Current Treatment Strategies and Nanoparticle-Mediated Drug Delivery Systems for Pulmonary Arterial Hypertension
Abstract
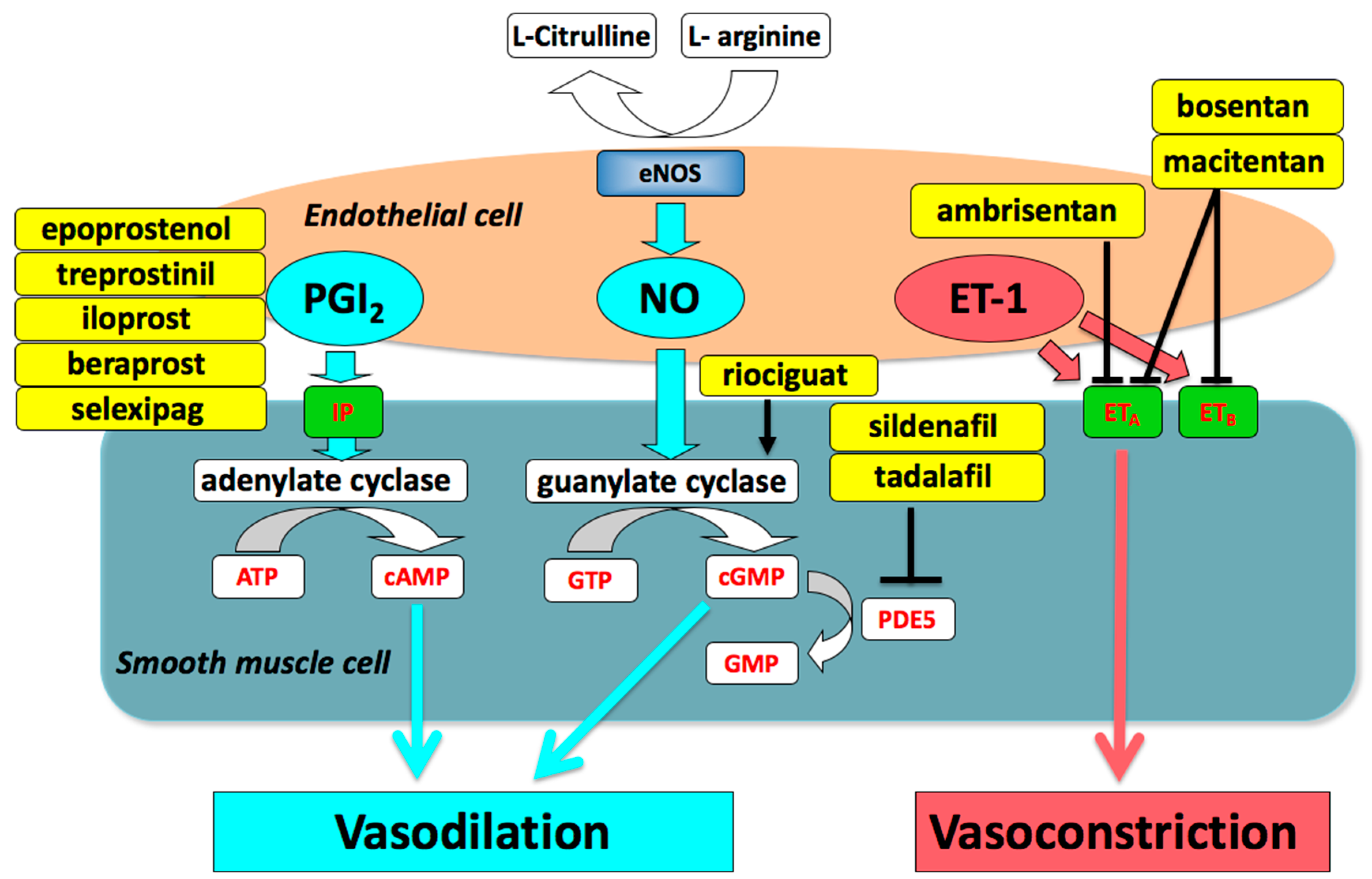
:1. Introduction
2. Medical Treatment of Pulmonary Arterial Hypertension (PAH)
2.1. Prostacyclin (PGI2)
2.2. Phosphodiesterase Type 5 (PDE5) Inhibitors and Soluble Guanylate Cyclase (sGC) Stimulator
2.3. Endothelin Receptor Antagonists (ERAs)
2.4. Combination Therapy
2.5. Current Status and Future Perspectives
3. Nanoparticle-Mediated Drug Delivery Systems (Nano-DDS)
4. Nano-DDS for PAH Treatment
4.1. Prostacyclin (PGI2) Analogue-Loaded Nanoparticles (NPs)
4.2. PDE5 Inhibitor-Loaded NPs
4.3. ERA-Loaded NPs
4.4. Others
5. Future Challenges and Possible Nanomedicine-Based DDS Solutions
5.1. Targeting
5.2. Risk Assessment Strategy for Novel Nanomaterials
5.3. Novel Therapeutic Targets and Potential Drugs for PAH
6. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ET-1 | Endothelin-1 |
| ETA | endothelin type A receptor |
| ETB | endothelin type B receptor |
| ERA | endothelin receptor antagonists |
| FITC | fluorescein isothiocyanate |
| GlcA | glucuronic acid |
| GLUT-1 | glucose transport-1 |
| HMG-CoA | 3-Hydroxy-3-methylglutanyl coenzyme A |
| IPAH | idiopathic pulmonary arterial hypertension |
| MCT | monocrotaline |
| mPAP | mean pulmonary arterial pressure |
| nano-DDS | nanoparticle-mediated drug delivery systems |
| NPs | nanoparticles |
| PAH | pulmonary arterial hypertension |
| PASMCs | pulmonary artery smooth muscle cells |
| PDE5 | phosphodiesterase type-5 |
| PDGF | platelet-derived growth factor |
| PEG | poly-(ethyleneglycol)-block |
| PGI2 | prostaglandin I2 |
| PH | pulmonary hypertension |
| PLA | poly(lactide) |
| PLGA | poly(lactide-co-glycolic acid) |
| PVR | pulmonary vascular resistance |
| RCTs | randomized clinical trials |
| sGC | soluble guanylate cyclase |
References
- Archer, S.; Rich, S. Primary pulmonary hypertension: a vascular biology and translational research “Work in progress”. Circulation 2000, 102, 2781–2791. [Google Scholar] [CrossRef]
- Miura, A.; Nakamura, K.; Kusano, K.F.; Matsubara, H.; Ogawa, A.; Akagi, S.; Oto, T.; Murakami, T.; Ohtsuka, A.; Yutani, C.; et al. Three-dimensional structure of pulmonary capillary vessels in patients with pulmonary hypertension. Circulation 2010, 121, 2151–2153. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Guignabert, C.; Bonnet, S.; Dorfmuller, P.; Klinger, J.R.; Nicolls, M.R.; Olschewski, A.J.; Pullamsetti, S.S.; Schermuly, R.T.; Stenmark, K.R.; et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur. Respir. J. 2019, 53. [Google Scholar] [CrossRef] [PubMed]
