iTRAQ-Based Protein Profiling and Biochemical Analysis of Two Contrasting Rice Genotypes Revealed Their Differential Responses to Salt Stress
Abstract
:1. Introduction
2. Results
2.1. Na+ and Cl− in the Soil
2.2. Na+ and Cl− in the Rice Plants
2.3. Cell Membrane Injury (CMI) in Rice Flag Leaves
2.4. Rice Root Activity
2.5. iTRAQ-Based Protein Identification at the Rice Maximum Tillering Stage
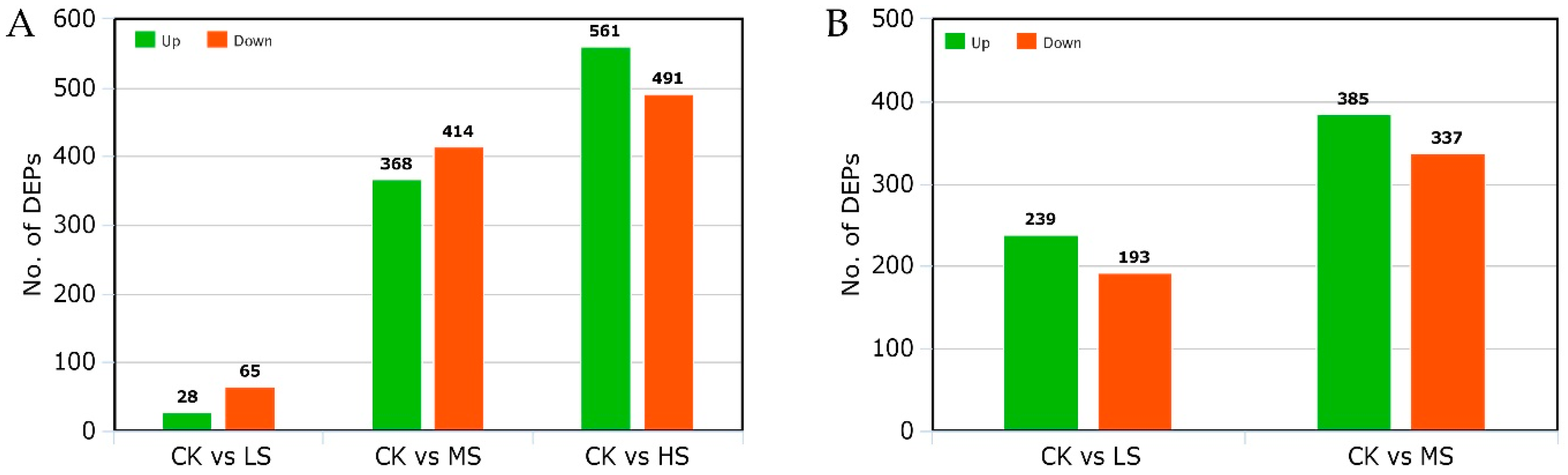
2.6. Identification of Differential Expressive Proteins in LYP9 and NPBA Subjected to Different Salt Stress Levels
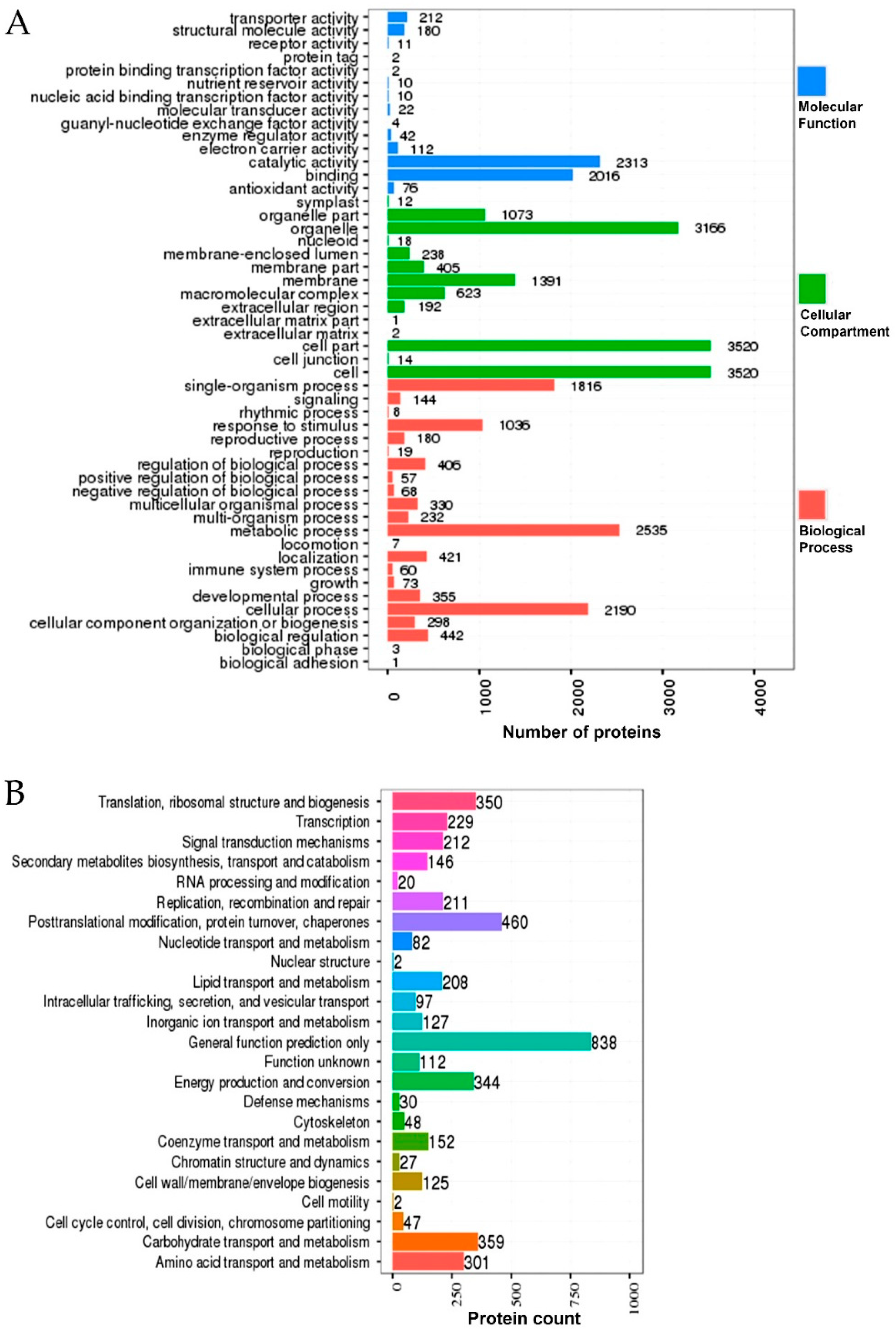
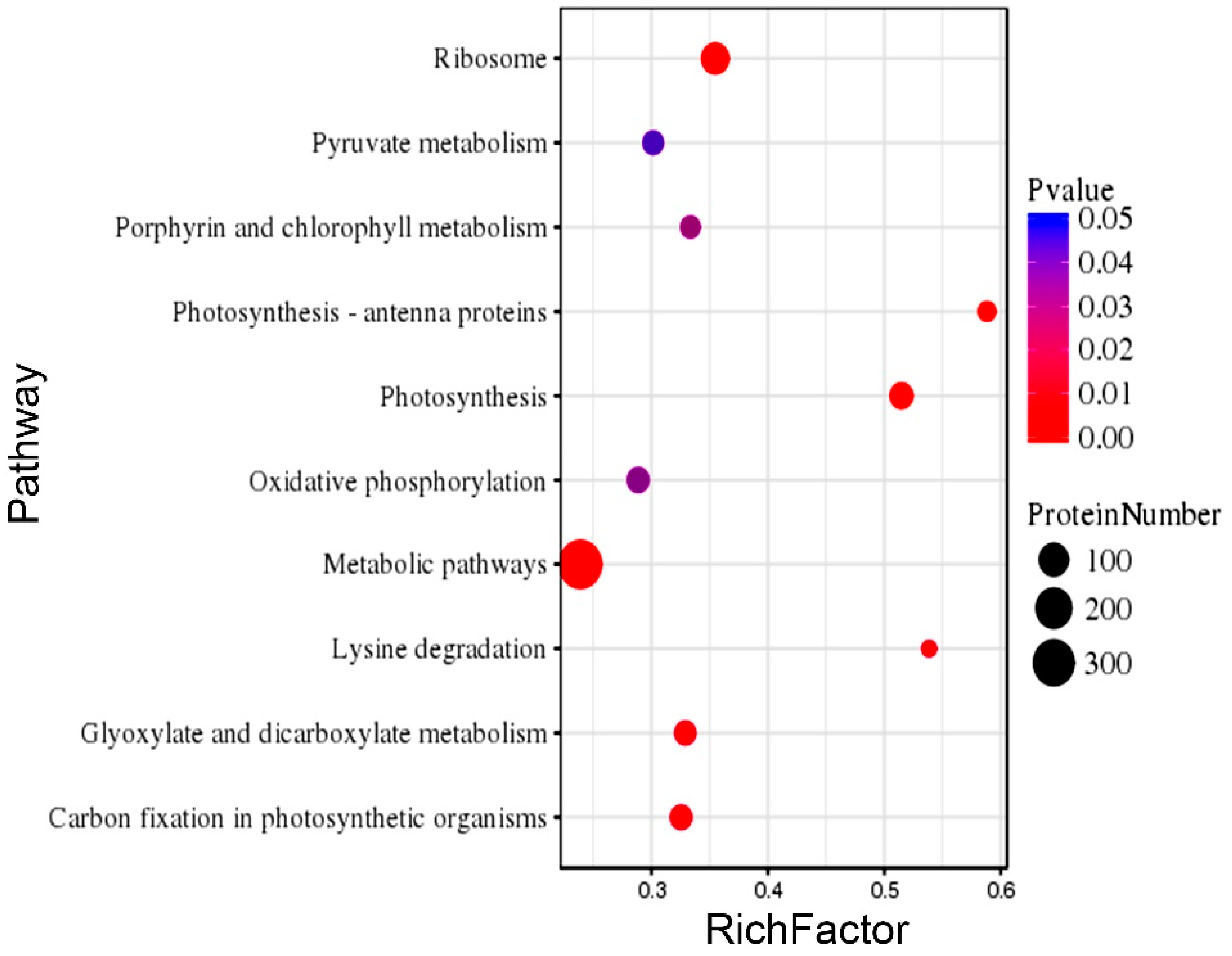
2.7. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment of the DEPs
3. Discussion
3.1. Biochemical Responses of Rice Plants to Salt Stress
3.2. Proteomic Analysis in the Rice Genotypes Under Salt Stress
3.2.1. Proteins Related to Salt Stress
3.2.2. Proteins Related to Redox Reactions
3.2.3. Proteins Related to Photosynthesis
3.2.4. Proteins Related to Carbohydrate Metabolism
3.2.5. Proteins Related to Osmotic Stress
3.2.6. Proteins Related to Other Metabolic Processes
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Soil and Plant Sampling
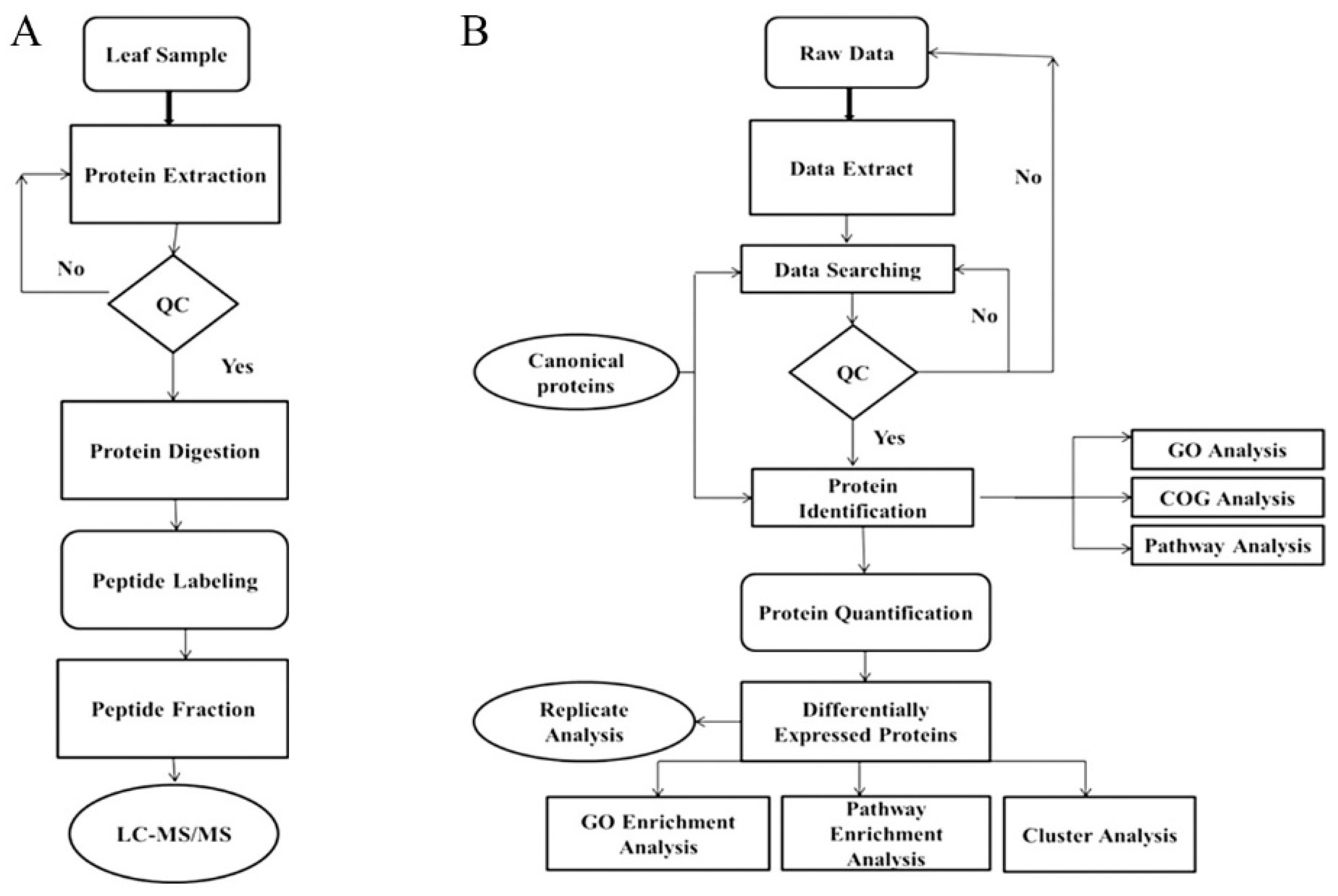
4.3. Leaf Proteomics Analysis Pipeline
4.3.1. Protein Extraction
4.3.2. Digestion of Proteins and Peptide Labeling
4.3.3. Peptide Fractionation and HPLC
4.3.4. Mass Spectrometer Detection
4.4. Cell Membrane Injury
4.5. Rice Root Activity
4.6. Na+ Concentration in the Soil and Plants
4.7. Cl− Concentration in the Soil and Plants
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| LYP9 | Liangyoupeijiu |
| NPBA | Nipponbare |
| iTRAQ | Isobaric tags for relative and absolute quantitation |
| CMI | Cell membrane injury |
| RRA | Rice root activity |
| DEPs | Differentially expressed proteins |
| GO | Gene ontology |
| KEGG | Kyoto encyclopedia of genes and genomes |
| PSI | Photosystem I |
| LS | Low salt stress |
| MS | Moderate salt stress |
| HS | High salt stress |
| COG | Cluster of orthologous groups |
References
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature response of photosynthesis in C3, C4, and CAM plants: Temperature acclimation and temperature adaptation. Photosynth. Res. 2013, 119, 101–117. [Google Scholar] [CrossRef] [PubMed]
- UNFPA. Linking Population, Poverty and Development. 2014. Available online: http://www.unfpa.org/pds/trends.htm (accessed on 28 January 2019).
- Ray, D.K.; Mueller, N.D.; West, P.C.; Foley, J.A. Yield trends are insufficient to double global crop production by 2050. PLoS ONE 2013, 8, e66428. [Google Scholar] [CrossRef] [PubMed]
- Nachimuthu, V.V.; Sabariappan, R.; Muthurajan, R.; Kumar, A. Breeding Rice Varieties for Abiotic Stress Tolerance: Challenges and Opportunities; Springer: Singapore, 2017. [Google Scholar]
- Pushpam, R.; Rangasamy, S.R.S. In vivo response of rice cultivars to salt stress. J. Ecol. 2002, 14, 177–182. [Google Scholar]
- Joseph, B.; Jini, D.; Sujatha, S. Biological and Physiological Perspectives of Specificity in Abiotic Salt Stress Response from Various Rice Plants. Asian J. Agric. Sci. 2010, 2, 99–105. [Google Scholar]
- Hussain, S.; Chu, Z.; Zhigang, B.; Xiaochuang, C.; Lianfeng, Z.; Azhar, H.; Chunquan, Z.; Shah, F.; Allen, B.J.; Junhua, Z.; et al. Effects of 1-Methylcyclopropene on Rice Growth Characteristics and Superior and Inferior Spikelet Development Under Salt Stress. J. Plant Growth Regul. 2018, 37, 1368–1384. [Google Scholar] [CrossRef]
- Islam, T.; Manna, M.; Reddy, M.K. Glutathione peroxidase of Pennisetum glaucum (PgGPx) is a functional Cd21 dependent peroxiredoxin that enhances tolerance against salinity and drought stress. PLoS ONE 2015, 10, e0143344. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Yasmeen, T.; Ali, S.; Ali, B.; Farooq, M.A.; Gill, R.A. Priming-induced antioxidative responses in two wheat cultivars under saline stress. Acta Physiol. Plant. 2015, 37, 153–161. [Google Scholar] [CrossRef]
- Islam, F.; Yasmeen, T.; Arif, M.S.; Ali, S.; Ali, B.; Hameed, S.; Zhou, W. Plant growth promoting bacteria confer salt tolerance in Vigna radiata by up-regulating antioxidant defense and biological soil fertility. Plant Growth Regul. 2016, 80, 23–36. [Google Scholar] [CrossRef]
- Islam, F.; Ali, B.; Wang, J.; Farooq, M.A.; Gill, R.A.; Ali, S.; Wang, D.; Zhou, W. Combined herbicide and saline stress differentially modulates hormonal regulation and antioxidant defense system in Oryza sativa cultivars. Plant Physiol. Biochem. 2016, 107, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Naeem, M.S.; Jin, Z.L.; Wan, G.L.; Liu, D.; Liu, H.B.; Yoneyama, K.; Zhou, W.J. 5-Aminolevulinic acid improves photosynthetic gas exchange capacity and ion uptake under salinity stress in oilseed rape (Brassica napus L.). Plant Soil 2010, 332, 405–415. [Google Scholar] [CrossRef]
- Hussain, S.; Xiaochuang, C.; Chu, Z.; Lianfeng, Z.; Maqsood, A.K.; Sajid, F.; Junhua, Z.; Qianyu, J. Sodium chloride stress during early growth stages altered physiological and growth characteristics of rice. Chil. J. Agric. Res. 2018, 78, 183–197. [Google Scholar] [CrossRef]
- Islam, F.; Ali, S.; Farooq, M.A.; Wang, J.; Gill, R.A.; Zhu, J.; Ali, B.; Zhou, W. Butachlor-induced alterations in ultrastructure, antioxidant, and stress-responsive gene regulations in rice cultivars. Clean—Soil Air Water 2017, 45, 1500851. [Google Scholar] [CrossRef]
- Islam, F.; Farooq, M.A.; Gill, R.A.; Wang, J.; Yang, C.; Ali, B.; Wang, G.X.; Zhou, W. 2,4-D attenuates salinity-induced toxicity by mediating anatomical changes, antioxidant capacity and cation transporters in the roots of rice cultivars. Sci. Rep. 2017, 7, 10443. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Liu, H.; Islam, F.; Li, L.; Farooq, M.A.; Ruan, S.; Zhou, W. OsPEX11, a peroxisomal biogenesis factor 11, contributes to salt stress tolerance in Oryza sativa. Front. Plant Sci. 2016, 7, 1357. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.; Chauhan, R.; Gill, T.; Swarnkar, M.K.; Sreenivasulu, Y.; Kumar, S.; Kumar, N.; Shankar, R.; Ahuja, P.S.; Singh, A.K. Expression of SOD and APX genes positively regulates secondary cell wall biosynthesis and promotes plant growth and yield in Arabidopsis under salt stress. Plant Mol. Biol. 2015, 87, 615–631. [Google Scholar] [CrossRef]
