]
- International Silkworm Genome Consortium. The genome of a lepidopteran model insect, the silkworm Bombyx mori. Insect Biochem. Mol. Biol. 2008, 38, 1036–1045. [Google Scholar] [CrossRef] [PubMed]
- Steiner, F.A.; Henikoff, S. Holocentromeres are dispersed point centromeres localized at transcription factor hotspots. Elife 2014, 3, e02025. [Google Scholar] [CrossRef] [PubMed]
- Redemann, S.; Baumgart, J.; Lindow, N.; Shelley, M.; Nazockdast, E.; Kratz, A.; Prohaska, S.; Brugues, J.; Furthauer, S.; Muller-Reichert, T. C. elegans chromosomes connect to centrosomes by anchoring into the spindle network. Nat. Commun. 2017, 8, 15288. [Google Scholar] [CrossRef] [PubMed]
- Oegema, K.; Desai, A.; Rybina, S.; Kirkham, M.; Hyman, A.A. Functional analysis of kinetochore assembly in Caenorhabditis elegans. J. Cell Biol. 2001, 153, 1209–1226. [Google Scholar] [CrossRef]
- Mon, H.; Lee, J.M.; Mita, K.; Goldsmith, M.R.; Kusakabe, T. Chromatin-induced spindle assembly plays an important role in metaphase congression of silkworm holocentric chromosomes. Insect Biochem. Mol. Biol. 2014, 45, 40–50. [Google Scholar] [CrossRef]
- Mon, H.; Lee, J.M.; Sato, M.; Kusakabe, T. Identification and functional analysis of outer kinetochore genes in the holocentric insect Bombyx mori. Insect Biochem. Mol. Biol. 2017, 86, 1–8. [Google Scholar] [CrossRef]
- Kato, H.; Jiang, J.; Zhou, B.R.; Rozendaal, M.; Feng, H.; Ghirlando, R.; Xiao, T.S.; Straight, A.F.; Bai, Y. A conserved mechanism for centromeric nucleosome recognition by centromere protein CENP-C. Science 2013, 340, 1110–1113. [Google Scholar] [CrossRef]
- Tian, T.; Li, X.; Liu, Y.; Wang, C.; Liu, X.; Bi, G.; Zhang, X.; Yao, X.; Zhou, Z.H.; Zang, J. Molecular basis for CENP-N recognition of CENP-A nucleosome on the human kinetochore. Cell Res. 2018, 28, 374–378. [Google Scholar] [CrossRef] [PubMed]
- Chittori, S.; Hong, J.; Saunders, H.; Feng, H.; Ghirlando, R.; Kelly, A.E.; Bai, Y.; Subramaniam, S. Structural mechanisms of centromeric nucleosome recognition by the kinetochore protein CENP-N. Science 2018, 359, 339–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pentakota, S.; Zhou, K.; Smith, C.; Maffini, S.; Petrovic, A.; Morgan, G.P.; Weir, J.R.; Vetter, I.R.; Musacchio, A.; Luger, K. Decoding the centromeric nucleosome through CENP-N. Elife 2017, 6, e33442. [Google Scholar] [CrossRef] [PubMed]
- McKinley, K.L.; Sekulic, N.; Guo, L.Y.; Tsinman, T.; Black, B.E.; Cheeseman, I.M. The CENP-L-N Complex Forms a Critical Node in an Integrated Meshwork of Interactions at the Centromere-Kinetochore Interface. Mol. Cell 2015, 60, 886–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, J.; Liu, Y.; Wei, Y.; Deng, W.; Yu, Z.; Huang, L.; Teng, Y.; Yao, T.; You, Q.; Ruan, H.; et al. Structural transitions of centromeric chromatin regulate the cell cycle-dependent recruitment of CENP-N. Genes Dev. 2015, 29, 1058–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasten, M.; Giordano, A. Cdk10, a Cdc2-related kinase, associates with the Ets2 transcription factor and modulates its transactivation activity. Oncogene 2001, 20, 1832–1838. [Google Scholar] [CrossRef] [Green Version]
- Delalle, I.; Pfleger, C.M.; Buff, E.; Lueras, P.; Hariharan, I.K. Mutations in the Drosophila orthologs of the F-actin capping protein alpha- and beta-subunits cause actin accumulation and subsequent retinal degeneration. Genetics 2005, 171, 1757–1765. [Google Scholar] [CrossRef] [Green Version]
- Diehl, J.A.; Yang, W.; Rimerman, R.A.; Xiao, H.; Emili, A. Hsc70 regulates accumulation of cyclin D1 and cyclin D1-dependent protein kinase. Mol. Cell Biol. 2003, 23, 1764–1774. [Google Scholar] [CrossRef] [Green Version]
- Samejima, I.; Spanos, C.; Alves Fde, L.; Hori, T.; Perpelescu, M.; Zou, J.; Rappsilber, J.; Fukagawa, T.; Earnshaw, W.C. Whole-proteome genetic analysis of dependencies in assembly of a vertebrate kinetochore. J. Cell Biol. 2015, 211, 1141–1156. [Google Scholar] [CrossRef]
- Hoischen, C.; Yavas, S.; Wohland, T.; Diekmann, S. CENP-C/H/I/K/M/T/W/N/L and hMis12 but not CENP-S/X participate in complex formation in the nucleoplasm of living human interphase cells outside centromeres. PLoS ONE 2018, 13, e0192572. [Google Scholar] [CrossRef] [Green Version]
