Dysregulation of Adenosinergic Signaling in Systemic and Organ-Specific Autoimmunity
Abstract
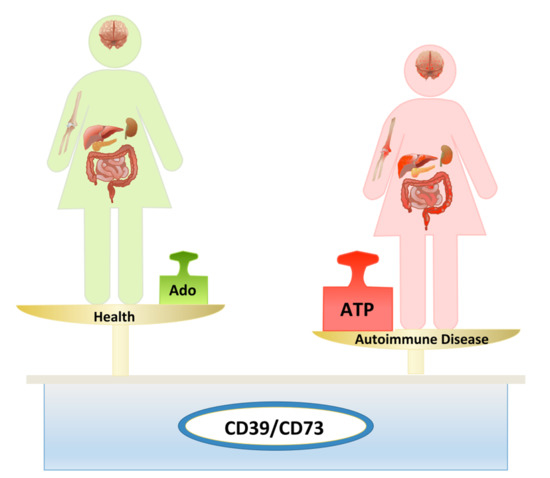
:1. Introduction
2. Systemic Autoimmune Disorders
2.1. Systemic Lupus Erythematosus
2.2. Rheumatoid Arthritis
2.3. Type 1 Diabetes
2.4. Autoimmune Hepatitis and Cholestatic Liver Disorders
2.5. Inflammatory Bowel Disease
2.6. Multiple Sclerosis
3. Therapeutic Implications
4. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Burnstock, G. Purinergic signalling: From discovery to current developments. Exp. Physiol. 2014, 99, 16–34. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Vuerich, M. Purinergic signaling in the immune system. Auton. Neurosci. 2015, 191, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Sitkovsky, M.V.; Robson, S.C. Purinergic signaling during inflammation. N. Engl. J. Med. 2012, 367, 2322–2333. [Google Scholar] [CrossRef] [PubMed]
- Longhi, M.S.; Moss, A.; Jiang, Z.G.; Robson, S.C. Purinergic signaling during intestinal inflammation. J. Mol. Med. 2017, 95, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Yegutkin, G.G. Enzymes involved in metabolism of extracellular nucleotides and nucleosides: Functional implications and measurement of activities. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 473–497. [Google Scholar] [CrossRef] [PubMed]
- Allard, B.; Longhi, M.S.; Robson, S.C.; Stagg, J. The ectonucleotidases CD39 and CD73: Novel checkpoint inhibitor targets. Immunol. Rev. 2017, 276, 121–144. [Google Scholar] [CrossRef] [Green Version]
- Longhi, M.S.; Vuerich, M.; Kalbasi, A.; Kenison, J.E.; Yeste, A.; Csizmadia, E.; Vaughn, B.; Feldbrugge, L.; Mitsuhashi, S.; Wegiel, B.; et al. Bilirubin suppresses Th17 immunity in colitis by upregulating CD39. JCI Insight 2017, 2. [Google Scholar] [CrossRef] [Green Version]
- Xie, A.; Robles, R.J.; Mukherjee, S.; Zhang, H.; Feldbrugge, L.; Csizmadia, E.; Wu, Y.; Enjyoji, K.; Moss, A.C.; Otterbein, L.E.; et al. HIF-1alpha-induced xenobiotic transporters promote Th17 responses in Crohn’s disease. J. Autoimmun. 2018, 94, 122–133. [Google Scholar] [CrossRef]
- Goettel, J.A.; Gandhi, R.; Kenison, J.E.; Yeste, A.; Murugaiyan, G.; Sambanthamoorthy, S.; Griffith, A.E.; Patel, B.; Shouval, D.S.; Weiner, H.L.; et al. AHR Activation Is Protective against Colitis Driven by T Cells in Humanized Mice. Cell Rep. 2016, 17, 1318–1329. [Google Scholar] [CrossRef] [Green Version]
- Deaglio, S.; Dwyer, K.M.; Gao, W.; Friedman, D.; Usheva, A.; Erat, A.; Chen, J.F.; Enjyoji, K.; Linden, J.; Oukka, M.; et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 2007, 204, 1257–1265. [Google Scholar] [CrossRef] [Green Version]
- Dwyer, K.M.; Hanidziar, D.; Putheti, P.; Hill, P.A.; Pommey, S.; McRae, J.L.; Winterhalter, A.; Doherty, G.; Deaglio, S.; Koulmanda, M.; et al. Expression of CD39 by human peripheral blood CD4+ CD25+ T cells denotes a regulatory memory phenotype. Am. J. Transplant. 2010, 10, 2410–2420. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.S.; Kurtz, C.C.; Rowlett, R.M.; Reuter, B.K.; Wiznerowicz, E.; Das, S.; Linden, J.; Crowe, S.E.; Ernst, P.B. CD73 is expressed by human regulatory T helper cells and suppresses proinflammatory cytokine production and Helicobacter felis-induced gastritis in mice. J. Infect. Dis. 2009, 199, 494–504. [Google Scholar] [CrossRef] [PubMed]
- Kobie, J.J.; Shah, P.R.; Yang, L.; Rebhahn, J.A.; Fowell, D.J.; Mosmann, T.R. T regulatory and primed uncommitted CD4 T cells express CD73, which suppresses effector CD4 T cells by converting 5’-adenosine monophosphate to adenosine. J. Immunol. 2006, 177, 6780–6786. [Google Scholar] [CrossRef]
- Bao, R.; Hou, J.; Li, Y.; Bian, J.; Deng, X.; Zhu, X.; Yang, T. Adenosine promotes Foxp3 expression in Treg cells in sepsis model by activating JNK/AP-1 pathway. Am. J. Transl. Res. 2016, 8, 2284–2292. [Google Scholar] [PubMed]
