Hexadecenoic Fatty Acid Positional Isomers and De Novo PUFA Synthesis in Colon Cancer Cells
Abstract
1. Introduction
2. Results
2.1. Effect of C16 Fatty Acid Supplementation on Cell Viability
2.2. Effect of C16 Fatty Acid Supplementation on Cell Morphology
2.3. Evaluation of Death Pathways after C16 Fatty Acid Supplementation
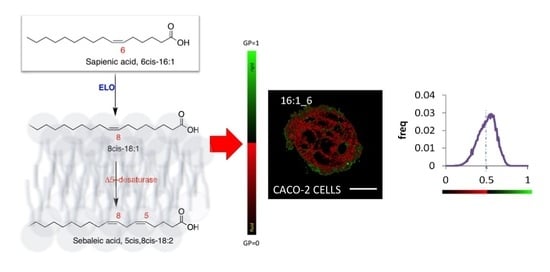
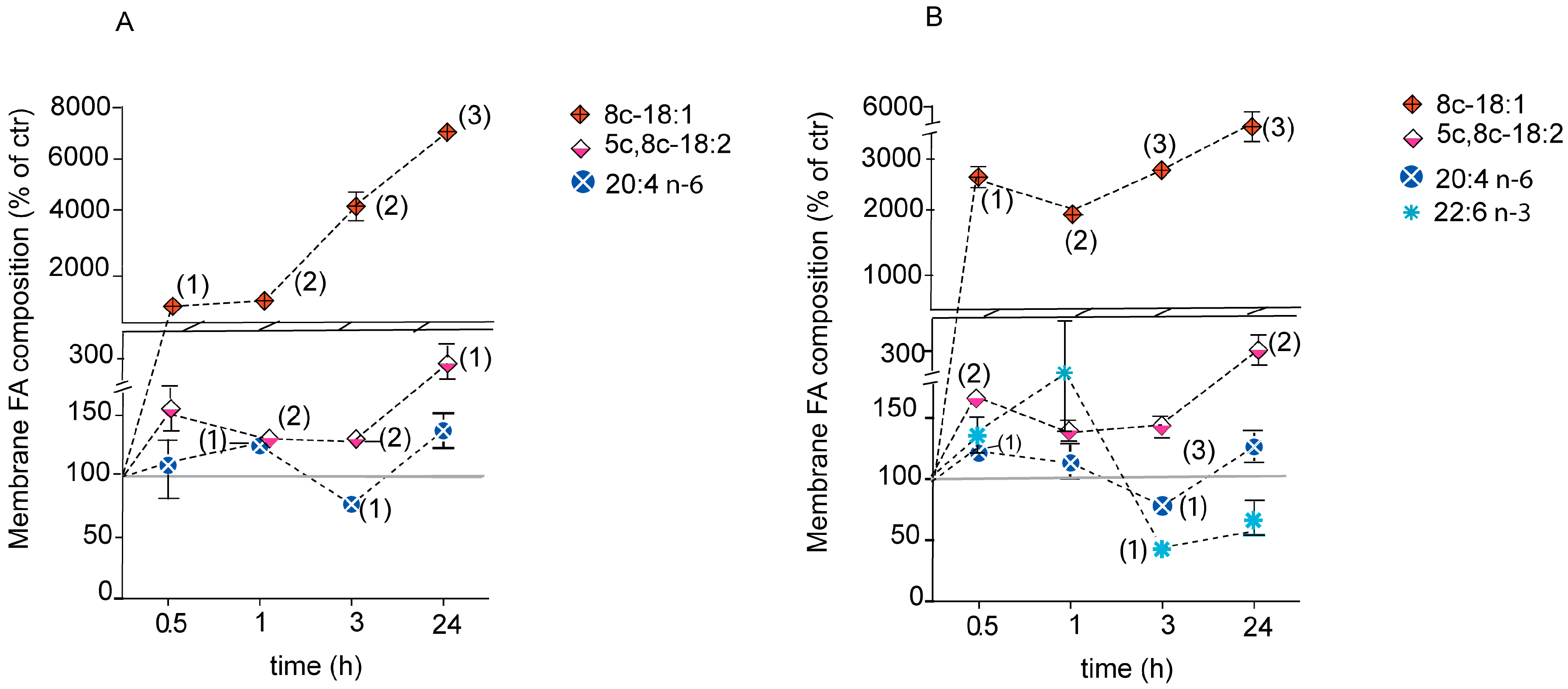
2.4. Fatty Acid-Based Membrane Lipidomic Monitoring
2.5. Generalized Polarization (GP) of Plasma Membrane in Caco-2 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Cell Viability Evaluation
4.3. Caspase Activation
4.4. Western Blot Analysis of p38 and cPLA2
4.5. Lipid Extraction and Fatty Acid Analysis
4.6. DMDS Derivatization
4.7. Laurdan Two-Photon Microscopy
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CE | Cholesteryl ester |
| DMDS | Dimethyl disulfide |
| FADS | Fatty acid desaturase |
| FAME | Fatty acid methyl ester |
| GC | Gas chromatography |
| FCS | Fetal calf serum |
| MUFA | Monounsaturated fatty acid |
| Nec | Necrostatin |
| GP | Generalized polarization |
| PL | Phospholipid |
| PLA2 | Phospholipase A2 |
| PUFA | Polyunsaturated fatty acid |
| SFA | Saturated fatty acid |
| SCD | Stearoyl CoA desaturase |
| TG | Triglyceride |
| TLC | Thin layer chromatography |
References
- Baenke, F.; Peck, B.; Meiss, H.; Schulze, A. Hooked on fat: The role of lipid synthesis in cancer metabolism and tumour development. Dis. Model. Mech. 2013, 6, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Wymann, M.P.; Schneiter, R. Lipid signalling in disease. Nat. Rev. Mol. Cell Biol. 2008, 9, 162–176. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, C.; Chatgilialoglu, C. Membrane Lipidomics for Personalized Health; John Wiley & Sons: New York, NY, USA, 2015. [Google Scholar]
- Nagle, J.F.; Tristram-Nagle, S. Structure of lipid bilayers. Biochim. Biophys. Acta 2000, 1469, 159–195. [Google Scholar] [CrossRef]
- Maulucci, G.; Cohen, O.; Daniel, B.; Sansone, A.; Petropoulou, P.I.; Filou, S.; Spyridonidis, A.; Pani, G.; De Spirito, M.; Chatgilialoglu, C.; et al. Fatty acid-related modulations of membrane fluidity in cells: Detection and implications. Free Radic. Res. 2016, 50, S40–S50. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Omega-3 polyunsaturated fatty acids and inflammatory processes: Nutrition or pharmacology? Br. J. Clin. Pharmacol. 2013, 75, 645–662. [Google Scholar] [CrossRef] [PubMed]
- Chiang, N.; Fredman, G.; Backhed, F.; Oh, S.F.; Vickery, T.; Schmidt, B.A.; Serhan, C.N. Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 2012, 484, 524–528. [Google Scholar] [CrossRef]
- Listemberger, L.L.; Han, X.; Lewis, S.E.; Cases, S.; Farese, R.V., Jr.; Ory, D.S.; Shaffer, J.E. Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Nat. Acad. Sci. USA 2003, 100, 3077–3082. [Google Scholar] [CrossRef]
- Cheon, H.G.; Cho, Y.S. Protection of palmitic acid-mediated lipotoxicity by arachidonic acid via channelling of palmitic acid into triglycerides in C2C12. J. Biomed. Sci. 2014, 21, 1–11. [Google Scholar] [CrossRef]