- Akagi, S.; Matsubara, H.; Nakamura, K.; Ito, H. Modern treatment to reduce pulmonary arterial pressure in pulmonary arterial hypertension. J. Cardiol. 2018, 72, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Tamura, Y.; Kumamaru, H.; Satoh, T.; Miyata, H.; Ogawa, A.; Tanabe, N.; Hatano, M.; Yao, A.; Abe, K.; Tsujino, I.; et al. Effectiveness and Outcome of Pulmonary Arterial Hypertension-Specific Therapy in Japanese Patients With Pulmonary Arterial Hypertension. Circ. J. 2017, 82, 275–282. [Google Scholar] [CrossRef]
- Humbert, M.; Sitbon, O.; Simonneau, G. Treatment of pulmonary arterial hypertension. N. Engl. J. Med. 2004, 351, 1425–1436. [Google Scholar] [CrossRef]
- Akagi, S.; Nakamura, K.; Miyaji, K.; Ogawa, A.; Kusano, K.F.; Ito, H.; Matsubara, H. Marked hemodynamic improvements by high-dose epoprostenol therapy in patients with idiopathic pulmonary arterial hypertension. Circ. J. 2010, 74, 2200–2205. [Google Scholar] [CrossRef]
- Saito, Y.; Nakamura, K.; Akagi, S.; Sarashina, T.; Ejiri, K.; Miura, A.; Ogawa, A.; Matsubara, H.; Ito, H. Epoprostenol sodium for treatment of pulmonary arterial hypertension. Vasc. Health Risk Manag. 2015, 11, 265–270. [Google Scholar] [CrossRef]
- Galie, N.; Corris, P.A.; Frost, A.; Girgis, R.E.; Granton, J.; Jing, Z.C.; Klepetko, W.; McGoon, M.D.; McLaughlin, V.V.; Preston, I.R.; et al. Updated treatment algorithm of pulmonary arterial hypertension. J. Am. Coll Cardiol. 2013, 62, D60–D72. [Google Scholar] [CrossRef]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart J. 2016, 37, 67–119. [Google Scholar] [CrossRef]
- Matsubara, H.; Ogawa, A. Treatment of idiopathic/hereditary pulmonary arterial hypertension. J. Cardiol. 2014, 64, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.J.; Mendoza, J.; Hood, M.; McGoon, M.; Barst, R.; Williams, W.B.; Diehl, J.H.; Crow, J.; Long, W. Treatment of primary pulmonary hypertension with continuous intravenous prostacyclin (epoprostenol). Results of a randomized trial. Ann. Intern. Med. 1990, 112, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Barst, R.J.; Rubin, L.J.; Long, W.A.; McGoon, M.D.; Rich, S.; Badesch, D.B.; Groves, B.M.; Tapson, V.F.; Bourge, R.C.; Brundage, B.H.; et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N. Engl. J. Med. 1996, 334, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Badesch, D.B.; Tapson, V.F.; McGoon, M.D.; Brundage, B.H.; Rubin, L.J.; Wigley, F.M.; Rich, S.; Barst, R.J.; Barrett, P.S.; Kral, K.M.; et al. Continuous intravenous epoprostenol for pulmonary hypertension due to the scleroderma spectrum of disease. A randomized, controlled trial. Ann. Intern. Med. 2000, 132, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Sitbon, O.; Channick, R.; Chin, K.M.; Frey, A.; Gaine, S.; Galie, N.; Ghofrani, H.A.; Hoeper, M.M.; Lang, I.M.; Preiss, R.; et al. Selexipag for the Treatment of Pulmonary Arterial Hypertension. N. Engl. J. Med. 2015, 373, 2522–2533. [Google Scholar] [CrossRef] [PubMed]
