The Role of NOX4 in Parkinson’s Disease with Dementia
Abstract
:1. Introduction
2. Results
2.1. Motor Deficit, Loss of TH+ Cells, Increased Expression of α-Synuclein and NOX1 in the 6-OHDA-Lesion Side of the Striatum and Substantia Nigra
2.2. Cognitive Deficit Induced by MFB Injury Caused by 6-OHDA Injection
2.3. Levels of Alpha-Synuclein and Amyloid Beta Pathology in Animals with MFB Injury Caused by 6-OHDA Injection
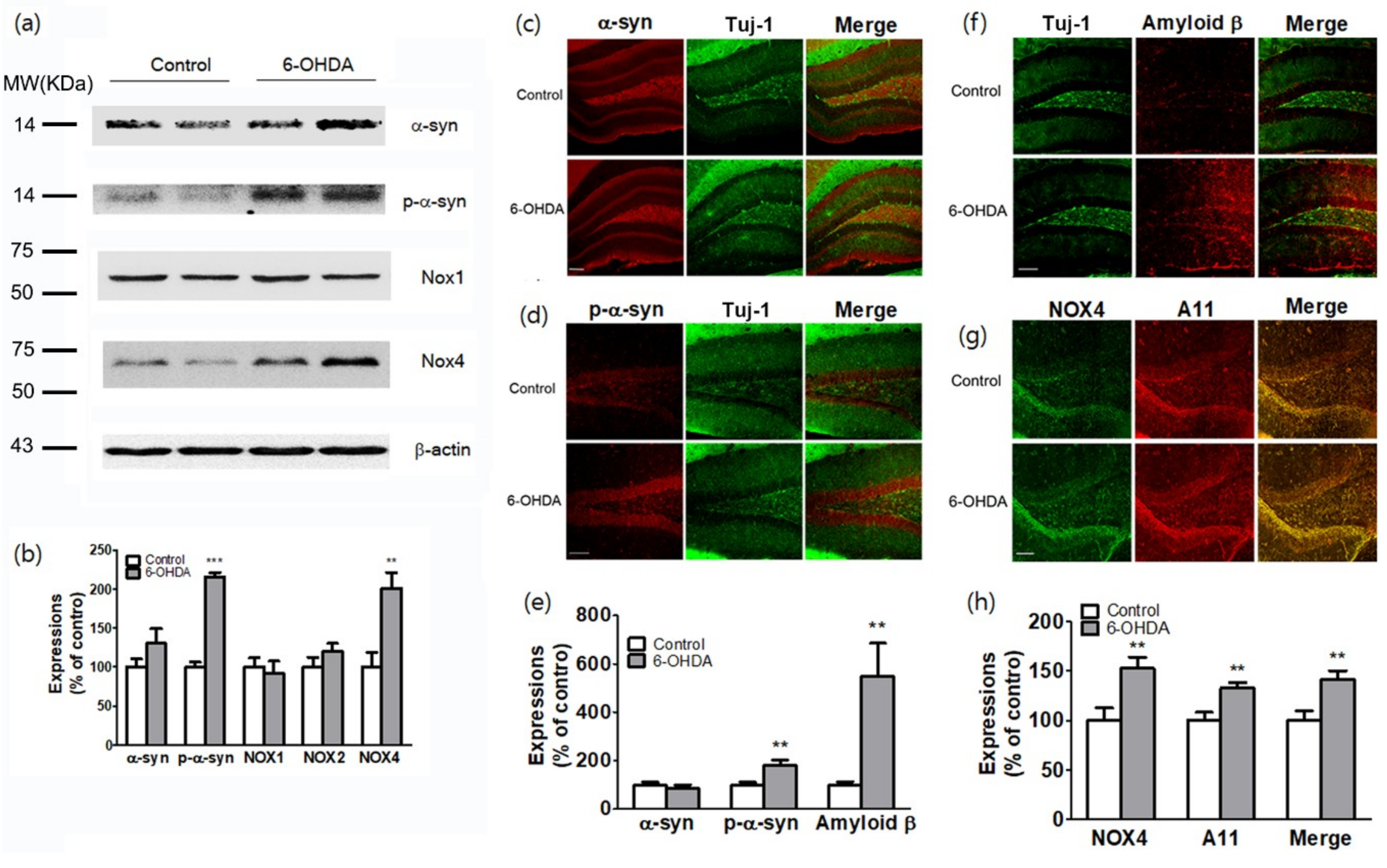
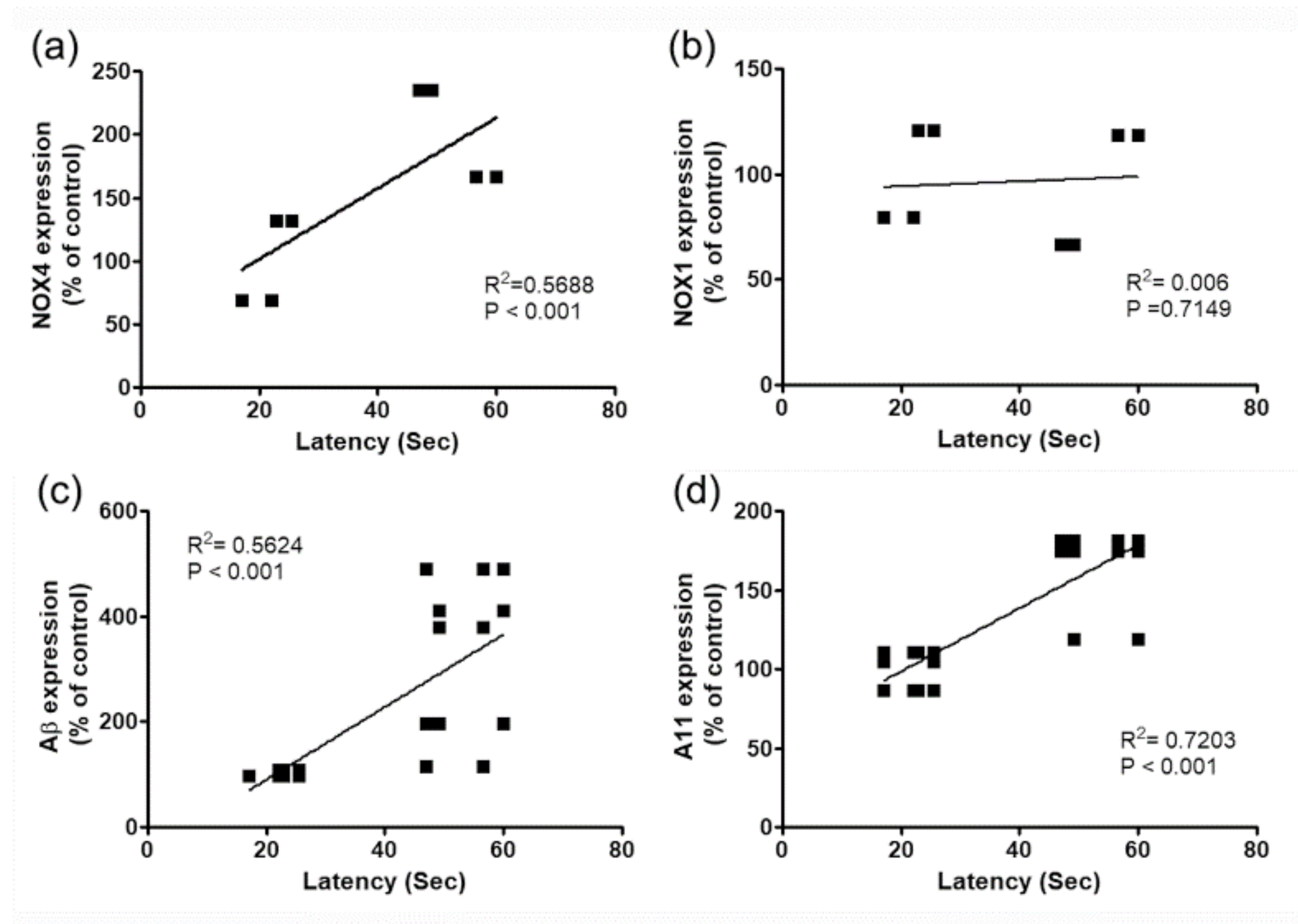