- Schenk, U.; Frascoli, M.; Proietti, M.; Geffers, R.; Traggiai, E.; Buer, J.; Ricordi, C.; Westendorf, A.M.; Grassi, F. ATP inhibits the generation and function of regulatory T cells through the activation of purinergic P2X receptors. Sci. Signal. 2011, 4, ra12. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Apasov, S.; Koshiba, M.; Sitkovsky, M. Role of A2a extracellular adenosine receptor-mediated signaling in adenosine-mediated inhibition of T-cell activation and expansion. Blood 1997, 90, 1600–1610. [Google Scholar] [PubMed]
- Liao, H.; Hyman, M.C.; Baek, A.E.; Fukase, K.; Pinsky, D.J. cAMP/CREB-mediated transcriptional regulation of ectonucleoside triphosphate diphosphohydrolase 1 (CD39) expression. J. Biol. Chem. 2010, 285, 14791–14805. [Google Scholar] [CrossRef] [PubMed]
- Kinsey, G.R.; Huang, L.; Jaworska, K.; Khutsishvili, K.; Becker, D.A.; Ye, H.; Lobo, P.I.; Okusa, M.D. Autocrine adenosine signaling promotes regulatory T cell-mediated renal protection. J. Am. Soc. Nephrol. 2012, 23, 1528–1537. [Google Scholar] [CrossRef]
- Ohta, A.; Kini, R.; Ohta, A.; Subramanian, M.; Madasu, M.; Sitkovsky, M. The development and immunosuppressive functions of CD4+ CD25+ FoxP3+ regulatory T cells are under influence of the adenosine-A2A adenosine receptor pathway. Front. Immunol. 2012, 3, 190. [Google Scholar] [CrossRef]
- Liang, D.; Woo, J.I.; Shao, H.; Born, W.K.; O’Brien, R.L.; Kaplan, H.J.; Sun, D. Ability of gammadelta T cells to modulate the Foxp3 T cell response is dependent on adenosine. PLoS ONE 2018, 13, e0197189. [Google Scholar] [CrossRef]
- Ring, S.; Pushkarevskaya, A.; Schild, H.; Probst, H.C.; Jendrossek, V.; Wirsdorfer, F.; Ledent, C.; Robson, S.C.; Enk, A.H.; Mahnke, K. Regulatory T cell-derived adenosine induces dendritic cell migration through the Epac-Rap1 pathway. J. Immunol. 2015, 194, 3735–3744. [Google Scholar] [CrossRef] [PubMed]
- Ehrentraut, H.; Westrich, J.A.; Eltzschig, H.K.; Clambey, E.T. Adora2b adenosine receptor engagement enhances regulatory T cell abundance during endotoxin-induced pulmonary inflammation. PLoS ONE 2012, 7, e32416. [Google Scholar] [CrossRef] [PubMed]
- Mascanfroni, I.D.; Takenaka, M.C.; Yeste, A.; Patel, B.; Wu, Y.; Kenison, J.E.; Siddiqui, S.; Basso, A.S.; Otterbein, L.E.; Pardoll, D.M.; et al. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-alpha. Nat. Med. 2015, 21, 638–646. [Google Scholar] [CrossRef] [PubMed]
- McGeachy, M.J.; Bak-Jensen, K.S.; Chen, Y.; Tato, C.M.; Blumenschein, W.; McClanahan, T.; Cua, D.J. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 2007, 8, 1390–1397. [Google Scholar] [CrossRef] [PubMed]
- Flores-Santibanez, F.; Fernandez, D.; Meza, D.; Tejon, G.; Vargas, L.; Varela-Nallar, L.; Arredondo, S.; Guixe, V.; Rosemblatt, M.; Bono, M.R.; et al. CD73-mediated adenosine production promotes stem cell-like properties in mouse Tc17 cells. Immunology 2015, 146, 582–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bono, M.R.; Fernandez, D.; Flores-Santibanez, F.; Rosemblatt, M.; Sauma, D. CD73 and CD39 ectonucleotidases in T cell differentiation: Beyond immunosuppression. FEBS Lett. 2015, 589, 3454–3460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.K.; Godec, J.; Wolski, D.; Adland, E.; Yates, K.; Pauken, K.E.; Cosgrove, C.; Ledderose, C.; Junger, W.G.; Robson, S.C.; et al. CD39 Expression Identifies Terminally Exhausted CD8+ T Cells. PLoS Pathog. 2015, 11, e1005177. [Google Scholar] [CrossRef]
- Hindupur, S.K.; Gonzalez, A.; Hall, M.N. The opposing actions of target of rapamycin and AMP-activated protein kinase in cell growth control. Cold Spring Harb. Perspect. Biol. 2015, 7, a019141. [Google Scholar] [CrossRef]