- Pulido, T.; Adzerikho, I.; Channick, R.N.; Delcroix, M.; Galie, N.; Ghofrani, H.A.; Jansa, P.; Jing, Z.C.; Le Brun, F.O.; Mehta, S.; et al. Macitentan and morbidity and mortality in pulmonary arterial hypertension. N. Engl. J. Med. 2013, 369, 809–818. [Google Scholar] [CrossRef] [PubMed]
- Sarashina, T.; Nakamura, K.; Akagi, S.; Oto, T.; Oe, H.; Ejiri, K.; Nakagawa, K.; Nishii, N.; Matsubara, H.; Kobayashi, M.; et al. Reverse Right Ventricular Remodeling After Lung Transplantation in Patients With Pulmonary Arterial Hypertension Under Combination Therapy of Targeted Medical Drugs. Circ. J. 2017, 81, 383–390. [Google Scholar] [CrossRef]
- Fukuda, K.; Date, H.; Doi, S.; Fukumoto, Y.; Fukushima, N.; Hatano, M.; Ito, H.; Kuwana, M.; Matsubara, H.; Momomura, S.I.; et al. Guidelines for the Treatment of Pulmonary Hypertension (JCS 2017/JPCPHS 2017). Circ. J. 2019, 83, 842–945. [Google Scholar] [CrossRef]
- Galie, N.; Barbera, J.A.; Frost, A.E.; Ghofrani, H.A.; Hoeper, M.M.; McLaughlin, V.V.; Peacock, A.J.; Simonneau, G.; Vachiery, J.L.; Grunig, E.; et al. Initial Use of Ambrisentan plus Tadalafil in Pulmonary Arterial Hypertension. N. Engl. J. Med. 2015, 373, 834–844. [Google Scholar] [CrossRef]
- Kanwar, M.K.; Thenappan, T.; Vachiery, J.L. Update in treatment options in pulmonary hypertension. J. Heart Lung Transpl. 2016, 35, 695–703. [Google Scholar] [CrossRef]
- Sitbon, O.; Gomberg-Maitland, M.; Granton, J.; Lewis, M.I.; Mathai, S.C.; Rainisio, M.; Stockbridge, N.L.; Wilkins, M.R.; Zamanian, R.T.; Rubin, L.J. Clinical trial design and new therapies for pulmonary arterial hypertension. Eur. Respir. J. 2019, 53. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.Y.; Rutka, J.T.; Chan, W.C. Nanomedicine. N. Engl. J. Med. 2010, 363, 2434–2443. [Google Scholar] [CrossRef] [PubMed]
- Matoba, T.; Egashira, K. Nanoparticle-mediated drug delivery system for cardiovascular disease. Int. Heart J. 2014, 55, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, B.; Gupta, V. Novel therapeutic approaches for pulmonary arterial hypertension: Unique molecular targets to site-specific drug delivery. J. Control. Release 2015, 211, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Segura-Ibarra, V.; Wu, S.; Hassan, N.; Moran-Guerrero, J.A.; Ferrari, M.; Guha, A.; Karmouty-Quintana, H.; Blanco, E. Nanotherapeutics for Treatment of Pulmonary Arterial Hypertension. Front. Physiol 2018, 9, 890. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Matsubara, H.; Akagi, S.; Sarashina, T.; Ejiri, K.; Kawakita, N.; Yoshida, M.; Miyoshi, T.; Watanabe, A.; Nishii, N.; et al. Nanoparticle-Mediated Drug Delivery System for Pulmonary Arterial Hypertension. J. Clin. Med. 2017, 6, E48. [Google Scholar] [CrossRef]
- Ishihara, T.; Hayashi, E.; Yamamoto, S.; Kobayashi, C.; Tamura, Y.; Sawazaki, R.; Tamura, F.; Tahara, K.; Kasahara, T.; Ishihara, T.; et al. Encapsulation of beraprost sodium in nanoparticles: analysis of sustained release properties, targeting abilities and pharmacological activities in animal models of pulmonary arterial hypertension. J. Control. Release 2015, 197, 97–104. [Google Scholar] [CrossRef]