2.4. Increased Levels of Phosphorylated Alpha-Synuclein, Aggregated Amyloid Beta, and NADPH Oxidase-4 in the Hippocampus
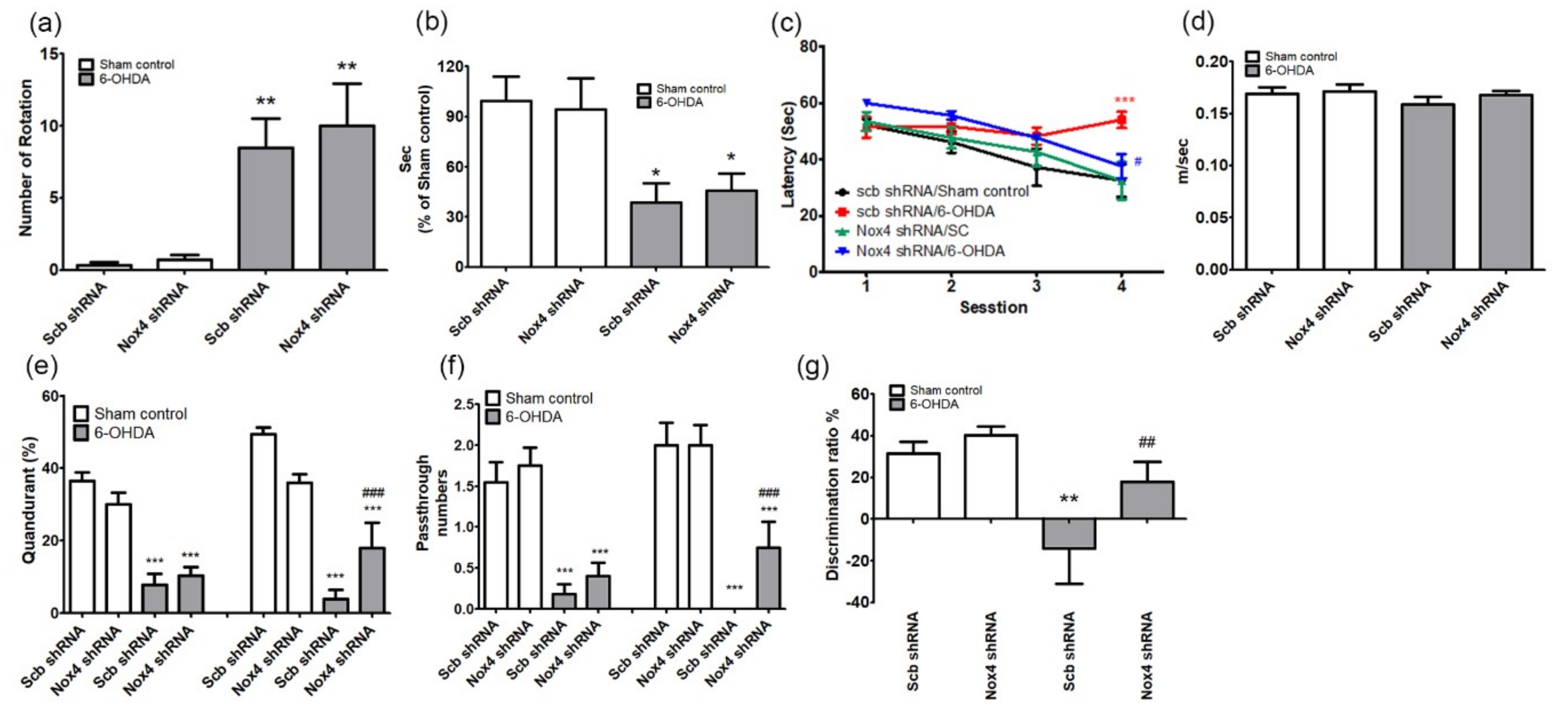
2.5. Inhibition of NADPH Oxidase-4 in Hippocampal Neurons Reduced Cognitive Impairment in a PD Animal Model