- Farez, M.F.; Mascanfroni, I.D.; Mendez-Huergo, S.P.; Yeste, A.; Murugaiyan, G.; Garo, L.P.; Balbuena Aguirre, M.E.; Patel, B.; Ysrraelit, M.C.; Zhu, C.; et al. Melatonin Contributes to the Seasonality of Multiple Sclerosis Relapses. Cell 2015, 162, 1338–1352. [Google Scholar] [CrossRef] [Green Version]
- Mann, E.H.; Chambers, E.S.; Chen, Y.H.; Richards, D.F.; Hawrylowicz, C.M. 1alpha,25-dihydroxyvitamin D3 acts via transforming growth factor-beta to up-regulate expression of immunosuppressive CD73 on human CD4+ Foxp3− T cells. Immunology 2015, 146, 423–431. [Google Scholar] [CrossRef]
- Regateiro, F.S.; Howie, D.; Nolan, K.F.; Agorogiannis, E.I.; Greaves, D.R.; Cobbold, S.P.; Waldmann, H. Generation of anti-inflammatory adenosine by leukocytes is regulated by TGF-beta. Eur. J. Immunol. 2011, 41, 2955–2965. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Blandizzi, C.; Pacher, P.; Hasko, G. Immunity, inflammation and cancer: A leading role for adenosine. Nat. Rev. Cancer 2013, 13, 842–857. [Google Scholar] [CrossRef]
- Gsandtner, I.; Charalambous, C.; Stefan, E.; Ogris, E.; Freissmuth, M.; Zezula, J. Heterotrimeric G protein-independent signaling of a G protein-coupled receptor. Direct binding of ARNO/cytohesin-2 to the carboxyl terminus of the A2A adenosine receptor is necessary for sustained activation of the ERK/MAP kinase pathway. J. Biol. Chem. 2005, 280, 31898–31905. [Google Scholar] [CrossRef] [PubMed]
- Peter, D.; Jin, S.L.; Conti, M.; Hatzelmann, A.; Zitt, C. Differential expression and function of phosphodiesterase 4 (PDE4) subtypes in human primary CD4+ T cells: Predominant role of PDE4D. J. Immunol. 2007, 178, 4820–4831. [Google Scholar] [CrossRef]
- Keuerleber, S.; Gsandtner, I.; Freissmuth, M. From cradle to twilight: The carboxyl terminus directs the fate of the A(2A)-adenosine receptor. Biochim. Biophys. Acta 2011, 1808, 1350–1357. [Google Scholar] [CrossRef] [PubMed]
- Kreth, S.; Ledderose, C.; Kaufmann, I.; Groeger, G.; Thiel, M. Differential expression of 5′-UTR splice variants of the adenosine A2A receptor gene in human granulocytes: Identification, characterization, and functional impact on activation. FASEB J. 2008, 22, 3276–3286. [Google Scholar] [CrossRef]
- Zhang, L.; Yang, N.; Wang, S.; Huang, B.; Li, F.; Tan, H.; Liang, Y.; Chen, M.; Li, Y.; Yu, X. Adenosine 2A receptor is protective against renal injury in MRL/lpr mice. Lupus 2011, 20, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Knight, J.S.; Mazza, L.F.; Yalavarthi, S.; Sule, G.; Ali, R.A.; Hodgin, J.B.; Kanthi, Y.; Pinsky, D.J. Ectonucleotidase-Mediated Suppression of Lupus Autoimmunity and Vascular Dysfunction. Front. Immunol. 2018, 9, 1322. [Google Scholar] [CrossRef] [PubMed]
- Loza, M.J.; Anderson, A.S.; O’Rourke, K.S.; Wood, J.; Khan, I.U. T-cell specific defect in expression of the NTPDase CD39 as a biomarker for lupus. Cell Immunol. 2011, 271, 110–117. [Google Scholar] [CrossRef] [Green Version]
- Kammer, G.M.; Birch, R.E.; Polmar, S.H. Impaired immunoregulation in systemic lupus erythematosus: Defective adenosine-induced suppressor T lymphocyte generation. J. Immunol. 1983, 130, 1706–1712. [Google Scholar]
- Schultz, L.A.; Kammer, G.M.; Rudolph, S.A. Characterization of the human T lymphocyte adenosine receptor: Comparison of normal and systemic lupus erythematosus cells. FASEB J. 1988, 2, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Bortoluzzi, A.; Vincenzi, F.; Govoni, M.; Padovan, M.; Ravani, A.; Borea, P.A.; Varani, K. A2A adenosine receptor upregulation correlates with disease activity in patients with systemic lupus erythematosus. Arthritis Res. Ther. 2016, 18, 192. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Peres, R.S.; Donate, P.B.; Talbot, J.; Cecilio, N.T.; Lobo, P.R.; Machado, C.C.; Lima, K.W.A.; Oliveira, R.D.; Carregaro, V.; Nakaya, H.I.; et al. TGF-beta signalling defect is linked to low CD39 expression on regulatory T cells and methotrexate resistance in rheumatoid arthritis. J. Autoimmun. 