- Akagi, S.; Nakamura, K.; Matsubara, H.; Kondo, M.; Miura, D.; Matoba, T.; Egashira, K.; Ito, H. Intratracheal Administration of Prostacyclin Analogue-incorporated Nanoparticles Ameliorates the Development of Monocrotaline and Sugen-Hypoxia-induced Pulmonary Arterial Hypertension. J. Cardiovasc. Pharmacol. 2016, 67, 290–298. [Google Scholar] [CrossRef]
- Leifer, F.G.; Konicek, D.M.; Chen, K.J.; Plaunt, A.J.; Salvail, D.; Laurent, C.E.; Corboz, M.R.; Li, Z.; Chapman, R.W.; Perkins, W.R.; et al. Inhaled Treprostinil-Prodrug Lipid Nanoparticle Formulations Provide Long-Acting Pulmonary Vasodilation. Drug Res. 2018, 68, 605–614. [Google Scholar] [CrossRef]
- Jain, P.P.; Leber, R.; Nagaraj, C.; Leitinger, G.; Lehofer, B.; Olschewski, H.; Olschewski, A.; Prassl, R.; Marsh, L.M. Liposomal nanoparticles encapsulating iloprost exhibit enhanced vasodilation in pulmonary arteries. Int. J. Nanomed. 2014, 9, 3249–3261. [Google Scholar] [CrossRef]
- Li, B.; He, W.; Ye, L.; Zhu, Y.; Tian, Y.; Chen, L.; Yang, J.; Miao, M.; Shi, Y.; Azevedo, H.S.; et al. Targeted Delivery of Sildenafil for Inhibiting Pulmonary Vascular Remodeling. Hypertension 2019, 73, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Varshosaz, J.; Taymouri, S.; Hamishehkar, H.; Vatankhah, R.; Yaghubi, S. Development of dry powder inhaler containing tadalafil-loaded PLGA nanoparticles. Res. Pharm. Sci. 2017, 12, 222–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gimenez, V.M.; Sperandeo, N.; Faudone, S.; Noriega, S.; Manucha, W.; Kassuha, D. Preparation and characterization of bosentan monohydrate/epsilon-polycaprolactone nanoparticles obtained by electrospraying. Biotechnol. Prog. 2019, 35, e2748. [Google Scholar] [CrossRef] [PubMed]
- Kimura, S.; Egashira, K.; Chen, L.; Nakano, K.; Iwata, E.; Miyagawa, M.; Tsujimoto, H.; Hara, K.; Morishita, R.; Sueishi, K.; et al. Nanoparticle-mediated delivery of nuclear factor kappaB decoy into lungs ameliorates monocrotaline-induced pulmonary arterial hypertension. Hypertension 2009, 53, 877–883. [Google Scholar] [CrossRef] [PubMed]
- McLendon, J.M.; Joshi, S.R.; Sparks, J.; Matar, M.; Fewell, J.G.; Abe, K.; Oka, M.; McMurtry, I.F.; Gerthoffer, W.T. Lipid nanoparticle delivery of a microRNA-145 inhibitor improves experimental pulmonary hypertension. J. Control. Release 2015, 210, 67–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Nakano, K.; Kimura, S.; Matoba, T.; Iwata, E.; Miyagawa, M.; Tsujimoto, H.; Nagaoka, K.; Kishimoto, J.; Sunagawa, K.; et al. Nanoparticle-mediated delivery of pitavastatin into lungs ameliorates the development and induces regression of monocrotaline-induced pulmonary artery hypertension. Hypertension 2011, 57, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Ichimura, K.; Matoba, T.; Koga, J.I.; Nakano, K.; Funamoto, D.; Tsutsui, H.; Egashira, K. Nanoparticle-Mediated Targeting of Pitavastatin to Small Pulmonary Arteries and Leukocytes by Intravenous Administration Attenuates the Progression of Monocrotaline-Induced Established Pulmonary Arterial Hypertension in Rats. Int. Heart J. 2018, 59, 1432–1444. [Google Scholar] [CrossRef] [Green Version]