- Biswas, M.S.; Mano, J.I. Lipid peroxide-derived short-chain carbonyls mediate hydrogen peroxide induced and salt-induced programmed cell death in plants. Plant Physiol. 2015, 168, 885–898. [Google Scholar] [CrossRef]
- Ali, I.; Liu, B.; Farooq, M.A.; Islam, F.; Azizullah, A.; Yu, C.; Su, W.; Gan, Y. Toxicological effects of bisphenol A on growth and antioxidant defense system in Oryza sativa as revealed by ultrastructure analysis. Ecotoxicol. Environ. Saf. 2016, 124, 277–284. [Google Scholar] [CrossRef]
- Ali, I.; Jan, M.; Wakeel, A.; Azizullah, A.; Liu, B.; Islam, F.; Ali, A.; Daud, M.K.; Liu, Y.; Gan, Y. Biochemical responses and ultrastructural changes in ethylene insensitive mutants of Arabidopsis thialiana subjected to bisphenol A exposure. Ecotoxicol. Environ. Saf. 2017, 144, 62–71. [Google Scholar] [CrossRef]
- Li, H.; Chang, J.; Chen, H.; Wang, Z.; Gu, X.; Wei, C.; Zhang, Y.; Ma, J.; Yang, J.; Zhang, X. Exogenous Melatonin Confers Salt Stress Tolerance to Watermelon by Improving Photosynthesis and Redox Homeostasis. Front. Plant Sci. 2017, 8, 295. [Google Scholar] [CrossRef]
- Khare, T.; Kumar, V.; Kishor, P.K. Na+ and Cl- ions show additive effects under NaCl stress on induction of oxidative stress and the responsive antioxidative defense in rice. Protoplasma 2015, 252, 1149–1165. [Google Scholar] [CrossRef]
- Li, W.; Zhao, F.; Fang, W.; Xie, D.; Hou, J.; Yang, X.; Zhao, Y.; Tang, Z.; Nie, L.; Lv, S. Identification of early salt stress responsive proteins in seedling roots of upland cotton (Gossypium hirsutum L.) employing iTRAQ-based proteomic technique. Front. Plant Sci. 2015, 6, 732. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Xu, X.Y.; Gong, Q.Q.; Xie, C.; Fan, W.; Yang, J.L.; Lin, Q.S.; Zheng, S.J. Root proteome of rice studied by iTRAQ provides integrated insight into aluminum stress tolerance mechanisms in plants. J. Proteom. 2014, 98, 189–205. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Li, N.; Wu, L.; Li, C.; Li, C.; Zhang, L.; Liu, T.; Wang, W. Quantitative iTRAQ-based proteomic analysis of phosphoproteins and ABA regulated phosphoproteins in maize leaves under osmotic stress. Sci. Rep. 2015, 27, 15626. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Ge, P.; Ma, C.; Li, X.; Lv, D.; Wang, S.; Ma, W.; Yan, Y. Comparative proteomic analysis of salt response proteins in seedling roots of two wheat varieties. J. Proteom. 2012, 75, 1867–1885. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Zhang, C.; Li, X.; Wen, D.; Wang, S.; Shi, Q.; Wang, X. Identification of NaCl and NaHCO3 stress-responsive proteins in tomato roots using iTRAQ-based analysis. Biochem. Biophys. Res. Commun. 2014, 446, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Rakwal, R.; Agrawal, G.K.; Jung, Y.H.; Shibato, J.; Jwa, N.S.; Iwahashi, Y.; Iwahashi, H.; Kim, D.H.; Shim, I.S.; et al. A hydroponic rice seedling culture model system for investigating proteome of salt stress in rice leaf. Electrophoresis 2005, 26, 4521–4539. [Google Scholar] [CrossRef] [PubMed]
- Dooki, A.D.; Mayer-Posner, F.J.; Askari, H.; Zaiee, A.; Salekdeh, G.H. Proteomic responses of rice young panicles to salinity. Proteomics 2006, 6, 6498–6507. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.G.; Kee, W.P.; Jae, Y.A.; Young, G.S.; Jung, K.H.; Hak, Y.K.; Dong, W.B.; Kyung, H.L.; Nam, J.K.; Byung-Hyun, L.; et al. Proteomics analysis of salt-induced leaf proteins in two rice germplasms with different salt sensitivity. Can. J. Plant Sci. 2011, 91, 337–349. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, G.; Bian, X.; Zhao, Q. Effects of root interaction and nitrogen fertilization on the chlorophyll content, root activity, photosynthetic characteristics of intercropped soybean and microbial quantity in the rhizosphere. Plant Soil Environ. 2013, 59, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Reddy, I.N.B.L.; Kim, S.M.; Kim, B.K.; Yoon, I.S.; Kwon, T.R. Identification of rice accessions associated with K1/Na1 ratio and salt tolerance based on physiological and molecular responses. Rice Sci. 2017, 24, 36–364. [Google Scholar] [CrossRef]
- Amirjani, M.R. Effect of salinity stress on growth, sugar content, pigments and enzyme activity of rice. Int. J. Bot. 2011, 7, 73–81. [Google Scholar] [CrossRef]
- Hinsinger, P.; Betencourt, E.; Bernard, L.; Brauman, A.; Plassard, C.; Shen, J.; Tang, X.; Zhang, F. P for two, sharing a scarce resource: Soil phosphorus acquisition in the rhizosphere of intercropped species. Plant Physiol. 2011, 156, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.N.; Zhang, F.S.; Marschner, P.; Fan, F.L.; Gao, H.M.; Bao, X.G.; Sun, J.H.; Li, L. Effect of intercropping on crop yield and chemical and microbiological properties in rhizosphere of wheat (Triticum aestivum L.), maize (Zea mays L.), and faba bean (Vicia faba L.). Biol. Fertil. Soils 2007, 43, 565–574. [Google Scholar] [CrossRef]
- Zhang, N.N.; Sun, Y.M.; Li, L.; Wang, E.T.; Chen, W.X.; Yuan, H.L. Effects of intercropping and Rhizobium inoculation on yield and rhizosphere bacterial community of faba bean (Vicia faba L.). Biol. Fertil. Soils 2010, 46, 625–639. [Google Scholar] [CrossRef]
- Salekdeh, G.H.; Siopongco, J.; Wade, L.J.; Ghareyazie, B.; Bennett, J. A proteomic approach to analyzing drough and salt responsiveness in rice. Field Crop Res. 2002, 76, 199–219. [Google Scholar] [CrossRef]
- Jiang, Q.; Xiaojuan, L.; Fengjuan, N.; Xianjun, S.; Zheng, H.; Hui, Z. iTRAQ-based quantitative proteomic analysis of wheat roots in response to salt stress. Proteomics 2017, 17, 1600265. [Google Scholar] [CrossRef]