2018, 90, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Peres, R.S.; Liew, F.Y.; Talbot, J.; Carregaro, V.; Oliveira, R.D.; Almeida, S.L.; Franca, R.F.; Donate, P.B.; Pinto, L.G.; Ferreira, F.I.; et al. Low expression of CD39 on regulatory T cells as a biomarker for resistance to methotrexate therapy in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2015, 112, 2509–2514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hider, S.L.; Thomson, W.; Mack, L.F.; Armstrong, D.J.; Shadforth, M.; Bruce, I.N. Polymorphisms within the adenosine receptor 2a gene are associated with adverse events in RA patients treated with MTX. Rheumatology 2008, 47, 1156–1159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veras, F.P.; Peres, R.S.; Saraiva, A.L.; Pinto, L.G.; Louzada-Junior, P.; Cunha, T.M.; Paschoal, J.A.; Cunha, F.Q.; Alves-Filho, J.C. Fructose 1,6-bisphosphate, a high-energy intermediate of glycolysis, attenuates experimental arthritis by activating anti-inflammatory adenosinergic pathway. Sci. Rep. 2015, 5, 15171. [Google Scholar] [CrossRef] [Green Version]
- Thiolat, A.; Semerano, L.; Pers, Y.M.; Biton, J.; Lemeiter, D.; Portales, P.; Quentin, J.; Jorgensen, C.; Decker, P.; Boissier, M.C.; et al. Interleukin-6 receptor blockade enhances CD39+ regulatory T cell development in rheumatoid arthritis and in experimental arthritis. Arthritis Rheumatol. 2014, 66, 273–283. [Google Scholar] [CrossRef]
- Nie, H.; Zheng, Y.; Li, R.; Guo, T.B.; He, D.; Fang, L.; Liu, X.; Xiao, L.; Chen, X.; Wan, B.; et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-alpha in rheumatoid arthritis. Nat. Med. 2013, 19, 322–328. [Google Scholar] [CrossRef]
- Dos Santos Jaques, J.A.; Becker, L.V.; Souza Vdo, C.; Leal, C.A.; Bertoldo, T.M.; de Vargas Pinheiro, K.; Morsch, V.M.; Schetinger, M.R.; Leal, D.B. Activities of enzymes that hydrolyze adenine nucleotides in lymphocytes from patients with rheumatoid arthritis. Cell Biochem. Funct. 2013, 31, 395–399. [Google Scholar] [CrossRef]
- Herrath, J.; Chemin, K.; Albrecht, I.; Catrina, A.I.; Malmstrom, V. Surface expression of CD39 identifies an enriched Treg-cell subset in the rheumatic joint, which does not suppress IL-17A secretion. Eur. J. Immunol. 2014, 44, 2979–2989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrobak, P.; Charlebois, R.; Rejtar, P.; El Bikai, R.; Allard, B.; Stagg, J. CD73 plays a protective role in collagen-induced arthritis. J. Immunol. 2015, 194, 2487–2492. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Wang, W.; Wu, J.; Ma, L.; Guo, J. Identification of CD4+ T-cell-derived CD161+ CD39+ and CD39+CD73+ microparticles as new biomarkers for rheumatoid arthritis. Biomark. Med. 2017, 11, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Madi, L.; Cohen, S.; Ochayin, A.; Bar-Yehuda, S.; Barer, F.; Fishman, P. Overexpression of A3 adenosine receptor in peripheral blood mononuclear cells in rheumatoid arthritis: Involvement of nuclear factor-kappaB in mediating receptor level. J. Rheumatol. 2007, 34, 20–26. [Google Scholar] [PubMed]
- Ochaion, A.; Bar-Yehuda, S.; Cohen, S.; Barer, F.; Patoka, R.; Amital, H.; Reitblat, T.; Reitblat, A.; Ophir, J.; Konfino, I.; et al. The anti-inflammatory target A(3) adenosine receptor is over-expressed in rheumatoid arthritis, psoriasis and Crohn’s disease. Cell Immunol. 2009, 258, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Varani, K.; Padovan, M.; Vincenzi, F.; Targa, M.; Trotta, F.; Govoni, M.; Borea, P.A. A2A and A3 adenosine receptor expression in rheumatoid arthritis: Upregulation, inverse correlation with disease activity score and suppression of inflammatory cytokine and metalloproteinase release. Arthritis Res. Ther. 2011, 13, R197. [Google Scholar] [CrossRef] [PubMed]