- Akagi, S.; Nakamura, K.; Miura, D.; Saito, Y.; Matsubara, H.; Ogawa, A.; Matoba, T.; Egashira, K.; Ito, H. Delivery of imatinib-incorporated nanoparticles into lungs suppresses the development of monocrotaline-induced pulmonary arterial hypertension. Int. Heart J. 2015, 56, 354–359. [Google Scholar] [CrossRef] [Green Version]
- Segura-Ibarra, V.; Amione-Guerra, J.; Cruz-Solbes, A.S.; Cara, F.E.; Iruegas-Nunez, D.A.; Wu, S.; Youker, K.A.; Bhimaraj, A.; Torre-Amione, G.; Ferrari, M.; et al. Rapamycin nanoparticles localize in diseased lung vasculature and prevent pulmonary arterial hypertension. Int. J. Pharm. 2017, 524, 257–267. [Google Scholar] [CrossRef]
- Gupta, V.; Gupta, N.; Shaik, I.H.; Mehvar, R.; McMurtry, I.F.; Oka, M.; Nozik-Grayck, E.; Komatsu, M.; Ahsan, F. Liposomal fasudil, a rho-kinase inhibitor, for prolonged pulmonary preferential vasodilation in pulmonary arterial hypertension. J. Control. Release 2013, 167, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Tuder, R.M.; Cool, C.D.; Geraci, M.W.; Wang, J.; Abman, S.H.; Wright, L.; Badesch, D.; Voelkel, N.F. Prostacyclin synthase expression is decreased in lungs from patients with severe pulmonary hypertension. Am. J. Respir. Crit. Care Med. 1999, 159, 1925–1932. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, N.; Ogawa, A.; Ito, H.; Matsubara, H. Rapid and high-dose titration of epoprostenol improves pulmonary hemodynamics and clinical outcomes in patients with idiopathic and heritable pulmonary arterial hypertension. J. Cardiol. 2016, 68, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.O.; Park, Y.; Seo, J.H.; Jeong, M.H.; Chae, S.C.; Ahn, T.H.; Jang, W.Y.; Kim, W.; Park, E.J.; Choi, B.G.; et al. Time-dependent prognostic effect of high sensitivity C-reactive protein with statin therapy in acute myocardial infarction. J. Cardiol. 2019, 74, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Fujisue, K.; Shirakawa, T.; Nakamura, S.; Yamamoto, N.; Oshima, S.; Matsumura, T.; Tsunoda, R.; Hirai, N.; Tayama, S.; Nakamura, N.; et al. Dose-dependent INhibitory effect of rosuVastatin In Japanese patienTs with Acute myocardial infarcTION on serum concentration of matrix metalloproteinases - INVITATION trial. J. Cardiol. 2018, 72, 350–355. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, T.; Nakamura, K.; Akagi, S.; Kusano, K.F.; Matsubara, H.; Fujio, H.; Ogawa, A.; Miura, A.; Miura, D.; Oto, T.; et al. Inhibitory effects of simvastatin on platelet-derived growth factor signaling in pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. J. Cardiovasc. Pharmacol. 