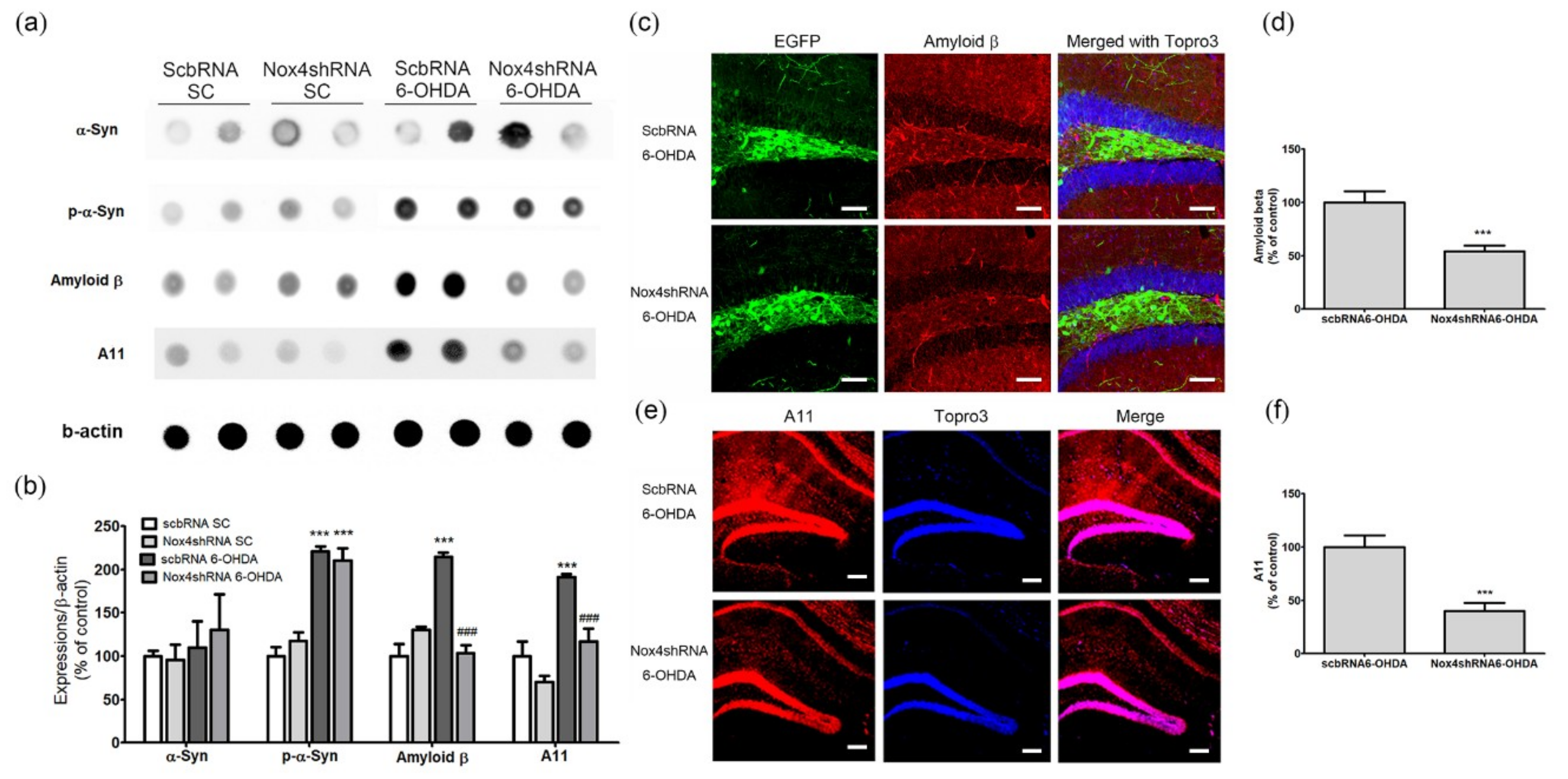
2.6. NOX4 Knockdown Reduces 6-OHDA-Mediated Amyloid Beta and A11 Oligomer Upregulation in the Hippocampus
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Animal Treatment and Unilateral 6-OHDA Lesion in MFB