- Ravani, A.; Vincenzi, F.; Bortoluzzi, A.; Padovan, M.; Pasquini, S.; Gessi, S.; Merighi, S.; Borea, P.A.; Govoni, M.; Varani, K. Role and Function of A2A and A(3) Adenosine Receptors in Patients with Ankylosing Spondylitis, Psoriatic Arthritis and Rheumatoid Arthritis. Int. J. Mol. Sci. 2017, 18, 697. [Google Scholar] [CrossRef]
- Chia, J.S.; McRae, J.L.; Thomas, H.E.; Fynch, S.; Elkerbout, L.; Hill, P.; Murray-Segal, L.; Robson, S.C.; Chen, J.F.; d’Apice, A.J.; et al. The protective effects of CD39 overexpression in multiple low-dose streptozotocin-induced diabetes in mice. Diabetes 2013, 62, 2026–2035. [Google Scholar] [CrossRef]
- Nemeth, Z.H.; Bleich, D.; Csoka, B.; Pacher, P.; Mabley, J.G.; Himer, L.; Vizi, E.S.; Deitch, E.A.; Szabo, C.; Cronstein, B.N.; et al. Adenosine receptor activation ameliorates type 1 diabetes. FASEB J. 2007, 21, 2379–2388. [Google Scholar] [CrossRef] [Green Version]
- Akesson, K.; Tompa, A.; Ryden, A.; Faresjo, M. Low expression of CD39+/CD45RA+ on regulatory T cells (Treg) cells in type 1 diabetic children in contrast to high expression of CD101+/CD129+ on Treg cells in children with coeliac disease. Clin. Exp. Immunol. 2015, 180, 70–82. [Google Scholar] [CrossRef]
- Tai, N.; Wong, F.S.; Wen, L. TLR9 deficiency promotes CD73 expression in T cells and diabetes protection in nonobese diabetic mice. J. Immunol. 2013, 191, 2926–2937. [Google Scholar] [CrossRef] [PubMed]
- Grant, C.R.; Liberal, R.; Holder, B.S.; Cardone, J.; Ma, Y.; Robson, S.C.; Mieli-Vergani, G.; Vergani, D.; Longhi, M.S. Dysfunctional CD39(POS) regulatory T cells and aberrant control of T-helper type 17 cells in autoimmune hepatitis. Hepatology 2014, 59, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Liberal, R.; Grant, C.R.; Ma, Y.; Csizmadia, E.; Jiang, Z.G.; Heneghan, M.A.; Yee, E.U.; Mieli-Vergani, G.; Vergani, D.; Robson, S.C.; et al. CD39 mediated regulation of Th17-cell effector function is impaired in juvenile autoimmune liver disease. J. Autoimmun. 2016, 72, 102–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeffery, H.C.; Braitch, M.K.; Bagnall, C.; Hodson, J.; Jeffery, L.E.; Wawman, R.E.; Wong, L.L.; Birtwistle, J.; Bartlett, H.; Lohse, A.W.; et al. Changes in natural killer cells and exhausted memory regulatory T Cells with corticosteroid therapy in acute autoimmune hepatitis. Hepatol. Commun. 2018, 2, 421–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Chen, P.; Wang, T.; Zhan, Y.; Zhou, M.; Xia, L.; Cheng, R.; Guo, Y.; Zhu, L.; Zhang, J. Loss of A(1) adenosine receptor attenuates alpha-naphthylisothiocyanate-induced cholestatic liver injury in mice. Toxicol. Sci. 2013, 131, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, E.G.; Fausther, M.; Goree, J.R.; Dranoff, J.A. The Cholangiocyte Adenosine-IL-6 Axis Regulates Survival During Biliary Cirrhosis. Gene Expr. 2017, 17, 327–340. [Google Scholar] [CrossRef]
- Bernuzzi, F.; Fenoglio, D.; Battaglia, F.; Fravega, M.; Gershwin, M.E.; Indiveri, F.; Ansari, A.A.; Podda, M.; Invernizzi, P.; Filaci, G. Phenotypical and functional alterations of CD8 regulatory T cells in primary biliary cirrhosis. J. Autoimmun. 2010, 35, 176–180. [Google Scholar] [CrossRef] [Green Version]
- Taylor, A.E.; Carey, A.N.; Kudira, R.; Lages, C.S.; Shi, T.; Lam, S.; Karns, R.; Simmons, J.; Shanmukhappa, K.; Almanan, M.; et al. Interleukin 2 Promotes Hepatic Regulatory T Cell Responses and Protects From Biliary Fibrosis in Murine Sclerosing Cholangitis. Hepatology 2018, 68, 1905–1921. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.W.; Rothweiler, S.; Wei, G.; Ikenaga, N.; Liu, S.B.; Sverdlov, D.Y.; Vaid, K.A.; Longhi, M.S.; Kuang, M.; Robson, S.C.; et al. The ectonucleotidase ENTPD1/CD39 limits biliary injury and fibrosis in mouse models of sclerosing cholangitis. Hepatol. Commun. 