2010, 55, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, T.; Faul, J.L.; Berry, G.J.; Vaszar, L.T.; Qiu, D.; Pearl, R.G.; Kao, P.N. Simvastatin attenuates smooth muscle neointimal proliferation and pulmonary hypertension in rats. Am. J. Respir. Crit. Care Med. 2002, 166, 1403–1408. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, T.; Vaszar, L.T.; Faul, J.L.; Zhao, G.; Berry, G.J.; Shi, L.; Qiu, D.; Benson, G.; Pearl, R.G.; Kao, P.N. Simvastatin rescues rats from fatal pulmonary hypertension by inducing apoptosis of neointimal smooth muscle cells. Circulation 2003, 108, 1640–1645. [Google Scholar] [CrossRef] [Green Version]
- Nakano, K.; Matoba, T.; Koga, J.I.; Kashihara, Y.; Fukae, M.; Ieiri, I.; Shiramoto, M.; Irie, S.; Kishimoto, J.; Todaka, K.; et al. Safety, Tolerability, and Pharmacokinetics of NK-104-NP. Int. Heart J. 2018, 59, 1015–1025. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, S.; Patra, C.R. Therapeutic application of anti-angiogenic nanomaterials in cancers. Nanoscale 2016, 8, 12444–12470. [Google Scholar] [CrossRef]
- Avram, S.; Buiu, C.; Duda-Seiman, D.M.; Duda-Seiman, C.; Mihailescu, D. 3D-QSAR design of new escitalopram derivatives for the treatment of major depressive disorders. Sci. Pharm. 2010, 78, 233–248. [Google Scholar] [CrossRef] [Green Version]
- Avram, S.; Buiu, C.; Duda-Seiman, D.; Duda-Seiman, C.; Borcan, F.; Mihailescu, D. Evaluation of the pharmacological descriptors related to the induction of antidepressant activity and its prediction by QSAR/QRAR methods. Mini Rev. Med. Chem. 2012, 12, 467–476. [Google Scholar] [CrossRef]
- Nakamura, K.; Akagi, S.; Ogawa, A.; Kusano, K.F.; Matsubara, H.; Miura, D.; Fuke, S.; Nishii, N.; Nagase, S.; Kohno, K.; et al. Pro-apoptotic effects of imatinib on PDGF-stimulated pulmonary artery smooth muscle cells from patients with idiopathic pulmonary arterial hypertension. Int. J. Cardiol. 2012, 159, 100–106. [Google Scholar] [CrossRef]
- Arita, Y.; Sakata, Y.; Sudo, T.; Maeda, T.; Matsuoka, K.; Tamai, K.; Higuchi, K.; Shioyama, W.; Nakaoka, Y.; Kanakura, Y.; et al. The efficacy of tocilizumab in a patient with pulmonary arterial hypertension associated with Castleman’s disease. Heart Vessel. 2010, 25, 444–447. [Google Scholar] [CrossRef]
- Nakamura, K.; Sakaguchi, M.; Matsubara, H.; Akagi, S.; Sarashina, T.; Ejiri, K.; Akazawa, K.; Kondo, M.; Nakagawa, K.; Yoshida, M.; et al. Crucial role of RAGE in inappropriate increase of smooth muscle cells from patients with pulmonary arterial hypertension. PLoS ONE 2018, 13, e0203046. [Google Scholar] [CrossRef] [PubMed]