- Abbasi, F.M.; Komatsu, S. A proteomic approach to analyze salt-responsive proteins in rice leaf sheath. Proteomics 2004, 4, 2072–2081. [Google Scholar] [CrossRef]
- Wen, B.; Zhou, R.; Feng, Q.; Wang, Q.; Wang, J.; Liu, S. IQuant: An automated pipeline for quantitative proteomics based upon isobaric tags. Proteomics 2014, 14, 2280–2285. [Google Scholar] [CrossRef]
- Eprintsev, A.T.; Fedorina, O.S.; Bessmeltseva, Y.S. Response of the Malate Dehydrogenase System of Maize Mesophyll and Bundle Sheath to Salt Stress. Russ. J. Plant Physiol. 2011, 58, 448–453. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, W.; Yang, J.; Ismail, A.M. Role of ABA in integrating plant responses to drought and salt stresses. Field Crops Res. 2006, 97, 111–119. [Google Scholar] [CrossRef]
- Su, Y.; Wang, Z.; Liu, F.; Li, Z.; Peng, Q.; Guo, J.; Xu, Q.Y. Isolation and Characterization of ScGluD2, a New Sugarcane beta-1,3-Glucanase D Family Gene Induced by Sporisorium scitamineum, ABA, H2O2, NaCl, and CdCl2 Stresses. Front. Plant Sci. 2016, 7, 1348. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Yu, G.; Han, X.; Han, J.; Li, W.; Wang, B.; Huang, S.; Cheng, X. ThPP1 gene, encodes an inorganic pyrophosphatase in Thellungiella halophila, enhanced the tolerance of the transgenic rice to alkali stress. Plant Cell Rep. 2017, 36, 1929. [Google Scholar] [CrossRef] [PubMed]
- Karan, R.; Prasanta, K.S. Overexpression of a nascent polypeptide associated complex gene (SabNAC) of Spartina alterniflora improves tolerance to salinity and drought in transgenic Arabidopsis. Bioch. Biophys. Res. Commun. 2012, 424, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, C.; Tripathi, A.K. A SAM-dependent methyltransferase cotranscribed with arsenate reductase alters resistance to peptidyl transferase center-binding antibiotics in Azospirillum brasilense Sp7. Appl. Microbiol. Biotechnol. 2014, 98, 4625–4636. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.L.S.; Passaia, G.; Lobo, A.K.M.; Jardim-Messeder, D.; Silveira, J.A.; Margis-Pinheiro, M. Mitochondrial glutathione peroxidase (OsGPX3) has a crucial role in rice protection against salt stress. Environ. Exp. Bot. 2018, 158, 12–21. [Google Scholar] [CrossRef]
- Zeng, Y.; Tan, X.; Zhang, L.; Long, H.; Wang, B.; Li, Z.; Yuan, Z. A fructose-1,6-biphosphate aldolase gene from Camellia oleifera: Molecular characterization and impact on salt stress tolerance. Mol. Breed. 2015, 35, 1–17. [Google Scholar] [CrossRef]
- Lu, W.; Tang, X.; Huo, Y.; Xu, R.; Qi, S.; Huang, J.; Zheng, C.; Wu, C.A. Identification and characterization of fructose 1,6-bisphosphate aldolase genes in Arabidopsis reveal a gene family with diverse responses to abiotic stresses. Gene 2012, 503, 65–74. [Google Scholar] [CrossRef]
- Minhas, D.; Grover, A. Transcript levels of genes encoding various glycolytic and fermentation enzymes change in response to abiotic stresses. Plant Sci. 1991, 146, 41–51. [Google Scholar] [CrossRef]
- Sharma, S.; Mustafiz, A.; Singla-Pareek, S.L.; Shankar, S.P.; Sopory, S.K. Characterization of stress and methylglyoxal inducible triose phosphate isomerase (OscTPI) from rice. Plant Signal. Behav. 2012, 7, 1337–1345. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.K. Plant Salt Stress; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Habib, S.H.; Kausar, H.; Saud, H.M. Plant growthpromoting Rhizobacteria enhance salinity stress tolerance in Okra through ROS-scavenging enzymes. Biomed. Res. Int. 2016, 2016, 6284547. [Google Scholar]
- Hossain, M.S.; Dietz, K.J. Tuning of Redox Regulatory Mechanisms, Reactive Oxygen Species and Redox Homeostasis under Salinity Stress. Front. Plant Sci. 2016, 7, 548. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakl, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012, 2012, 217037. [Google Scholar] [CrossRef]
- Rejeb, K.B.; Benzarti, M.; Debez, A.; Bailly, C.; Savoure, A.; Abdelly, C. NADPH oxidase-dependent H2O2 production is required for saltinduced antioxidant defense in Arabidopsis thaliana. J. Plant Physiol. 2015, 174, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Han-Wei, J.; En-Jung, H.; Hsing-Yu, C.; Ching-Te, C.; Hsu-Liang, H.; Tsan-Piao, L. Drought and Salt Stress Tolerance of an Arabidopsis Glutathione S-Transferase U17 Knockout Mutant Are Attributed to the Combined Effect of Glutathione and Abscisic Acid. Plant Physiol. 2012, 158, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Kumari, B.; Dubey, R.S. Differential responses of antioxidative defense system to prolonged salinity stress in salt-tolerant and salt-sensitive Indica rice (Oryza sativa L.) seedlings. Protoplasma 2013, 250, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Li, C.R.; Liang, D.D.; Li, J.; Duan, Y.B.; Li, H.; Yang, Y.C.; Qin, R.Y.; Li, L.I.; Wei, P.C.; Yang, J.B. Unravelling mitochondrial retrograde regulation in the abiotic stress induction of rice Alternative oxidase 1 gene. Plant Cell Environ. 2013, 36, 775–788. [Google Scholar] [CrossRef]
- Gao, Y.; Cui, Y.; Long, R.; Sun, Y.; Zhang, T.; Yang, Q.; Kang, J. Salt-stress induced proteomic changes of two contrasting alfalfa cultivars during germination stage. J. Sci. Food Agric. 2019, 99, 1384–1396. [Google Scholar] [CrossRef]
- Belghith, I.; Jennifer, S.; Tatjana, H.; Chedly, A.; Hans-Peter, B.; Ahmed, D. Comparative analysis of salt-induced changes in the root proteome of two accessions of the halophyte Cakile maritime. Plant Physiol. Biochem. 2018, 130, 20–29. [Google Scholar] [CrossRef]
- Soares, A.L.C.; Christoph-Martin, G.; Sebastien, C.C. Genotype-Specific Growth and Proteomic Responses of Maize Toward Salt Stress. Front Plant Sci. 2018, 9, 661. [Google Scholar] [CrossRef]
- Fatehi, F.; Abdolhadi, H.; Houshang, A.; Tahereh, B.; Paul, C.S. The proteome response of salt-resistant and salt-sensitive barley genotypes to long-term salinity stress. Mol. Biol. Rep. 2012, 39, 6387. [Google Scholar] [CrossRef]