4.3. Apomorphine-Induced Rotation.
4.4. Immunohistochemistry
4.5. Western Blot and Dot Blot Analysis
4.6. Behavior Test
4.7. Double-Fluorescence Immunostaining of Tissues
4.8. Quantitative Analysis
4.9. Establishment of U6-NOX4 shRNA-CMV-EGFP/AAV and AAV Vector and AAV Viral Package.
4.10. Adeno-Associated Virus 2-Mediated NOX4 Knockdown
4.11. NOX Activity Assay
4.12. Novel Object Recognition Test
4.13. Data Analysis and Statistics
Author Contributions
Acknowledgments
Conflicts of Interest
Abbreviations
| NOX4 | NADPH oxidase-4 |
| NADPH | Nicotinamide adenine sinucleotide phosphate |
| PD | Parkinson’s disease |
| PDD | Parkinson’s disease with dementia |
| AD | Alzheimer disease |
| MCI | Mild cognitive impairment |
| MWM | Morris water maze |
| NOR | Novel object recognition |
| α-syn | Alpha synuclein |
| p-α-syn | Phosphorylated alpha-synuclein |
| Aβ | Amyloid beta |
| Tuj-1 | Neuron-specific class III beta-tubulin |
| 6-OHDA | 6-hydroxydopamine |
| DG | Dentate gyrus |
| SN | Substantia nigra |
| TH | Tyrosine hydroxylase |
| MFB | Medial forebrain bundle |
| APO | Apomorphine |
| Scb shRNA | Scrambled shRNA/AAV |
| rAAV | Recombinant AAV |
| EGFP | Enhanced green fluorescent protein |
| ANOVA | Analysis of variance |
| h | hour(s) |
References
- Calabresi, P.; Castrioto, A.; Di Filippo, M.; Picconi, B. New experimental and clinical links between the hippocampus and the dopaminergic system in parkinson’s disease. Lancet Neurol. 2013, 12, 811–821. [Google Scholar] [CrossRef]
- Monchi, O.; Hanganu, A.; Bellec, P. Markers of cognitive decline in pd: The case for heterogeneity. Parkinsonism Relat. Disord. 2016, 24, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.J.; White, M.T.; Toledo, J.B.; Xie, S.X.; Robinson, J.L.; Van Deerlin, V.; Lee, V.M.; Leverenz, J.B.; Montine, T.J.; Duda, J.E.; et al. Neuropathologic substrates of parkinson disease dementia. Ann. Neurol. 2012, 72, 587–598. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.J.; Lee, V.M.; Trojanowski, J.Q. Parkinson’s disease dementia: Convergence of alpha-synuclein, tau and amyloid-beta pathologies. Nat. Rev. Neurosci. 2013, 14, 626–636. [Google Scholar] [CrossRef] [PubMed]
- Compta, Y.; Parkkinen, L.; O’Sullivan, S.S.; Vandrovcova, J.; Holton, J.L.; Collins, C.; Lashley, T.; Kallis, C.; Williams, D.R.; de Silva, R.; et al. Lewy- and alzheimer-type pathologies in parkinson’s disease dementia: Which is more important? Brain 2011, 134, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A.; Attems, J. Prevalence and impact of vascular and alzheimer pathologies in lewy body disease. Acta Neuropathol. 2008, 115, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Kovari, E.; Gold, G.; Herrmann, F.R.; Canuto, A.; Hof, P.R.; Bouras, C.; Giannakopoulos, P. Lewy body densities in the entorhinal and anterior cingulate cortex predict cognitive deficits in parkinson’s disease. Acta Neuropathol. 2003, 106, 83–88. [Google Scholar] [PubMed]
- Jellinger, K.A. Morphological substrates of parkinsonism with and without dementia: A retrospective clinico-pathological study. In Neuropsychiatric Disorders An Integrative Approach; Springer: Vienna, Austria, 2007; pp. 91–104. [Google Scholar]
- Jellinger, K.A.; Seppi, K.; Wenning, G.K.; Poewe, W. Impact of coexistent alzheimer pathology on the natural history of parkinson’s disease. J. Neural Transm. 2002, 109, 329–339. [Google Scholar] [CrossRef]
- Petrou, M.; Dwamena, B.A.; Foerster, B.R.; MacEachern, M.P.; Bohnen, N.I.; Muller, M.L.; Albin, R.L.; Frey, K.A. Amyloid deposition in parkinson’s disease and cognitive impairment: A systematic review. Mov. Disord. 2015, 30, 928–935. [Google Scholar] [CrossRef]
- Jellinger, K.A. Significance of brain lesions in parkinson disease dementia and lewy body dementia. Front. Neurol. Neurosci. 2009, 24, 114–125. [Google Scholar]
- Halliday, G.M.; Holton, J.L.; Revesz, T.; Dickson, D.W. Neuropathology underlying clinical variability in patients with synucleinopathies. Acta Neuropathol. 2011, 122, 187–204. [Google Scholar] [CrossRef]