- Berndt, J.; Kovacs, P.; Ruschke, K.; Klöting, N.; Fasshauer, M.; Schön, M.R.; Körner, A.; Stumvoll, M.; Blüher, M. Fatty acid synthase gene expression in human adipose tissue: Association with obesity and type 2 diabetes. Diabetologia 2007, 50, 1472–1480. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of US adults. New Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef]
- Wang, D.; Dubois, R.N. Associations between obesity and cancer. The role of fatty acid synthase. J. Natl. Cancer Inst. 2012, 104, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Igal, R.A. Roles of Stearoyl Coa Desaturase-1 in the Regulation of Cancer Growth, Survival and tumorigenesis. Cancer 2011, 3, 2462–2477. [Google Scholar] [CrossRef] [PubMed]
- Kamphorst, J.J.; Cross, J.R.; Fan, J.; Methew, R.; White, E.P.; Thompson, C.B.; Rabinowitz, J.D. Hypoxic and Ras-transformed cells support growth by scavenging unsaturated fatty acids from lysophospholipids. Proc. Natl. Acad. Sci. USA 2013, 110, 8882–8887. [Google Scholar] [CrossRef] [PubMed]
- Peck, B.; Schug, Z.T.; Zhang, Q.; Dankworth, B.; Jones, D.T.; Smethurst, E.; Patel, R.; Mason, S.; Jiang, M.; Saunders, R.; et al. Inhibition of fatty acid desaturation is detrimental to cancer cell survival in metabolically compromised environments. Cancer Metab. 2016, 4, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Scaglia, N.; Igal, R.A. Inibition of Stearoyl-CoA Desaturase 1 expression in human lung adenocarcinoma cells impairs tumorigenesis. Int. J. Oncol. 2008, 33, 839–850. [Google Scholar] [CrossRef]
- Roongta, U.V.; Pabalan, J.G.; Wang, X.; Ryseck, R.P.; Fargnoli, J.; Henley, B.J.; Yang, W.P.; Zhu, J.; Madireddi, M.T.; Lawrence, R.M.; et al. Cancer cell dependence on unsaturated fatty acids implicates stearoyl-CoA desaturase as a target for cancer therapy. Cancer Res. 2011, 9, 1551–1561. [Google Scholar] [CrossRef]
- Chatgilialoglu, C.; Ferreri, C.; Melchiorre, M.; Sansone, A.; Torreggiani, A. Lipid geometrical isomerism: From chemistry to biology and diagnostics. Chem Rev. 2014, 114, 255–284. [Google Scholar] [CrossRef]
- Ge, L.; Gordon, J.S.; Hsuan, C.; Stenn, K.; Stephen, M.; Prouty, S.M. Identification of the Δ-6 Desaturase of Human Sebaceous Glands: Expression and Enzyme Activity. J. Investig. Dermatol. 2003, 120, 707–714. [Google Scholar] [CrossRef]
- Prouty, M.S.; Pappas, A. Sapienic Acid: Species-Specific Fatty Acid Metabolism of the Human Sebaceous Gland. Lipids and Skin Health; Springer Science + Business Media: New York, NY, USA, 2015; pp. 139–157. [Google Scholar]
- Sansone, A.; Melchiorre, M.; Chatgilialoglu, C.; Ferreri, C. Hexadecenoic fatty acid isomers: A chemical biology approach for human plasma biomarker development. Chem. Res. Toxicol. 2013, 26, 1703–1709. [Google Scholar] [CrossRef]
- Sansone, A.; Tolika, E.; Louka, M.; Sunda, V.; Deplano, S.; Melchiorre, M.; Anagnostopoulos, D.; Chatgilialoglu, C.; Formisano, C.; Di Micco, R.; et al. Hexadecenoic Fatty Acid Isomers in Human Blood Lipids and Their Relevance for the Interpretation of Lipidomic Profiles. PLoS ONE 2016, 11, e0152378. [Google Scholar] [CrossRef]