- Gao, L.; Yan, X.; Li, X.; Guo, G.; Hu, Y.; Ma, W.; Yan, Y. Proteome analysis of wheat leaf under salt stress by two-dimensional difference gel electrophoresis (2D-DIGE). Phytochemistry 2011, 72, 1180–1191. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Tester, M.; Gilliham, M. Chloride on the move. Trends Plant Sci. 2017, 22, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Moolna, A.; Bowsher, C.G. The physiological importance of photosynthetic ferredoxin NADP+ oxidoreductase (FNR) isoforms in wheat. J. Exp. Bot. 2010, 61, 2669–2681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngara, R.; Roya, N.; Jonas, B.J.; Ole, N.J.; Bongani, N. Identification and profiling of salinity stress-responsive proteins in Sorghum bicolor seedlings. J. Proteom. 2012, 75, 4139–4150. [Google Scholar] [CrossRef] [Green Version]
- Bai, J.; Yan, Q.; Jinghui, L.; Yuqing, W.; Rula, S.; Na, Z.; Ruizong, J. Proteomic response of oat leaves to long-term salinity stress. Environ. Sci. Pollut. Res. 2017, 24, 3387. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Natan, G.; Bruria, H. Proteomic analysis of salt-stressed tomato (Solanum lycopersicum) seedlings: Effect of genotype and exogenous application of glycinebetaine. J. Exp. Bot. 2009, 60, 2005–2019. [Google Scholar] [CrossRef] [PubMed]
- Turan, S.; Baishnab, C.T. Salt-stress induced modulation of chlorophyll biosynthesis during de-etiolation of rice seedlings. Physiol. Plantarum. 2015, 153, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, H.; Shao, H.; Tang, X. Recent advances in utilizing transcription factors to improve plant abiotic stress tolerance by transgenic technology. Front. Plant Sci. 2017, 7, 4563. [Google Scholar] [CrossRef] [PubMed]
- Boriboonkaset, T.; Theerawitaya, C.; Yamada, N.; Pichakum, A.; Supaibulwatana, K.; Cha-um, S.; Takabe, T.; Protoplasma, C.K. Regulation of some carbohydrate metabolism-related genes, starch and soluble sugar contents, photosynthetic activities and yield attributes of two contrasting rice genotypes subjected to salt stress. Protoplasma 2013, 250, 1157–1167. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Jin-Long, W.; Rui-Jun, F.; Shan-Jia, L.; Chun-Mei, W. iTRAQ-Based Comparative Proteomic Analysis Provides Insights into Molecular Mechanisms of Salt Tolerance in Sugar Beet (Beta vulgaris L.). Int. J. Mol. Sci. 2018, 19, 3866. [Google Scholar] [CrossRef]
- Cho, S.K.; Jee, E.K.; Jong-A, P.; Tae, J.E. Woo Taek KimConstitutive expression of abiotic stress-inducible hot pepper CaXTH3, which encodes a xyloglucan endotransglucosylase/hydrolase homolog, improves drought and salt tolerance in transgenic Arabidopsis plants. FEBS Lett. 2006, 580, 3136–3144. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.Y.; Seo, Y.S.; Kim, S.J.; Kim, W.T.; Shin, J.S. Constitutive expression of CaXTH3, a hot pepper xyloglucan endotransglucosylase/hydrolase, enhanced tolerance to salt and drought stresses without phenotypic defects in tomato plants (Solanum lycopersicum cv. Dotaerang). Plant Cell Rep. 2011, 30, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.; Sauter, A.; Wichert, K.; Messdaghi, D.; Hartung, W. Extracellular β-glucosidase activity in barley involved in the hydrolysis of ABA glucose conjugate in leaves. J. Exp. Bot. 2000, 51, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Hermosa, R.; Leticia, B.; Emma, K.; Jesus, A.J.; Marta, M.B.; Vicent, A.; Aurelio, G.-C.; Enrique, M.; Carlos, N. The overexpression in Arabidopsis thaliana of a Trichoderma harzianum gene that modulates glucosidase activity, and enhances tolerance to salt and osmotic stresses. J. Plant Physiol. 2011, 168, 1295–1302. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Renu, S.; Ping, C.; Stephen, H.H. Salt stress responses in Arabidopsis utilize a signal transduction pathway related to endoplasmic reticulum stress signaling. Plant J. 2007, 51, 897–909. [Google Scholar] [CrossRef]
- Takaaki, T.; Muneharu, E. Induction of a Novel XIP-Type Xylanase Inhibitor by External Ascorbic Acid Treatment and Differential Expression of XIP-Family Genes in Rice. Plant Cell Phys. 2007, 48, 700–714. [Google Scholar] [Green Version]
- Upadhaya, H.; Sahoo, L.; Panda, S.K. Molecular Physiology of Osmotic Stress in Plants. In Molecular Stress Physiology of Plants; Rout, G., Das, A., Eds.; Springer: India, New Delhi, 2013. [Google Scholar]
- Torres-Schumann, S.; Godoy, J.A.; Pintor-Toro, J.A. A probable lipid transfer protein gene is induced by NaCl in stems of tomato plants. Plant Mol. Biol. 1992, 18, 749–757. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Li, J.; Jiao, L.; Li, C.; Zhu, D.; Yu, J. A Non-specific Setaria italica Lipid Transfer Protein Gene Plays a Critical Role under Abiotic Stress. Front. Plant Sci. 2016, 7, 1752. [Google Scholar] [CrossRef]
- Dejardin, A.; Sokolov, L.N.; Kleczkowski, L.A. An Arabidopsis Stress-Responsive Sucrose Synthase Gene is UP-Regulated by Low Water Potential. In Photosynthesis: Mechanisms and Effects; Garab, G., Ed.; Springer: Dordrecht, The Netherlands, 1998. [Google Scholar]
- Vastarelli, P.; Moschella, A.; Pacifico, D.; Mandolino, G. Water Stress in Beta vulgaris: Osmotic Adjustment Response and Gene Expression Analysis in ssp. Vulgaris and maritima. Am. J. Plant Sci. 2013, 4, 11–16. [Google Scholar] [CrossRef]
- Sobhanian, H.; Razavizadeh, R.; Nanjo, Y.; Ehsanpour, A.A.; Jazii, F.R.; Motamed, N.; Komatsu, S. Proteome analysis of soybean leaves, hypocotyls and roots under salt stress. Proteome Sci. 2010, 8, 19. [Google Scholar] [CrossRef] [Green Version]
- Sampath, K.I.; Ramgopal, R.S.; Vardhini, B.V. Role of Phytohormones during Salt Stress Tolerance in plants. Curr. Trends Biotechnol. Pharm. 2015, 9, 334–343. [Google Scholar]