2017, 1, 957–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujino, S.; Andoh, A.; Bamba, S.; Ogawa, A.; Hata, K.; Araki, Y.; Bamba, T.; Fujiyama, Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut 2003, 52, 65–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longhi, M.S.; Moss, A.; Bai, A.; Wu, Y.; Huang, H.; Cheifetz, A.; Quintana, F.J.; Robson, S.C. Characterization of human CD39+ Th17 cells with suppressor activity and modulation in inflammatory bowel disease. PLoS ONE 2014, 9, e87956. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.J.; Kunzli, B.M.; YI, A.R.; Sevigny, J.; Berberat, P.O.; Enjyoji, K.; Csizmadia, E.; Friess, H.; Robson, S.C. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc. Natl. Acad. Sci. USA 2009, 106, 16788–16793. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.J.; Elliott, L.; McDermott, E.; Tosetto, M.; Keegan, D.; Byrne, K.; Martin, S.T.; Rispens, T.; Cullen, G.; Mulcahy, H.E.; et al. Heightened Expression of CD39 by Regulatory T Lymphocytes Is Associated with Therapeutic Remission in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 2806–2814. [Google Scholar] [CrossRef] [PubMed]
- Bai, A.; Moss, A.; Kokkotou, E.; Usheva, A.; Sun, X.; Cheifetz, A.; Zheng, Y.; Longhi, M.S.; Gao, W.; Wu, Y.; et al. CD39 and CD161 modulate Th17 responses in Crohn’s disease. J. Immunol. 2014, 193, 3366–3377. [Google Scholar] [CrossRef] [PubMed]
- Bai, A.; Moss, A.; Rothweiler, S.; Longhi, M.S.; Wu, Y.; Junger, W.G.; Robson, S.C. NADH oxidase-dependent CD39 expression by CD8+ T cells modulates interferon gamma responses via generation of adenosine. Nat. Commun. 2015, 6, 8819. [Google Scholar] [CrossRef] [PubMed]
- Bynoe, M.S.; Waickman, A.T.; Mahamed, D.A.; Mueller, C.; Mills, J.H.; Czopik, A. CD73 is critical for the resolution of murine colonic inflammation. J. Biomed. Biotechnol. 2012, 2012, 260983. [Google Scholar] [CrossRef] [PubMed]
- Doherty, G.A.; Bai, A.; Hanidziar, D.; Longhi, M.S.; Lawlor, G.O.; Putheti, P.; Csizmadia, E.; Nowak, M.; Cheifetz, A.S.; Moss, A.C.; et al. CD73 is a phenotypic marker of effector memory Th17 cells in inflammatory bowel disease. Eur. J. Immunol. 2012, 42, 3062–3072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Odashima, M.; Bamias, G.; Rivera-Nieves, J.; Linden, J.; Nast, C.C.; Moskaluk, C.A.; Marini, M.; Sugawara, K.; Kozaiwa, K.; Otaka, M.; et al. Activation of A2A adenosine receptor attenuates intestinal inflammation in animal models of inflammatory bowel disease. Gastroenterology 2005, 129, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Kolachala, V.L.; Vijay-Kumar, M.; Dalmasso, G.; Yang, D.; Linden, J.; Wang, L.; Gewirtz, A.; Ravid, K.; Merlin, D.; Sitaraman, S.V. A2B adenosine receptor gene deletion attenuates murine colitis. Gastroenterology 2008, 135, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Aherne, C.M.; Collins, C.B.; Rapp, C.R.; Olli, K.E.; Perrenoud, L.; Jedlicka, P.; Bowser, J.L.; Mills, T.W.; Karmouty-Quintana, H.; Blackburn, M.R.; et al. Coordination of ENT2-dependent adenosine transport and signaling dampens mucosal inflammation. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Jaraquemada, D.; Flerlage, M.; Richert, J.; Whitaker, J.; Long, E.O.; McFarlin, D.E.; McFarland, H.F. Fine specificity and HLA restriction of myelin basic protein-specific cytotoxic T cell lines from multiple sclerosis patients and healthy individuals. J. Immunol. 1990, 145, 540–548. [Google Scholar] [PubMed]