- Kalaitzakis, M.E.; Graeber, M.B.; Gentleman, S.M.; Pearce, R.K. Striatal beta-amyloid deposition in parkinson disease with dementia. J. Neuropathol. Exp. Neurol. 2008, 67, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Klunk, W.E.; Engler, H.; Nordberg, A.; Wang, Y.; Blomqvist, G.; Holt, D.P.; Bergstrom, M.; Savitcheva, I.; Huang, G.F.; Estrada, S.; et al. Imaging brain amyloid in alzheimer’s disease with pittsburgh compound-b. Ann. Neurol. 2004, 55, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Foo, H.; Mak, E.; Chander, R.J.; Ng, A.; Au, W.L.; Sitoh, Y.Y.; Tan, L.C.; Kandiah, N. Associations of hippocampal subfields in the progression of cognitive decline related to parkinson’s disease. Neuroimage Clin. 2017, 14, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Kehagia, A.A.; Barker, R.A.; Robbins, T.W. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with parkinson’s disease. Lancet Neurol. 2010, 9, 1200–1213. [Google Scholar] [CrossRef]
- Lisman, J.; Grace, A.A.; Duzel, E. A neohebbian framework for episodic memory; role of dopamine-dependent late ltp. Trends Neurosci. 2011, 34, 536–547. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Sgobio, C.; Siliquini, S.; Tozzi, A.; Tantucci, M.; Ghiglieri, V.; Di Filippo, M.; Pendolino, V.; de Iure, A.; Marti, M.; et al. Mechanisms underlying the impairment of hippocampal long-term potentiation and memory in experimental parkinson’s disease. Brain 2012, 135, 1884–1899. [Google Scholar] [CrossRef] [PubMed]
- Churchyard, A.; Lees, A.J. The relationship between dementia and direct involvement of the hippocampus and amygdala in parkinson’s disease. Neurology 1997, 49, 1570–1576. [Google Scholar] [CrossRef]
- Camicioli, R.; Moore, M.M.; Kinney, A.; Corbridge, E.; Glassberg, K.; Kaye, J.A. Parkinson’s disease is associated with hippocampal atrophy. Mov. Disord. 2003, 18, 784–790. [Google Scholar] [CrossRef]
- Tam, C.W.; Burton, E.J.; McKeith, I.G.; Burn, D.J.; O’Brien, J.T. Temporal lobe atrophy on mri in parkinson disease with dementia: A comparison with alzheimer disease and dementia with lewy bodies. Neurology 2005, 64, 861–865. [Google Scholar] [CrossRef]
- Jokinen, P.; Bruck, A.; Aalto, S.; Forsback, S.; Parkkola, R.; Rinne, J.O. Impaired cognitive performance in parkinson’s disease is related to caudate dopaminergic hypofunction and hippocampal atrophy. Parkinsonism Relat. Disord. 2009, 15, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Lee, K.H.; Kim, J.H.; Seo, J.H.; Kim, H.Y.; Shin, C.Y.; Han, J.S.; Han, S.H.; Kim, Y.S.; Lee, J. Nadph oxidase 1, a novel molecular source of ros in hippocampal neuronal death in vascular dementia. Antioxid. Redox Signal. 2014, 21, 533–550. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Lee, J. A mini-review of the nadph oxidases in vascular dementia: Correlation with noxs and risk factors for vad. Int. J. Mol. Sci. 2017, 18, 2500. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Scheff, S.W. Nadph-oxidase activation and cognition in alzheimer disease progression. Free Radic. Biol. Med. 2011, 51, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Bruce-Keller, A.J.; Gupta, S.; Parrino, T.E.; Knight, A.G.; Ebenezer, P.J.; Weidner, A.M.; LeVine, H., 3rd; Keller, J.N.; Markesbery, W.R. Nox activity is increased in mild cognitive impairment. Antioxid. Redox Signal. 2010, 12, 1371–1382. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Cristovao, A.C.; Guhathakurta, S.; Lee, J.; Joh, T.H.; Beal, M.F.; Kim, Y.S. Nadph oxidase 1-mediated oxidative stress leads to dopamine neuron death in parkinson’s disease. Antioxid. Redox Signal. 2012, 16, 1033–1045. [Google Scholar] [CrossRef]
- Cristovao, A.C.; Choi, D.H.; Baltazar, G.; Beal, M.F.; Kim, Y.S. The role of nadph oxidase 1-derived reactive oxygen species in paraquat-mediated dopaminergic cell death. Antioxid. Redox Signal. 2009, 11, 2105–2118. [Google Scholar] [CrossRef]
- Cristovao, A.C.; Guhathakurta, S.; Bok, E.; Je, G.; Yoo, S.D.; Choi, D.H.; Kim, Y.S. Nadph oxidase 1 mediates alpha-synucleinopathy in parkinson’s disease. J. Neurosci. 2012, 32, 14465–14477. [Google Scholar] [CrossRef]