- Mukhopadhyay, A.; Shubha, V.; Akhilesh, K.T. Overexpression of a zinc-finger protein gene from rice confers tolerance to cold, dehydration, and salt stress in transgenic tobacco. Proc. Natl. Acad. Sci. USA 2004, 101, 6309–6314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Huanhuan, L.; Ce, S.; Qibin, M.; Huaiyu, B.; Kang, C.; Yunyuan, X. A C2H2 zinc-finger protein OsZFP213 interacts with OsMAPK3 to enhance salt tolerance in rice. J. Plant Phys. 2018, 229, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Jamil, M.; Iqbal, W.; Bangash, A.; Rehman, S.; Imran, Q.M.; Rha, E.S. Constitutive Expression of OSC3H33, OSC3H50 AND OSC3H37 Genes in Rice under Salt Stress. Pak. J. Bot. 2010, 42, 4003–4009. [Google Scholar]
- Wang, X.K.; Zhang, W.H.; Hao, Z.B.; Li, X.R.; Zhang, Y.Q.; Wang, S.M. Principles and Techniques of Plant Physiological Biochemical Experiment; Higher Education Press: Beijing, China, 2006; pp. 118–119. (In Chinese) [Google Scholar]
- U.S. Salinity Laboratory Staff. Diagnosis and Improvement of Saline and Alkali Soils; Richards, L.A., Ed.; U.S. Goverment Publishing Office: Washington, DC, USA, 1954. [Google Scholar]





| Cultivars | Treatments | Na+ (mg/g) | Cl− (mg/g) | Na+ (mg/g) | Cl− (mg/g) | ||
|---|---|---|---|---|---|---|---|
| Root | Leaf | Root | Leaf | Soil | Soil | ||
| LYP9 | CK | 0.7 ± 0.05d | 0.2 ± 0.03c | 0.5 ± 0.3d | 6.8 ± 0.4d | 0.2 ± 0.01d | 0.04 ± 0.01e |
| LS | 1.1 ± 0.03bc | 0.5 ± 0.08b | 1.6 ± 0.7cd | 12.4 ± 1.7bcd | 1.0 ± 0.03c | 0.6 ± 0.06de | |
| MS | 1.5 ± 0.04b | 0.8 ± 0.10a | 7.9 ± 1.3ab | 17.6 ± 2.4ab | 1.7 ± 0.04b | 2.2 ± 0.06bc | |
| HS | 1.6 ± 0.09a | 0.9 ± 0.11a | 9.5 ± 1.6a | 19.1 ± 2.9a | 2.0 ± 0.07a | 2.4 ± 0.33b | |
| NPBA | CK | 0.7 ± 0.03d | 0.15 ± 0.01cd | 1.0 ± 0.3d | 9.9 ± 0.9cd | 0.2 ± 0.01d | 0.01 ± 0.01e |
| LS | 1.0 ± 0.07c | 0.3 ± 0.01c | 3.8 ± 0.4c | 14.7 ± 2.8abc | 1.0 ± 0.02c | 0.7 ± 0.08e | |
| MS | 1.3 ± 0.07b | 0.9 ± 0.02a | 6.3 ± 0.6b | 18.7 ± 2.4ab | 1.6 ± 0.12b | 1.6 ± 0.035d | |
| HS | - | - | - | - | 2.2 ± 0.07a | 3.0 ± 0.23a | |
| Total Spectra | Spectra | Unique Spectra | Peptides | Unique Peptide |
|---|---|---|---|---|
| 402,823 | 71,146 | 53,833 | 21,741 | 18,899 |
| Protein ID | NCBI Accession | Protein Name | NPBA | LYP9 | |||
|---|---|---|---|---|---|---|---|
| LS vs. CK | MS vs. CK | LS vs. CK | MS vs. CK | HS vs. CK | |||
| Salt responsive | |||||||
| tr|B9FWE4|B9FWE4_ORYSJ | gi|222636749 | Uncharacterized protein | 1.516 | 1.415 | 0.906 | 1.234 | 1.255 |
| tr|A2Y7R4|A2Y7R4_ORYSI | gi|115465579 | Malate dehydrogenase | 1.393 | 2 | 1.014 | 1.488 | 1.573 |
| tr|B8BBS3|B8BBS3_ORYSI | gi|115476908 | Os08g0478200 protein | 1.389 | 1.593 | 0.951 | 1.402 | 2.706 |
| tr|A2WT84|A2WT84_ORYSI | gi|115438875 | Malate dehydrogenase | 1.897 | 2.835 | 1.027 | 1.871 | 2.006 |
| tr|A0A0P0VS15|A0A0P0VS15_ORYSJ | gi|115450217 | Nascent polypeptide-associated complex subunit β (Fragment) | 2.523 | 2.558 | 1.017 | 1.594 | 1.384 |
| tr|A2XA10|A2XA10_ORYSI | gi|46805452 | Os02g0768600 protein | 1.506 | 2.225 | 1.071 | 2.213 | 2.212 |
| tr|A0A190X658|A0A190X658_ORYSI | gi|115477769 | l-isoaspartate methyltransferase | 1.575 | 2.403 | 0.901 | 1.591 | 1.69 |
| sp|Q43008|SODM_ORYSJ | gi|115463191 | Superoxide dismutase | 1.775 | 2.06 | 1.071 | 1.534 | 1.828 |
| sp|Q9FE01|APX2_ORYSJ | gi|115474285 | Ascorbate peroxidase | 1.308 | 1.26 | 0.966 | 1.227 | 1.119 |
| sp|Q07661|NDK1_ORYSJ | gi|61679782 | Nucleoside diphosphate kinase 1 | 1.295 | 1.816 | 0.909 | 1.068 | 1.435 |
| sp|Q5N725|ALFC3_ORYSJ | gi|297598143 | Fructose-bisphosphate aldolase 3 | 1.399 | 1.639 | 1.023 | 1.089 | 1.532 |
| sp|Q7XDC8|MDHC_ORYSJ | gi|115482534 | Malate dehydrogenase | 1.37 | 1.749 | 1.004 | 1.284 | 1.523 |
| tr|A2X753|A2X753_ORYSI | gi|115447273 | Os02g0612900 protein | 1.441 | 1.552 | 1.036 | 1.506 | 1.597 |
| tr|A2X7X9|A2X7X9_ORYSI | gi|125540544 | Putative uncharacterized protein | 1.152 | 1.502 | 0.882 | 1.378 | 1.25 |
| tr|A0A0P0VTX8|A0A0P0VTX8_ORYSJ | gi|108706531 | Os03g0182600 protein | 0.852 | 1.876 | 0.908 | 0.949 | 1.367 |
| tr|E0X6V4|E0X6V4_ORYSJ | gi|306415973 | Triosephosphate isomerase | 1.003 | 1.232 | 1.027 | 1.107 | 1.256 |
| tr|A2ZAA7|A2ZAA7_ORYSI | gi|115483468 | Nucleoside diphosphate kinase | 1.197 | 2.406 | 0.882 | 1.08 | 1.768 |
| tr|Q9ATR3|Q9ATR3_ORYSA | gi|13249140 | Glucanase | 1.063 | 2.009 | 0.876 | 0.912 | 1.39 |
| tr|A2ZIH2|A2ZIH2_ORYSI | gi|115487556 | Expressed protein | 1.048 | 1.561 | 0.957 | 1.204 | 1.424 |
| tr|B9FV80|B9FV80_ORYSJ | gi|222636335 | Peroxidase | 0.888 | 1.812 | 0.966 | 1.298 | 1.814 |
| tr|B8B893|B8B893_ORYSI | gi|218199240 | Plasma membrane ATPase | 1.404 | 1.435 | 0.906 | 0.716 | 0.86 |
| tr|A2XA20|A2XA20_ORYSI | gi|115448935 | Proteasome subunit β type | 0.862 | 1.109 | 0.946 | 1.067 | 1.059 |
| tr|A2Y628|A2Y628_ORYSI | gi|125552829 | Cysteine proteinase inhibitor | 0.96 | 1.775 | 1.056 | 1.438 | 2.205 |
| tr|Q9ZNZ1|Q9ZNZ1_ORYSA | gi|4097938 | Beta-1,3-glucanase | 0.795 | 1.711 | 1.003 | 0.932 | 1.619 |
| tr|A2ZCK1|A2ZCK1_ORYSI | gi|148762354 | Alcohol dehydrogenase 2 | 0.63 | 0.866 | 1.055 | 1.019 | 1.019 |
| sp|A2XFC7|APX1_ORYSI | gi|158512874 | l-ascorbate peroxidase 1 | 1.216 | 1.343 | 0.942 | 1.21 | 1.327 |
| tr|A2X822|A2X822_ORYSI | gi|125540587 | Glutathione peroxidase | 0.717 | 0.617 | 0.924 | 1.77 | 1.567 |
| tr|A2XFD1|A2XFD1_ORYSI | gi|125543402 | Putative uncharacterized protein | 1.1 | 1.543 | 0.943 | 1.23 | 1.554 |
| tr|A2YLI3|A2YLI3_ORYSI | gi|115472191 | Os07g0495200 protein | 1.159 | 1.821 | 0.971 | 1.505 | 1.818 |
| tr|B8ADI1|B8ADI1_ORYSI | gi|218187601 | NADH-cytochrome b5 reductase | 0.771 | 0.72 | 1.081 | 2.182 | 2.316 |
| tr|A2YSB2|A2YSB2_ORYSI | gi|115475275 | Os08g0205400 protein | 1.587 | 2.217 | 0.908 | 1.597 | 1.194 |
| tr|B8AY35|B8AY35_ORYSI | gi|218196772 | Fructose-bisphosphate aldolase | 0.458 | 0.214 | 0.964 | 0.74 | 1.505 |
| tr|B8AY17|B8AY17_ORYSI | gi|218196757 | Putative uncharacterized protein | 0.725 | 0.849 | 0.996 | 1.174 | 1.649 |
| tr|Q9ZNZ1|Q9ZNZ1_ORYSA | gi|4097938 | Beta-1,3-glucanase | 0.795 | 1.711 | 1.003 | 0.932 | 1.619 |