- Oka, N.; Kasamatsu, A.; Endo-Sakamoto, Y.; Eizuka, K.; Wagai, S.; Koide-Ishida, N.; Miyamoto, I.; Iyoda, M.; Tanzawa, H.; Uzawa, K. Centromere Protein N Participates in Cellular Proliferation of Human Oral Cancer by Cell-Cycle Enhancement. J. Cancer 2019, 10, 3728–3734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellwig, D.; Emmerth, S.; Ulbricht, T.; Doring, V.; Hoischen, C.; Martin, R.; Samora, C.P.; McAinsh, A.D.; Carroll, C.W.; Straight, A.F.; et al. Dynamics of CENP-N kinetochore binding during the cell cycle. J. Cell Sci. 2011, 124, 3871–3883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niikura, Y.; Kitagawa, R.; Ogi, H.; Kitagawa, K. SGT1-HSP90 complex is required for CENP-A deposition at centromeres. Cell Cycle 2017, 16, 1683–1694. [Google Scholar] [CrossRef] [Green Version]
- Melters, D.P.; Bradnam, K.R.; Young, H.A.; Telis, N.; May, M.R.; Ruby, J.G.; Sebra, R.; Peluso, P.; Eid, J.; Rank, D.; et al. Comparative analysis of tandem repeats from hundreds of species reveals unique insights into centromere evolution. Genome Biol. 2013, 14, R10. [Google Scholar] [CrossRef] [PubMed]
- Alkan, C.; Cardone, M.F.; Catacchio, C.R.; Antonacci, F.; O’Brien, S.J.; Ryder, O.A.; Purgato, S.; Zoli, M.; Della Valle, G.; Eichler, E.E.; et al. Genome-wide characterization of centromeric satellites from multiple mammalian genomes. Genome Res. 2011, 21, 137–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navajas-Perez, R.; Paterson, A.H. Patterns of tandem repetition in plant whole genome assemblies. Mol. Genet. Genom. 2009, 281, 579–590. [Google Scholar] [CrossRef]
- Li, Z.; Mon, H.; Xu, J.; Zhu, L.; Lee, J.M.; Kusakabe, T. A conserved SUMOylation signaling for cell cycle control in a holocentric species Bombyx mori. Insect Biochem. Mol. Biol. 2014, 51, 71–79. [Google Scholar] [CrossRef]
- Mon, H.; Lee, J.; Kawaguchi, Y.; Kusakabe, T. Double-strand breaks repair by gene conversion in silkworm holocentric chromosomes. Mol. Genet. Genom. 2011, 286, 215–224. [Google Scholar] [CrossRef]
- Mon, H.; Kobayashi, I.; Ohkubo, S.; Tomita, S.; Lee, J.; Sezutsu, H.; Tamura, T.; Kusakabe, T. Effective RNA interference in cultured silkworm cells mediated by overexpression of Caenorhabditis elegans SID-1. RNA Biol. 2012, 9, 40–46. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Cui, Q.; Wang, X.; Li, B.; Zhao, D.; Xia, Q.; Zhao, P. Functions and substrates of NEDDylation during cell cycle in the silkworm, Bombyx mori. Insect Biochem. Mol. Biol. 2017, 90, 101–112. [Google Scholar] [CrossRef]
- Wang, X.; Li, Z.; Zhang, Q.; Li, B.; Lu, C.; Li, W.; Cheng, T.; Xia, Q.; Zhao, P. DNA methylation on N6-adenine in lepidopteran Bombyx mori. Biochim. Biophys. Acta Gene Regul. Mech. 2018, 1861, 815–825. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Wang, X.; Li, Z.; Lu, C.; Zhang, Q.; Chang, L.; Li, W.; Cheng, T.; Xia, Q.; Zhao, P. Transcriptome-wide analysis of N6-methyladenosine uncovers its regulatory role in gene expression in the lepidopteran Bombyx mori. Insect Mol. Biol. 2019, 28, 703–715. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cui, Q.; Xu, J.; Cheng, D.; Wang, X.; Li, B.; Lee, J.M.; Xia, Q.; Kusakabe, T.; Zhao, P. SUMOylation regulates the localization and activity of Polo-like kinase 1 during cell cycle in the silkworm, Bombyx mori. Sci. Rep. 2017, 7, 15536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Z.; Zhang, W.; Zhang, Y.; Zhang, X.; Zhao, P.; Xia, Q. Identification and Characterization of Novel Chitin-Binding Proteins from the Larval Cuticle of Silkworm, Bombyx mori. J. Proteome Res. 2016, 15, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Gotz, S.; Garcia-Gomez, J.M.; Terol, J.; Talon, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [Green Version]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, B.; Li, Z.; Lu, C.; Chang, L.; Zhao, D.; Shen, G.; Kusakabe, T.; Xia, Q.; Zhao, P. Heat Shock Cognate 70 Functions as A Chaperone for the Stability of Kinetochore Protein CENP-N in Holocentric Insect Silkworms. Int. J. Mol. Sci. 2019, 20, 5823. https://doi.org/10.3390/ijms20235823
Li B, Li Z, Lu C, Chang L, Zhao D, Shen G, Kusakabe T, Xia Q, Zhao P. Heat Shock Cognate 70 Functions as A Chaperone for the Stability of Kinetochore Protein CENP-N in Holocentric Insect Silkworms. International Journal of Molecular Sciences. 2019; 20(23):5823. https://doi.org/10.3390/ijms20235823
Chicago/Turabian StyleLi, Bingqian, Zhiqing Li, Chenchen Lu, Li Chang, Dongchao Zhao, Guanwang Shen, Takahiro Kusakabe, Qingyou Xia, and Ping Zhao. 2019. "Heat Shock Cognate 70 Functions as A Chaperone for the Stability of Kinetochore Protein CENP-N in Holocentric Insect Silkworms" International Journal of Molecular Sciences 20, no. 23: 5823. https://doi.org/10.3390/ijms20235823