- Harding, A.J.; Halliday, G.M. Cortical lewy body pathology in the diagnosis of dementia. Acta Neuropathol. 2001, 102, 355–363. [Google Scholar]
- Hurtig, H.I.; Trojanowski, J.Q.; Galvin, J.; Ewbank, D.; Schmidt, M.L.; Lee, V.M.; Clark, C.M.; Glosser, G.; Stern, M.B.; Gollomp, S.M.; et al. Alpha-synuclein cortical lewy bodies correlate with dementia in parkinson’s disease. Neurology 2000, 54, 1916–1921. [Google Scholar] [CrossRef]
- Ullman, M.T.; Pullman, M.Y. A compensatory role for declarative memory in neurodevelopmental disorders. Neurosci. Biobehav. Rev. 2015, 51, 205–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Bai, L.; Zhang, S.; Zhou, X.; Li, Y.; Bai, J. Trx-1 ameliorates learning and memory deficits in mptp-induced parkinson’s disease model in mice. Free Radic. Biol. Med. 2018, 124, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Hely, M.A.; Reid, W.G.; Adena, M.A.; Halliday, G.M.; Morris, J.G. The sydney multicenter study of parkinson’s disease: The inevitability of dementia at 20 years. Mov. Disord. 2008, 23, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Kempster, P.A.; O’Sullivan, S.S.; Holton, J.L.; Revesz, T.; Lees, A.J. Relationships between age and late progression of parkinson’s disease: A clinico-pathological study. Brain 2010, 133, 1755–1762. [Google Scholar] [CrossRef] [PubMed]
- Aarsland, D.; Andersen, K.; Larsen, J.P.; Lolk, A.; Kragh-Sorensen, P. Prevalence and characteristics of dementia in parkinson disease: An 8-year prospective study. Arch. Neurol. 2003, 60, 387–392. [Google Scholar] [CrossRef]
- Halliday, G.; Hely, M.; Reid, W.; Morris, J. The progression of pathology in longitudinally followed patients with parkinson’s disease. Acta Neuropathol. 2008, 115, 409–415. [Google Scholar] [CrossRef]
- Levy, G.; Schupf, N.; Tang, M.X.; Cote, L.J.; Louis, E.D.; Mejia, H.; Stern, Y.; Marder, K. Combined effect of age and severity on the risk of dementia in parkinson’s disease. Ann. Neurol. 2002, 51, 722–729. [Google Scholar] [CrossRef]
- Williams-Gray, C.H.; Evans, J.R.; Goris, A.; Foltynie, T.; Ban, M.; Robbins, T.W.; Brayne, C.; Kolachana, B.S.; Weinberger, D.R.; Sawcer, S.J.; et al. The distinct cognitive syndromes of parkinson’s disease: 5 year follow-up of the campaign cohort. Brain 2009, 132, 2958–2969. [Google Scholar] [CrossRef]
- Emre, M.; Aarsland, D.; Brown, R.; Burn, D.J.; Duyckaerts, C.; Mizuno, Y.; Broe, G.A.; Cummings, J.; Dickson, D.W.; Gauthier, S.; et al. Clinical diagnostic criteria for dementia associated with parkinson’s disease. Mov. Disord. 2007, 22, 1689–1707. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhan, M.; OuYang, L.; Li, Y.; Chen, S.; Wu, J.; Chen, J.; Luo, C.; Lei, W. The effects of unilateral 6-ohda lesion in medial forebrain bundle on the motor, cognitive dysfunctions and vulnerability of different striatal interneuron types in rats. Behav. Brain Res. 2014, 266, 37–45. [Google Scholar] [CrossRef]
- Apaydin, H.; Ahlskog, J.E.; Parisi, J.E.; Boeve, B.F.; Dickson, D.W. Parkinson disease neuropathology: Later-developing dementia and loss of the levodopa response. Arch. Neurol. 2002, 59, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Tsuboi, Y.; Uchikado, H.; Dickson, D.W. Neuropathology of parkinson’s disease dementia and dementia with lewy bodies with reference to striatal pathology. Parkinsonism Relat. Disord. 2007, 13, S221–S224. [Google Scholar] [CrossRef]