| sp|Q941Z0|NQR1_ORYSJ | gi|115442299 | Putative uncharacterized protein | 0.686 | 0.766 | 0.984 | 0.931 | 1.369 |
| tr|A2WWV4|A2WWV4_ORYSI | gi|125528336 | Putative uncharacterized protein | 0.518 | 0.55 | 1.031 | 1.159 | 1.304 |
| sp|P93438|METK2_ORYSJ | gi|3024122 | S-adenosylmethionine synthase | 1.282 | 1.017 | 1.013 | 1.226 | 1.092 |
| tr|A2XUB9|A2XUB9_ORY I | gi|90265194 | B0812A04.3 protein | 1.074 | 1.225 | 1.215 | 1.186 | 1.437 |
| tr|A2Z2Z0|A2Z2Z0_ORYSI | gi|125564321 | Putative uncharacterized protein | 1.01 | 0.776 | 0.921 | 1.202 | 1.102 |
| tr|B8AEU4|B8AEU4_ORYSI | gi|218191814 | Putative uncharacterized protein | 0.954 | 1.212 | 0.948 | 0.908 | 1.134 |
| tr|A0A0P0VTX8|A0A0P0VTX8_ORYSJ | gi|108706531 | Os03g0182600 protein | 0.852 | 1.876 | 0.908 | 0.949 | 1.367 |
| tr|Q688M9|Q688M9_ORYSJ | gi|51854423 | putative endo-1,31,4-β-D-glucanase | 1.16 | 1.14 | 0.992 | 1.111 | 1.144 |
| tr|B8ATW7|B8ATW7_ORYSI | gi|115460338 | Os04g0602100 protein | 1.386 | 1.494 | 1.115 | 1.448 | 1.482 |
| sp|Q7FAH2|G3PC2_ORYSJ | gi|115459078 | Glyceraldehyde-3-phosphate dehydrogenase 2 | 0.887 | 1.03 | 1.004 | 0.996 | 1.196 |
| tr|Q0JG30|Q0JG30_ORYSJ | gi|297598314 | Os01g0946500 protein | 0.95 | 0.844 | 0.995 | 0.799 | 0.959 |
| tr|Q6L5I4|Q6L5I4_ORYSJ | gi|47900421 | Putative aldehyde dehydrogenase | 0.735 | 0.737 | 0.911 | 1.167 | 1.008 |
| sp|A2XW22|DHE2_ORYSI | gi|81686712 | Glutamate dehydrogenase 2 | 1.177 | 1.142 | 0.912 | 0.8 | 1.184 |
| sp|Q7FAY6|RGP2_ORYSJ | gi|115461086 | Amylogenin | 1.357 | 1.021 | 0.776 | 0.558 | 0.683 |
| sp|Q259G4|PMM_ORYSI | gi|115461390 | Phosphomannomutase | 0.836 | 1.19 | 1.007 | 0.924 | 1.216 |
| Photosynthesis related | |||||||
| tr|A2YWS7|A2YWS7_ORYSI | gi|115477166 | Os08g0504500 protein | 1.317 | 2.22 | 0.883 | 1.852 | 1.77 |
| tr|Q2QWM7|Q2QWM7_ORYSJ | gi|108862278 | Os12g0190200 protein | 1.053 | 1.535 | 0.91 | 1.363 | 1.279 |
| tr|B8BCC6|B8BCC6_ORYSI | gi|115477246 | Os08g0512500 protein | 2.311 | 3.409 | 1.022 | 1.551 | 1.349 |
| tr|A2XZK1|A2XZK1_ORYSI | gi|125550552 | Putative uncharacterized protein | 1.246 | 1.015 | 1.301 | 2.61 | 2.439 |
| tr|B8AAX3|B8AAX3_ORYSI | gi|115440559 | Os01g0805300 protein | 1.418 | 2.178 | 0.986 | 1.943 | 1.703 |
| tr|Q0D6V8|Q0D6V8_ORYSJ | gi|297607127 | Os07g0435300 protein | 2.246 | 3.387 | 0.982 | 2.305 | 2.053 |
| tr|Q7XHS1|Q7XHS1_ORYSJ | gi|115472141 | 2Fe-2S iron-sulfur cluster protein-like | 1.016 | 1.414 | 0.936 | 1.62 | 1.548 |
| tr|A2X7M2|A2X7M2_ORYSI | gi|115447507 | Os02g0638300 protein | 1.096 | 1.611 | 1.14 | 1.73 | 1.825 |
| tr|B0FFP0|B0FFP0_ORYSJ | gi|115470529 | Chloroplast 23 kDa polypeptide of PS II (Fragment) | 1.319 | 1.705 | 0.997 | 1.747 | 1.609 |
| tr|Q7M1U9|Q7M1U9_ORYSA | gi|218186547 | Photosystem I 9K protein | 1.832 | 3.172 | 1.027 | 2.206 | 2.383 |
| tr|A0A0P0XF80|A0A0P0XF80_ORYSJ | gi|38636895 | Os08g0347500 protein | 1.642 | 2.347 | 0.926 | 1.756 | 1.81 |
| tr|Q7M1Y7|Q7M1Y7_ORYSA | gi|164375543 | Photosystem II oxygen-evolving complex protein 2 (Fragment) | 1.77 | 2.373 | 0.989 | 2.015 | 1.756 |
| tr|B8AJX7|B8AJX7_ORYSI | gi|115455221 | Serine hydroxymethyltransferase | 2.24 | 2.885 | 1.078 | 1.525 | 1.334 |
| tr|B8AY24|B8AY24_ORYSI | gi|218196765 | Putative uncharacterized protein | 1.288 | 1.56 | 1.026 | 1.639 | 1.326 |
| sp|Q6Z2T6|CHLP_ORYSJ | gi|297599916 | Geranylgeranyl reductase | 0.956 | 1.173 | 0.973 | 0.957 | 1.174 |
| sp|P0C420|PSBH_ORYSA | gi|11466818 | Photosystem II reaction center protein H | 0.795 | 0.694 | 1.029 | 0.864 | 1.14 |
| Oxidation reduction responsive | |||||||
| tr|A3BVS6|A3BVS6_ORYSJ | gi|125604340 | Superoxide dismutase | 1.512 | 1.903 | 0.96 | 1.618 | 1.684 |
| sp|Q6H7E4|TRXM1_ORYSJ | gi|115447527 | Putative uncharacterized protein | 0.941 | 1.681 | 1.049 | 1.75 | 2.489 |
| sp|Q9SDD6|PRX2F_ORYSJ | gi|115435844 | Peroxiredoxin-2F, mitochondrial | 1.363 | 1.772 | 1.021 | 1.642 | 1.687 |
| tr|B7FAE9|B7FAE9_ORYSJ | gi|215769368 | Glutathione peroxidase | 0.98 | 1.347 | 0.965 | 1.38 | 1.176 |
| tr|A2Y043|A2Y043_ORYSI | gi|125550744 | Peroxidase | 1.232 | 2.046 | 0.87 | 0.633 | 1.271 |
| tr|Q9FTN6|Q9FTN6_ORYSJ | gi|115434034 | Os01g0106300 protein | 0.732 | 1.977 | 0.788 | 0.606 | 1.476 |
| tr|A2X2T0|A2X2T0_ORYSI | gi|55700921 | Peroxidase | 0.775 | 1.122 | 0.913 | 0.85 | 1.697 |
| tr|O22440|O22440_ORYSA | gi|115474063 | Peroxidase | 1.763 | 2.554 | 0.963 | 2.051 | 1.612 |
| tr|A3A7Y3|A3A7Y3_ORYSJ | gi|125582491 | Uncharacterized protein | 1.099 | 1.361 | 1.101 | 1.555 | 2.4 |
| tr|B9FL20|B9FL20_ORYSJ | gi|115464801 | Uncharacterized protein | 1.175 | 1.416 | 0.965 | 1.159 | 1.356 |
| tr|Q9AS12|Q9AS12_ORYSJ | gi|115436300 | Peroxidase | 4.654 | 5.188 | 0.78 | 1.948 | 2.334 |
| tr|B8ATW7|B8ATW7_ORYSI | gi|115460338 | Os04g0602100 protein | 1.386 | 1.494 | 1.115 | 1.448 | 1.482 |
| tr|B9FCM4|B9FCM4_ORYSJ | gi|116309795 | OSIGBa0148A10.12 protein | 2.208 | 2.05 | 1.017 | 1.365 | 1.123 |
| tr|Q0JB49|Q0JB49_ORYSJ | gi|115459848 | Glutathione peroxidase | 1.449 | 1.435 | 0.933 | 1.537 | 1.271 |
| tr|Q43006|Q43006_ORYSA | gi|20286|emb | Peroxidase | 4.58 | 4.923 | 1.16 | 1.421 | 1.233 |
| tr|Q5Z7J7|Q5Z7J7_ORYSJ | gi|55701041 | Peroxidase | 5.025 | 5.222 | 0.802 | 2.469 | 2.659 |
| tr|Q25AK7|Q25AK7_ORYSA | gi|90265065 | H0510A06.15 protein | 1.326 | 1.047 | 0.91 | 1.209 | 1.022 |
| tr|Q6K4J4|Q6K4J4_ORYSJ | gi|115479691 | Peroxidase | 1.23 | 1.049 | 0.988 | 1.16 | 0.919 |
| tr|A2WJQ7|A2WJQ7_ORYSI | gi|115434036 | Os01g0106400 protein | 0.884 | 2.12 | 0.973 | 1.313 | 2.057 |
| sp|P41095|RLA0_ORYSJ | gi|115474653 | 60S acidic ribosomal protein | 1.312 | 1.231 | 1.073 | 0.882 | 0.83 |
| sp|B8AUI3|GLO3_ORYSI | gi|115460650 | Peroxisomal (S)-2-hydroxy-acid oxidase GLO3 | 0.627 | 0.615 | 1.305 | 0.833 | 0.972 |
| tr|A0A0N7KI36|A0A0N7KI36_ORYSJ | gi|55700967 | Peroxidase | 0.895 | 0.817 | 0.934 | 1.403 | 1.041 |
| tr|B8B5W7|B8B5W7_ORYSI | gi|218200254 | Peroxidase | 1.11 | 1.51 | 0.996 | 2.708 | 1.966 |