- Fletcher, J.M.; Lonergan, R.; Costelloe, L.; Kinsella, K.; Moran, B.; O’Farrelly, C.; Tubridy, N.; Mills, K.H. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J. Immunol. 2009, 183, 7602–7610. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Begum-Haque, S.; Telesford, K.M.; Ochoa-Reparaz, J.; Christy, M.; Kasper, E.J.; Kasper, D.L.; Robson, S.C.; Kasper, L.H. A commensal bacterial product elicits and modulates migratory capacity of CD39+ CD4 T regulatory subsets in the suppression of neuroinflammation. Gut Microbes 2014, 5, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Telesford, K.M.; Ochoa-Reparaz, J.; Haque-Begum, S.; Christy, M.; Kasper, E.J.; Wang, L.; Wu, Y.; Robson, S.C.; Kasper, D.L.; et al. An intestinal commensal symbiosis factor controls neuroinflammation via TLR2-mediated CD39 signalling. Nat. Commun. 2014, 5, 4432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borsellino, G.; Kleinewietfeld, M.; Di Mitri, D.; Sternjak, A.; Diamantini, A.; Giometto, R.; Hopner, S.; Centonze, D.; Bernardi, G.; Dell’Acqua, M.L.; et al. Expression of ectonucleotidase CD39 by Foxp3+ Treg cells: Hydrolysis of extracellular ATP and immune suppression. Blood 2007, 110, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Peelen, E.; Damoiseaux, J.; Smolders, J.; Knippenberg, S.; Menheere, P.; Tervaert, J.W.; Hupperts, R.; Thewissen, M. Th17 expansion in MS patients is counterbalanced by an expanded CD39+ regulatory T cell population during remission but not during relapse. J. NeuroImmunol. 2011, 240–241, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, S.; Schnermann, J.; Noorbakhsh, F.; Henry, S.; Yong, V.W.; Winston, B.W.; Warren, K.; Power, C. A1 adenosine receptor upregulation and activation attenuates neuroinflammation and demyelination in a model of multiple sclerosis. J. Neurosci. 2004, 24, 1521–1529. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.B.; Silva, C.; Gonzalez, G.; Holden, J.; Warren, K.G.; Metz, L.M.; Power, C. Diminished adenosine A1 receptor expression on macrophages in brain and blood of patients with multiple sclerosis. Ann. Neurol. 2001, 49, 650–658. [Google Scholar] [CrossRef]
- Mayne, M.; Shepel, P.N.; Jiang, Y.; Geiger, J.D.; Power, C. Dysregulation of adenosine A1 receptor-mediated cytokine expression in peripheral blood mononuclear cells from multiple sclerosis patients. Ann. Neurol. 1999, 45, 633–639. [Google Scholar] [CrossRef]
- Wan, P.; Liu, X.; Xiong, Y.; Ren, Y.; Chen, J.; Lu, N.; Guo, Y.; Bai, A. Extracellular ATP mediates inflammatory responses in colitis via P2 x 7 receptor signaling. Sci. Rep. 2016, 6, 19108. [Google Scholar] [CrossRef]
- Flye, M.W.; Yu, S. The synergistic effect of superoxide dismutase and adenosine triphosphate-MgCl2 on acute hepatic ischemia. Transplant. Proc. 1987, 19, 1324–1326. [Google Scholar] [PubMed]
- Ohana, G.; Cohen, S.; Rath-Wolfson, L.; Fishman, P. A3 adenosine receptor agonist, CF102, protects against hepatic ischemia/reperfusion injury following partial hepatectomy. Mol. Med. Rep. 2016, 14, 4335–4341. [Google Scholar] [CrossRef] [PubMed]
- Hart, M.L.; Gorzolla, I.C.; Schittenhelm, J.; Robson, S.C.; Eltzschig, H.K. SP1-dependent induction of CD39 facilitates hepatic ischemic preconditioning. J. Immunol. 2010, 184, 4017–4024. [Google Scholar] [CrossRef] [PubMed]
- Mediero, A.; Perez-Aso, M.; Cronstein, B.N. Activation of adenosine A(2A) receptor reduces osteoclast formation via PKA- and ERK1/2-mediated suppression of NFkappaB nuclear translocation. Br. J. Pharmacol. 2013, 169, 1372–1388. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, F.; Corciulo, C.; Targa, M.; Merighi, S.; Gessi, S.; Casetta, I.; Gentile, M.; Granieri, E.; Borea, P.A.; Varani, K. Multiple sclerosis lymphocytes upregulate A2A adenosine receptors that are antiinflammatory when stimulated. Eur. J. Immunol. 2013, 43, 2206–2216. [Google Scholar] [CrossRef] [PubMed]
- Varani, K.; Massara, A.; Vincenzi, F.; Tosi, A.; Padovan, M.; Trotta, F.; Borea, P.A. Normalization of A2A and A3 adenosine receptor up-regulation in rheumatoid arthritis patients by treatment with anti-tumor necrosis factor alpha but not methotrexate. Arthritis Rheum. 2009, 60, 2880–2891. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, F.; Padovan, M.; Targa, M.; Corciulo, C.; Giacuzzo, S.; Merighi, S.; Gessi, S.; Govoni, M.; Borea, P.A.; Varani, K. A(2A) adenosine receptors are differentially modulated by pharmacological treatments in rheumatoid arthritis patients and their stimulation ameliorates adjuvant-induced arthritis in rats. PLoS ONE 2013, 8, e54195. [Google Scholar] [CrossRef]