- Parkkinen, L.; Pirttila, T.; Alafuzoff, I. Applicability of current staging/categorization of alpha-synuclein pathology and their clinical relevance. Acta Neuropathol. 2008, 115, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Parkkinen, L.; Kauppinen, T.; Pirttila, T.; Autere, J.M.; Alafuzoff, I. Alpha-synuclein pathology does not predict extrapyramidal symptoms or dementia. Ann. Neurol. 2005, 57, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Pletnikova, O.; West, N.; Lee, M.K.; Rudow, G.L.; Skolasky, R.L.; Dawson, T.M.; Marsh, L.; Troncoso, J.C. Abeta deposition is associated with enhanced cortical alpha-synuclein lesions in lewy body diseases. Neurobiol. Aging 2005, 26, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Duda, J.E.; Giasson, B.I.; Mabon, M.E.; Lee, V.M.; Trojanowski, J.Q. Novel antibodies to synuclein show abundant striatal pathology in lewy body diseases. Ann. Neurol. 2002, 52, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Bruce-Keller, A.J.; Gupta, S.; Knight, A.G.; Beckett, T.L.; McMullen, J.M.; Davis, P.R.; Murphy, M.P.; Van Eldik, L.J.; St Clair, D.; Keller, J.N. Cognitive impairment in humanized appxps1 mice is linked to abeta(1-42) and nox activation. Neurobiol. Dis. 2011, 44, 317–326. [Google Scholar] [CrossRef]
- Kan, H.; Hu, W.; Wang, Y.; Wu, W.; Yin, Y.; Liang, Y.; Wang, C.; Huang, D.; Li, W. Nadph oxidase-derived production of reactive oxygen species is involved in learning and memory impairments in 16-month-old female rats. Mol. Med. Rep. 2015, 12, 4546–4553. [Google Scholar] [CrossRef]
- Han, B.H.; Zhou, M.L.; Johnson, A.W.; Singh, I.; Liao, F.; Vellimana, A.K.; Nelson, J.W.; Milner, E.; Cirrito, J.R.; Basak, J.; et al. Contribution of reactive oxygen species to cerebral amyloid angiopathy, vasomotor dysfunction, and microhemorrhage in aged tg2576 mice. Proc. Natl. Acad. Sci. USA 2015, 112, E881–E890. [Google Scholar] [CrossRef]
- Park, L.; Zhou, P.; Pitstick, R.; Capone, C.; Anrather, J.; Norris, E.H.; Younkin, L.; Younkin, S.; Carlson, G.; McEwen, B.S.; et al. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc. Natl. Acad. Sci. USA 2008, 105, 1347–1352. [Google Scholar] [CrossRef] [Green Version]
- Dugan, L.L.; Ali, S.S.; Shekhtman, G.; Roberts, A.J.; Lucero, J.; Quick, K.L.; Behrens, M.M. Il-6 mediated degeneration of forebrain gabaergic interneurons and cognitive impairment in aged mice through activation of neuronal nadph oxidase. PLoS ONE 2009, 4, e5518. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, L.; Jette, N.; Frolkis, A.; Steeves, T.; Pringsheim, T. The incidence of parkinson’s disease: A systematic review and meta-analysis. Neuroepidemiology 2016, 46, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Das, N.R.; Sharma, S.S. Cognitive impairment associated with parkinson’s disease: Role of mitochondria. Curr. Neuropharmacol. 2016, 14, 584–592. [Google Scholar] [CrossRef] [PubMed]
- Franklin, K.B.J.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Boix, J.; Padel, T.; Paul, G. A partial lesion model of parkinson’s disease in mice–characterization of a 6-ohda-induced medial forebrain bundle lesion. Behav. Brain Res. 2015, 284, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Kim, J.H.; Kim, S.M.; Kang, K.; Han, D.W.; Lee, J. Therapeutic potential of induced neural stem cells for parkinson’s disease. Int. J. Mol. Sci. 2017, 18, 224. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.H.; Kim, J.H.; Seo, J.H.; Lee, J.; Choi, W.S.; Kim, Y.S. Matrix metalloproteinase-3 causes dopaminergic neuronal death through nox1-regenerated oxidative stress. PLoS ONE 2014, 9, e115954. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.R.; Lee, S.R.; Han, J.S.; Woo, S.K.; Kim, K.M.; Choi, D.H.; Kwon, K.J.; Han, S.H.; Shin, C.Y.; Lee, J.; et al. Synergistic memory impairment through the interaction of chronic cerebral hypoperfusion and amlyloid toxicity in a rat model. Stroke 2011, 42, 2595–2604. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, D.-H.; Choi, I.-A.; Lee, C.S.; Yun, J.H.; Lee, J. The Role of NOX4 in Parkinson’s Disease with Dementia. Int. J. Mol. Sci. 2019, 20, 696. https://doi.org/10.3390/ijms20030696
Choi D-H, Choi I-A, Lee CS, Yun JH, Lee J. The Role of NOX4 in Parkinson’s Disease with Dementia. International Journal of Molecular Sciences. 2019; 20(3):696. https://doi.org/10.3390/ijms20030696
Chicago/Turabian StyleChoi, Dong-Hee, In-Ae Choi, Cheol Soon Lee, Ji Hee Yun, and Jongmin Lee. 2019. "The Role of NOX4 in Parkinson’s Disease with Dementia" International Journal of Molecular Sciences 20, no. 3: 696. https://doi.org/10.3390/ijms20030696