| tr|A2WPA1|A2WPA1_ORYSI | gi|125525683 | Peroxidase | 1.258 | 1.625 | 1.07 | 2.133 | 3.577 |
| tr|A2ZAA6|A2ZAA6_ORYSI | gi|115483466 | Putative peptide methionine sulfoxide reductase | 1.121 | 1.184 | 0.902 | 1.739 | 1.32 |
| tr|A2XVK6|A2XVK6_ORYSI | gi|125549044 | Putative uncharacterized protein | 0.844 | 0.93 | 0.946 | 1.321 | 1.19 |
| tr|B9F688|B9F688_ORYSJ | gi|222624472 | Uncharacterized protein | 2.063 | 3.091 | 1.018 | 2.407 | 2.351 |
| tr|B8AU10|B8AU10_ORYSI | gi|218194884 | Putative uncharacterized protein | 1.206 | 0.747 | 1.145 | 1.386 | 1.226 |
| tr|Q7F1J9|Q7F1J9_ORYSJ | gi|115477368 | Os08g0522400 protein | 1.225 | 1.347 | 1.072 | 1.309 | 1.121 |
| sp|Q6K471|FTRC_ORYSJ | gi|75125055 | Ferredoxin-thioredoxin reductase | 1.28 | 2.03 | 0.905 | 1.598 | 1.668 |
| tr|A0A0B4U1V7|A0A0B4U1V7_ORYSA | gi|115467518 | Aldehyde dehydrogenase ALDH2b | 1.178 | 1.029 | 1.016 | 1.006 | 1.233 |
| sp|Q6AV34|ARGC_ORYSJ | gi|218193315 | Probable N-acetyl-gamma-glutamyl-phosphate reductase | 1.046 | 1.075 | 0.971 | 0.966 | 1.237 |
| tr|Q2QV45|Q2QV45_ORYSJ | gi|115487998 | 70 kDa heat shock protein | 1.415 | 1.387 | 1.068 | 1.391 | 1.254 |
| sp|Q84VG0|CML7_ORYSJ | gi|115474531 | Putative uncharacterized protein | 1.351 | 1.579 | 0.859 | 1.35 | 1.392 |
| tr|A2Y8A8|A2Y8A8_ORYSI | gi|115465902 | Os06g0104300 protein | 0.877 | 2.193 | 1.078 | 1.336 | 1.654 |
| tr|A0A0P0X7V0|A0A0P0X7V0_ORYSJ | gi|115472943 | Os07g0573800 protein (Fragment) | 1.61 | 1.898 | 0.916 | 1.161 | 1.207 |
| tr|B8BAM3|B8BAM3_ORYSI | gi|115474739 | Os08g0139200 protein | 1.096 | 1.539 | 0.841 | 0.9 | 1.207 |
| sp|Q69TY4|PR2E1_ORYSJ | gi|115469028 | Putative uncharacterized protein | 1.289 | 1.212 | 0.944 | 1.336 | 1.361 |
| sp|Q8W3D9|PORB_ORYSJ | gi|75248671 | Protochlorophyllide reductase B | 0.881 | 1.621 | 0.891 | 1.192 | 2.065 |
| tr|B8AGN1|B8AGN1_ORYSI | gi|115445869 | Os02g0328300 protein | 1.63 | 2.925 | 0.927 | 1.814 | 1.808 |
| tr|B9F604|B9F604_ORYSJ | gi|222625905 | Uncharacterized protein | 1.474 | 1.82 | 1.086 | 1.62 | 1.613 |
| tr|Q7F229|Q7F229_ORYSJ | gi|115471449 | Os07g0260300 protein | 0.924 | 1.084 | 0.949 | 1.375 | 2.104 |
| tr|A6N0B2|A6N0B2_ORYSI | gi|149391329 | Mitochondrial formate dehydrogenase 1 (Fragment) | 0.993 | 1.084 | 0.931 | 0.99 | 1.212 |
| sp|Q10L32|MSRB5_ORYSJ | gi|115453111 | Putative uncharacterized protein | 1.116 | 1.471 | 0.84 | 1.272 | 1.479 |
| tr|Q941T6|Q941T6_ORYSJ | gi|15408884 | Os01g0847700 protein | 1.028 | 0.871 | 1.18 | 1.416 | 1.504 |
| tr|B8B2F2|B8B2F2_ORYSI | gi|218198209 | Formate dehydrogenase | 1.014 | 1.19 | 0.97 | 0.898 | 1.278 |
| sp|Q7XPL2|HEM6_ORYSJ | gi|75232919 | OSIGBa0152L12.9 protein | 0.993 | 1.224 | 0.873 | 0.963 | 1.253 |
| sp|P0C5D4|PRXQ_ORYSI | gi|115466906 | Peroxiredoxin Q, chloroplastic | 1.215 | 1.691 | 0.909 | 1.71 | 2.077 |
| tr|A0A0P0WR9|A0A0P0WWR9_ORYSJ | gi|300681235 | Os06g0472000 protein | 1.308 | 1.269 | 1.032 | 1.532 | 1.593 |
| tr|A2WL79|A2WL79_ORYSI | gi|125524611 | Peroxidase | 0.826 | 0.852 | 1.047 | 1.182 | 1.233 |
| sp|P37834|PER1_ORYSJ | gi|115464711 | Peroxidase | 0.702 | 0.999 | 0.819 | 0.742 | 1.764 |
| tr|Q01LB1|Q01LB1_ORYSA | gi|115458104 | OSJNBa0072K14.5 protein | 1.175 | 1.243 | 0.937 | 1.124 | 1.221 |
| sp|P0C0L1|APX6_ORYSJ | gi|115487636 | Putative uncharacterized protein | 1.127 | 1.213 | 1.031 | 1.282 | 1.477 |
| sp|Q7X8R5|TRXM2_ORYSJ | gi|115459582 | B1011H02.3 protein | 1.557 | 2.198 | 0.916 | 1.577 | 3.486 |
| tr|B7E4J4|B7E4J4_ORYSJ | gi|215704355 | Putative uncharacterized protein | 0.853 | 1.062 | 0.762 | 0.579 | 1.105 |
| tr|Q7XV08|Q7XV08_ORYSJ | gi|38567882 | OSJNBa0036B21.10 protein | 1.159 | 1.382 | 0.971 | 1.084 | 1.397 |
| Carbohydrate metabolism | |||||||
| sp|Q8L7J2|BGL06_ORYSJ | gi|218192323 | Beta-glucosidase 6 | 0.177 | 0.383 | 1.004 | 0.424 | 1.741 |
| sp|Q76BW5|XTH8_ORYSJ | gi|115475445 | Xyloglucan endotransglycosylase/hydrolase protein 8 | 0.953 | 2.101 | 0.939 | 1.074 | 1.369 |
| tr|Q01JC3|Q01JC3_ORYSA | gi|116310134 | Malate dehydrogenase | 0.795 | 0.74 | 0.995 | 0.591 | 0.889 |
| tr|Q0DCB1|Q0DCB1_ORYSJ | gi|115467998 | Os06g0356700 protein | 0.849 | 1.073 | 0.912 | 1.227 | 2.764 |
| tr|Q10CU4|Q10CU4_ORYSJ | gi|115455353 | GH family 3 N terminal domain containing protein, expressed | 0.72 | 2.799 | 0.66 | 0.663 | 2.234 |
| tr|Q9ZNZ1|Q9ZNZ1_ORYSA | gi|4097938 | Beta-1,3-glucanase | 0.795 | 1.711 | 1.003 | 0.932 | 1.619 |
| tr|H2KWT0|H2KWT0_ORYSJ | gi|108863034 | HIPL1 protein, putative, expressed | 1.106 | 2.099 | 0.908 | 1.231 | 2.014 |
| tr|B8AIS2|B8AIS2_ORYSI | gi|218191593 | Putative uncharacterized protein | 0.773 | 0.837 | 0.875 | 1.437 | 1.452 |
| sp|Q0INM3|BGA15_ORYSJ | gi|115488372 | Beta-galactosidase 15 | 1.348 | 1.602 | 0.924 | 1.187 | 1.56 |
| tr|B9FWS5|B9FWS5_ORYSJ | gi|222636880 | Uncharacterized protein | 0.838 | 1.177 | 1.122 | 0.866 | 1.141 |
| tr|Q0JG30|Q0JG30_ORYSJ | gi|297598314 | Os01g0946500 protein | 0.95 | 0.844 | 0.995 | 0.799 | 0.959 |
| tr|Q0J0Q9|Q0J0Q9_ORYSJ | gi|115479865 | Os09g0487600 protein | 0.829 | 1.294 | 0.88 | 1.272 | 1.594 |
| tr|A2XM08|A2XM08_ORYSI | gi|115455349 | GH family 3 N terminal domain containing protein, expressed | 0.859 | 1.124 | 0.866 | 0.78 | 1.387 |
| sp|Q10NX8|BGAL6_ORYSJ | gi|152013362 | Beta-galactosidase 6 | 1.063 | 1.684 | 0.938 | 1.413 | 1.814 |
| tr|B8AII1|B8AII1_ORYSI | gi|218190145 | Putative uncharacterized protein | 0.904 | 1.55 | 0.954 | 1.167 | 1.478 |
| tr|Q01IH0|Q01IH0_ORYSA | gi|116310092 | H0502G05.3 protein | 0.728 | 0.783 | 0.894 | 1.001 | 1.193 |
| tr|Q01JK3|Q01JK3_ORYSA | gi|116310050 | Aldose 1-epimerase | 0.823 | 1.226 | 0.939 | 1.515 | 1.515 |
| tr|B8BHM7|B8BHM7_ORYSI | gi|10140702 | Alpha-galactosidase | 0.723 | 1.32 | 1.006 | 1.331 | 1.544 |
| tr|A2Z9V6|A2Z9V6_ORYSI | gi|125532825 | Uncharacterized protein | 0.73 | 2.017 | 0.876 | 1.276 | 1.194 |