- Decking, U.K.; Schlieper, G.; Kroll, K.; Schrader, J. Hypoxia-induced inhibition of adenosine kinase potentiates cardiac adenosine release. Circ. Res. 1997, 81, 154–164. [Google Scholar] [CrossRef]
- Chouker, A.; Thiel, M.; Lukashev, D.; Ward, J.M.; Kaufmann, I.; Apasov, S.; Sitkovsky, M.V.; Ohta, A. Critical role of hypoxia and A2A adenosine receptors in liver tissue-protecting physiological anti-inflammatory pathway. Mol. Med. 2008, 14, 116–123. [Google Scholar] [CrossRef]
- Flogel, U.; Burghoff, S.; van Lent, P.L.; Temme, S.; Galbarz, L.; Ding, Z.; El-Tayeb, A.; Huels, S.; Bonner, F.; Borg, N.; et al. Selective activation of adenosine A2A receptors on immune cells by a CD73-dependent prodrug suppresses joint inflammation in experimental rheumatoid arthritis. Sci. Transl. Med. 2012, 4, 146ra108. [Google Scholar] [CrossRef]
- El-Tayeb, A.; Michael, S.; Abdelrahman, A.; Behrenswerth, A.; Gollos, S.; Nieber, K.; Muller, C.E. Development of Polar Adenosine A2A Receptor Agonists for Inflammatory Bowel Disease: Synergism with A2B Antagonists. ACS Med. Chem. Lett. 2011, 2, 890–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochaion, A.; Bar-Yehuda, S.; Cohen, S.; Amital, H.; Jacobson, K.A.; Joshi, B.V.; Gao, Z.G.; Barer, F.; Patoka, R.; Del Valle, L.; et al. The A3 adenosine receptor agonist CF502 inhibits the PI3K, PKB/Akt and NF-kappaB signaling pathway in synoviocytes from rheumatoid arthritis patients and in adjuvant-induced arthritis rats. Biochem. Pharmacol. 2008, 76, 482–494. [Google Scholar] [CrossRef] [PubMed]
- Silverman, M.H.; Strand, V.; Markovits, D.; Nahir, M.; Reitblat, T.; Molad, Y.; Rosner, I.; Rozenbaum, M.; Mader, R.; Adawi, M.; et al. Clinical evidence for utilization of the A3 adenosine receptor as a target to treat rheumatoid arthritis: Data from a phase II clinical trial. J. Rheumatol. 2008, 35, 41–48. [Google Scholar] [PubMed]
- Fishman, P.; Cohen, S. The A3 adenosine receptor (A3AR): Therapeutic target and predictive biological marker in rheumatoid arthritis. Clin. Rheumatol. 2016, 35, 2359–2362. [Google Scholar] [CrossRef] [PubMed]
- Bar-Yehuda, S.; Rath-Wolfson, L.; Del Valle, L.; Ochaion, A.; Cohen, S.; Patoka, R.; Zozulya, G.; Barer, F.; Atar, E.; Pina-Oviedo, S.; et al. Induction of an antiinflammatory effect and prevention of cartilage damage in rat knee osteoarthritis by CF101 treatment. Arthritis Rheum. 2009, 60, 3061–3071. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzman, J.; Yu, J.G.; Suntres, Z.; Bozarov, A.; Cooke, H.; Javed, N.; Auer, H.; Palatini, J.; Hassanain, H.H.; Cardounel, A.J.; et al. ADOA3R as a therapeutic target in experimental colitis: Proof by validated high-density oligonucleotide microarray analysis. Inflamm. Bowel Dis. 2006, 12, 766–789. [Google Scholar] [CrossRef] [PubMed]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vuerich, M.; Harshe, R.P.; Robson, S.C.; Longhi, M.S. Dysregulation of Adenosinergic Signaling in Systemic and Organ-Specific Autoimmunity. Int. J. Mol. Sci. 2019, 20, 528. https://doi.org/10.3390/ijms20030528
Vuerich M, Harshe RP, Robson SC, Longhi MS. Dysregulation of Adenosinergic Signaling in Systemic and Organ-Specific Autoimmunity. International Journal of Molecular Sciences. 2019; 20(3):528. https://doi.org/10.3390/ijms20030528
Chicago/Turabian StyleVuerich, Marta, Rasika P. Harshe, Simon C. Robson, and Maria Serena Longhi. 2019. "Dysregulation of Adenosinergic Signaling in Systemic and Organ-Specific Autoimmunity" International Journal of Molecular Sciences 20, no. 3: 528. https://doi.org/10.3390/ijms20030528