- Spiekerkoetter, E.; Tian, X.; Cai, J.; Hopper, R.K.; Sudheendra, D.; Li, C.G.; El-Bizri, N.; Sawada, H.; Haghighat, R.; Chan, R.; et al. FK506 activates BMPR2, rescues endothelial dysfunction, and reverses pulmonary hypertension. J. Clin. Investig. 2013, 123, 3600–3613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drake, K.M.; Dunmore, B.J.; McNelly, L.N.; Morrell, N.W.; Aldred, M.A. Correction of nonsense BMPR2 and SMAD9 mutations by ataluren in pulmonary arterial hypertension. Am. J. Respir. Cell Mol. Biol. 2013, 49, 403–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piao, L.; Fang, Y.H.; Cadete, V.J.; Wietholt, C.; Urboniene, D.; Toth, P.T.; Marsboom, G.; Zhang, H.J.; Haber, I.; Rehman, J.; et al. The inhibition of pyruvate dehydrogenase kinase improves impaired cardiac function and electrical remodeling in two models of right ventricular hypertrophy: resuscitating the hibernating right ventricle. J. Mol. Med. (Berl) 2010, 88, 47–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujio, H.; Nakamura, K.; Matsubara, H.; Kusano, K.F.; Miyaji, K.; Nagase, S.; Ikeda, T.; Ogawa, A.; Ohta-Ogo, K.; Miura, D.; et al. Carvedilol inhibits proliferation of cultured pulmonary artery smooth muscle cells of patients with idiopathic pulmonary arterial hypertension. J. Cardiovasc. Pharmacol. 2006, 47, 250–255. [Google Scholar] [CrossRef] [Green Version]
- Boucherat, O.; Chabot, S.; Paulin, R.; Trinh, I.; Bourgeois, A.; Potus, F.; Lampron, M.C.; Lambert, C.; Breuils-Bonnet, S.; Nadeau, V.; et al. HDAC6: A Novel Histone Deacetylase Implicated in Pulmonary Arterial Hypertension. Sci. Rep. 2017, 7, 4546. [Google Scholar] [CrossRef] [Green Version]
- Guignabert, C.; Izikki, M.; Tu, L.I.; Li, Z.; Zadigue, P.; Barlier-Mur, A.M.; Hanoun, N.; Rodman, D.; Hamon, M.; Adnot, S.; et al. Transgenic mice overexpressing the 5-hydroxytryptamine transporter gene in smooth muscle develop pulmonary hypertension. Circ. Res. 2006, 98, 1323–1330. [Google Scholar] [CrossRef]
- Shimokawa, H.; Satoh, K. 2015 ATVB Plenary Lecture: translational research on rho-kinase in cardiovascular medicine. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1756–1769. [Google Scholar] [CrossRef] [PubMed]
- Harada-Shiba, M.; Takamisawa, I.; Miyata, K.; Ishii, T.; Nishiyama, N.; Itaka, K.; Kangawa, K.; Yoshihara, F.; Asada, Y.; Hatakeyama, K.; et al. Intratracheal gene transfer of adrenomedullin using polyplex nanomicelles attenuates monocrotaline-induced pulmonary hypertension in rats. Mol. Ther.J. Am. Soc. Gene Ther. 2009, 17, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Kim, J. Apelin-APJ signaling: a potential therapeutic target for pulmonary arterial hypertension. Mol. Cells 2014, 37, 196–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Drug | Delivery System | Animal Model | Route of Admin. | Refs |
|---|---|---|---|---|
| Prostacyclin analogues | ||||
| Beraprost | Polymer (PLA and PEG-PLA) | MCT-induced rat model | Intravenous | [27] |
| Beraprost | Polymer (PLA and PEG-PLA) | Hypoxia mouse model | Intravenous | [27] |
| Beraprost | Polymer (PLGA) | MCT-induced rat model | Intratracheal | [28] |