| tr|Q0DTS9|Q0DTS9_ORYSJ | gi|297600575 | Os03g0227400 protein (Fragment) | 1.101 | 1.226 | 0.804 | 1.06 | 1.306 |
| tr|A2XME9|A2XME9_ORYSI | gi|115455637 | Malate dehydrogenase | 1.049 | 1.261 | 1.151 | 1.502 | 1.548 |
| tr|Q6Z8F4|Q6Z8F4_ORYSJ | gi|115448091 | Phosphoribulokinase | 1.143 | 1.318 | 1.066 | 1.144 | 1.249 |
| tr|A2YIJ5|A2YIJ5_ORYSI | gi|50509727 | Os07g0168600 protein | 0.779 | 0.93 | 0.952 | 1.118 | 1.317 |
| sp|Q75I93|BGL07_ORYSJ | gi|115454825 | Beta-glucosidase | 1.201 | 1.066 | 0.95 | 1.276 | 1.58 |
| tr|Q7XIV4|Q7XIV4_ORYSJ | gi|115474081 | Alpha-galactosidase | 0.786 | 1.367 | 0.919 | 1.115 | 1.491 |
| tr|A3A285|A3A285_ORYSJ | gi|115443693 | Uncharacterized protein | 0.83 | 1.101 | 0.843 | 1.151 | 1.262 |
| tr|A0A0P0XVT5|A0A0P0XVT5_ORYSJ | gi|297610712 | Alpha-galactosidase (Fragment) | 0.72 | 1.115 | 0.848 | 1.16 | 1.321 |
| tr|B7F946|B7F946_ORYSJ | gi|297605789 | Os06g0356800 protein | 0.681 | 1.016 | 0.7 | 1.159 | 2.984 |
| Stress responsive | |||||||
| tr|Q9AQU0|Q9AQU0_ORYSJ | gi|13486733 | Peptidyl-prolyl cis-trans isomerase | 1.249 | 1.825 | 0.965 | 1.578 | 1.772 |
| tr|Q8GTB0|Q8GTB0_ORYSJ | gi|27476086 | Putative heat shock 70 KD protein, mitochondrial | 1.294 | 1.354 | 0.92 | 1.027 | 1.208 |
| tr|Q84S20|Q84S20_ORYSJ | gi|28971968 | CHP-rich zinc finger protein-like | 2.605 | 2.416 | 0.869 | 1.374 | 1.439 |
| tr|Q5JKK9|Q5JKK9_ORYSJ | gi|115442153 | Os01g0940700 protein | 1.897 | 3.948 | 0.955 | 1.04 | 0.959 |
| sp|Q75HQ0|BIP4_ORYSJ | gi|115464027 | Heat shock 70 kDa protein BIP4 | 10 | 10 | 1.058 | 0.8 | 0.72 |
| tr|Q53NM9|Q53NM9_ORYSJ | gi|115486793 | DnaK-type molecular chaperone hsp70-rice | 1.87 | 1.487 | 1.009 | 0.821 | 0.793 |
| tr|Q10NA9|Q10NA9_ORYSJ | gi|115452223 | 70 kDa heat shock protein | 2.198 | 1.668 | 1.086 | 0.907 | 0.816 |
| sp|Q5VRY1|HSP18_ORYSJ | gi|115434946 | 17.5 kDa heat shock protein | 1.413 | 3.508 | 1.045 | 1.084 | 1.043 |
| tr|Q6YUA7|Q6YUA7_ORYSJ | gi|115476792 | Os08g0464000 protein | 1.323 | 1.3 | 1.041 | 0.866 | 1.034 |
| tr|A2YK26|A2YK26_ORYSI | gi|115471453 | Os07g0262200 protein | 1.096 | 1.252 | 0.994 | 0.995 | 1.314 |
| tr|B9FK56|B9FK56_ORYSJ | gi|222631026 | Uncharacterized protein | 1.028 | 1.106 | 1.031 | 1.314 | 1.25 |
| tr|A2Z3L9|A2Z3L9_ORYSI | gi|115480445 | Os09g0541700 protein | 1.1 | 1.218 | 0.999 | 1.144 | 1.342 |
| tr|O82143|O82143_ORYSJ | gi|115451853 | 26S proteasome regulatory particle | 1.138 | 1.146 | 0.981 | 1.293 | 1.427 |
| tr|Q5ZAV7|Q5ZAV7_ORYSJ | gi|115440349 | Os01g0783500 protein | 1.066 | 1.434 | 1.03 | 1.71 | 2.189 |
| tr|A2Y628|A2Y628_ORYSI | gi|125552829 | Cysteine proteinase inhibitor | 0.96 | 1.775 | 1.056 | 1.438 | 2.205 |
| Osmotic stress responsive | |||||||
| tr|A2XHR1|A2XHR1_ORYSI | gi|125544232 | Sucrose synthase | 0.82 | 1.102 | 0.545 | 0.366 | 1.005 |
| tr|B8B835|B8B835_ORYSI | gi|115473055 | NADH-dehydrogenase | 0.992 | 1.182 | 0.879 | 1.282 | 1.538 |
| tr|Q2RBD1|Q2RBD1_ORYSJ | gi|115483847 | Non-specific lipid-transfer protein | 0.988 | 1.244 | 0.894 | 1.274 | 2.009 |
| tr|Q0IQK7|Q0IQK7_ORYSJ | gi|297612544 | Non-specific lipid-transfer protein | 1.226 | 2.979 | 0.78 | 1.023 | 2.235 |
| tr|B8B936|B8B936_ORYSI | gi|218201512 | Putative uncharacterized protein | 0.871 | 1.281 | 0.93 | 0.976 | 1.51 |
| tr|B8AII1|B8AII1_ORYSI | gi|218190145 | Putative uncharacterized protein | 0.904 | 1.55 | 0.954 | 1.167 | 1.478 |
| tr|Q9SNL7|Q9SNL7_ORYSJ | gi|6006382 | Putative SAM-protoporphyrin IX methyltransferase | 0.935 | 0.974 | 0.965 | 1.05 | 1.216 |
| sp|Q10LR9|DCUP2_ORYSJ | gi|115452897 | Uroporphyrinogen decarboxylase 2 | 1.265 | 1.835 | 0.902 | 0.95 | 1.425 |
| tr|A2X8B7|A2X8B7_ORYSI | gi|242062934 | 2-C-methyl-d-erythritol 2,4-cyclodiphosphate synthase | 1.362 | 1.399 | 0.746 | 1.202 | 1.538 |
| tr|Q2RBD1|Q2RBD1_ORYSJ | gi|115483847 | Non-specific lipid-transfer protein | 0.988 | 1.244 | 0.894 | 1.274 | 2.009 |
| Ethylene responsive | |||||||
| tr|B9G3V3|B9G3V3_ORYSJ | gi|222641669 | Uncharacterized protein | 1.837 | 2.313 | 1.837 | 1.825 | 1.982 |
| sp|Q8W3D9|PORB_ORYSJ | gi|75248671 | Protochlorophyllide reductase B | 0.881 | 1.621 | 0.891 | 1.192 | 2.065 |
| tr|Q0IQK7|Q0IQK7_ORYSJ | gi|297612544 | Non-specific lipid-transfer protein (Fragment) | 1.226 | 2.979 | 0.78 | 1.023 | 2.235 |
| tr|Q2RBD1|Q2RBD1_ORYSJ | gi|115483847 | Non-specific lipid-transfer protein | 0.988 | 1.244 | 0.894 | 1.274 | 2.009 |
| Metabolic responsive | |||||||
| tr|Q0D572|Q0D572_ORYSJ | gi|297607511 | Os07g0577300 protein | 1.28 | 1.719 | 1.105 | 0.899 | 2.422 |
| tr|A2YIJ5|A2YIJ5_ORYSI | gi|50509727 | Os07g0168600 protein | 0.779 | 0.93 | 0.952 | 1.118 | 1.317 |
| tr|B9F240|B9F240_ORYSJ | gi|222622048 | Uncharacterized protein | 0.739 | 1.149 | 1.372 | 1.24 | 1.422 |
| tr|B9F7T1|B9F7T1_ORYSJ | gi|222624734 | Uncharacterized protein | 1.389 | 1.083 | 1.317 | 0.854 | 0.954 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, S.; Zhu, C.; Bai, Z.; Huang, J.; Zhu, L.; Cao, X.; Nanda, S.; Hussain, S.; Riaz, A.; Liang, Q.; et al. iTRAQ-Based Protein Profiling and Biochemical Analysis of Two Contrasting Rice Genotypes Revealed Their Differential Responses to Salt Stress. Int. J. Mol. Sci. 2019, 20, 547. https://doi.org/10.3390/ijms20030547
Hussain S, Zhu C, Bai Z, Huang J, Zhu L, Cao X, Nanda S, Hussain S, Riaz A, Liang Q, et al. iTRAQ-Based Protein Profiling and Biochemical Analysis of Two Contrasting Rice Genotypes Revealed Their Differential Responses to Salt Stress. International Journal of Molecular Sciences. 2019; 20(3):547. https://doi.org/10.3390/ijms20030547
Chicago/Turabian StyleHussain, Sajid, Chunquan Zhu, Zhigang Bai, Jie Huang, Lianfeng Zhu, Xiaochuang Cao, Satyabrata Nanda, Saddam Hussain, Aamir Riaz, Qingduo Liang, and et al. 2019. "iTRAQ-Based Protein Profiling and Biochemical Analysis of Two Contrasting Rice Genotypes Revealed Their Differential Responses to Salt Stress" International Journal of Molecular Sciences 20, no. 3: 547. https://doi.org/10.3390/ijms20030547