| Beraprost | Polymer (PLGA) | Sugen/hypoxia rat model | Intratracheal | [28] |
| Treprostinil | Lipid nanoparticle | Hypoxia rat model | Inhalation | [29] |
| Iloprost | Liposome | Isolated PA of mice | [30] | |
| PDE5 inhibitors | ||||
| Sildenafil | GlcA-modified liposome | MCT-induced rat model | Intravenous | [31] |
| Tadalafil | Polymer (PLGA) | In vitro study | [32] | |
| ERA | ||||
| Bosentan | Polymer (PCL) | In vitro study | [33] | |
| Others | ||||
| Pitavastatin | Polymer (PLGA) | MCT-induced rat model | Intratracheal | [36] |
| Pitavastatin | Polymer (PLGA) | MCT-induced rat model | Intravenous | [37] |
| Imatinib | Polymer (PLGA) | MCT-induced rat model | Intratracheal | [38] |
| Rapamycin | Polymer (PEG-PCL) | MCT-induced rat model | Intravenous | [39] |
| Fasudil | Liposome | MCT-induced rat model | Inhalation | [40] |
| Oligonucleotides | ||||
| NF-kB decoy | Polymer (PEG-PLGA) | MCT-induced rat model | Intratracheal | [34] |
| AntimiRNA-145 | Liposome | Sugen/hypoxia rat model | Intravenous | [35] |
| Pathways | Therapeutic Targets | Potential Drugs |
|---|---|---|
| Growth factor | PDGF, EGF, FGF and VEGF | tyrosine kinase inhibitors |
| Imatinib [52] | ||
| Inflammation | IL-6 | tocilizumab [53] |
| RAGE | RAGE aptamer, AS-1 [54] | |
| Nrf2 and NFkB | bardoxolone methyl [21] | |
| BMPR-II | BMPR2 and sma-9 | tacrolimus [55] |
| ataluren [56] | ||
| Metabolic modulators | glucose oxidation | dichloroacetate [57] |
| Neurohormonal activation | sympathetic nerve system | β-blockers [58] |
| DNA damage | BRCA1 and PARP | olaparib [21] |
| Epigenetic modification | HDAC6 | tubastatin A [59] |
| Vasoactive mediators | 5HT | 5HT-receptor antagonists [60] |
| rho A/ROCK | fasudil [61] | |
| adrenomedullin | adrenomedullin [62] | |
| Apelin | apelin [63] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamura, K.; Akagi, S.; Ejiri, K.; Yoshida, M.; Miyoshi, T.; Toh, N.; Nakagawa, K.; Takaya, Y.; Matsubara, H.; Ito, H. Current Treatment Strategies and Nanoparticle-Mediated Drug Delivery Systems for Pulmonary Arterial Hypertension. Int. J. Mol. Sci. 2019, 20, 5885. https://doi.org/10.3390/ijms20235885
Nakamura K, Akagi S, Ejiri K, Yoshida M, Miyoshi T, Toh N, Nakagawa K, Takaya Y, Matsubara H, Ito H. Current Treatment Strategies and Nanoparticle-Mediated Drug Delivery Systems for Pulmonary Arterial Hypertension. International Journal of Molecular Sciences. 2019; 20(23):5885. https://doi.org/10.3390/ijms20235885
Chicago/Turabian StyleNakamura, Kazufumi, Satoshi Akagi, Kentaro Ejiri, Masashi Yoshida, Toru Miyoshi, Norihisa Toh, Koji Nakagawa, Yoichi Takaya, Hiromi Matsubara, and Hiroshi Ito. 2019. "Current Treatment Strategies and Nanoparticle-Mediated Drug Delivery Systems for Pulmonary Arterial Hypertension" International Journal of Molecular Sciences 20, no. 23: 5885. https://doi.org/10.3390/ijms20235885