- Park, H.G.; Kothapalli, K.S.D.; Park, W.J.; DeAllie, C.; Liu, L.; Liang, A.; Lawrence, P.; Brenna, J.T. Palmitic acid (16:0) competes with omega-6 linoleic and omega-3 α-linolenic acids for FADS2 mediated Δ6-desaturation. Biochim. Biophys. Acta 2016, 1861, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Gerhold, K.; Mayers, J.R.; Wiest, M.M.; Watkins, S.M.; Hotamisligil, G.S. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 2008, 134, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Koeberle, A.; Shindou, H.; Harayama, T.; Shimizu, T. Palmitoleate is a mitogen, formed upon stimulation with growth factors, and converted to palmitoleoyl phosphatidylinositol. J. Biol. Chem. 2012, 287, 27244–27254. [Google Scholar] [CrossRef] [PubMed]
- Warensjo, E.; Rosell, M.; Hellenius, M.L.; Vessby, B.; De Faire, U.; Riserus, U. Associations between estimated fatty acid desaturase activities in serum lipids and adipose tissue in humans: Links to obesity and insulin resistance. Lipids Health Dis. 2009, 8, 37. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Vaarhorst, A.; Merry, A.H.; Dolle, M.E.; Hovenier, R.; Imholz, S.; Schouten, J.L.; Heijmans, B.T.; Müller, M.; Slagboom, P.E.; et al. Markers of endogenous desaturase activity and risk of coronary heart disease in the CAREMA cohort study. PLoS ONE 2012, 7, e41681. [Google Scholar] [CrossRef] [PubMed]
- Astudillo, A.M.; Meana, C.; Guijas, C.; Pereira, L.; Lebrebro, P.; Balboa, M.A.; Balsinde, J. Occurrence and biological activity of palmitoleic isomers in phagocytic cells. J. Lipid Res. 2018, 59, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Destaillats, F.; Guitard, M.; Cruz-Hernandez, C. Identification of Δ6-monounsaturated fatty acids in human hair and nail samples by gas-chromatography–mass-spectrometry using ionic-liquid coated capillary column. J. Chromatogr. A 2011, 1218, 9384–9389. [Google Scholar] [CrossRef]
- Guijas, C.; Meana, C.; Astudillo, A.M.; Balboa, M.A.; Balsinde, J. Foamy monocytes are enriched in cis-7-hexadecenoic fatty acid (16:1n-9), a possible biomarker for early detection of cardiovascular disease. Cell Chem. Biol. 2016, 23, 689–699. [Google Scholar] [CrossRef]
- Lands, W.E. Metabolism of glycerolipides; a comparison of lecithin and triglyceride synthesis. J. Biol. Chem. 1958, 231, 883–888. [Google Scholar]
- Prasinou, P.; Dafnis, I.; Giacometti, G.; Ferreri, C.; Chroni, A.; Chatgilialoglu, C. Fatty acid-based lipidomics and membrane remodeling induced by ApoE3 and ApoE4 in human neuroblastoma cells. BBA Biomembr. 2017, 1859, 1967–1973. [Google Scholar] [CrossRef]
- Cort, A.; Ozben, T.; Melchiorre, M.; Chatgilialoglu, C.; Ferreri, C.; Sansone, A. Effects of bleomycin and antioxidants on the fatty acid profile of testicular cancer cell membranes. BBA Biomembr. 2016, 1858, 434–441. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Shamni, O.; Avrahami, Y.; Cohen, O.; Broner, E.C.; Filippov-Levy, N.; Chatgilialoglu, C.; Ferreri, C.; Kaiser, N.; Sasson, S. Beta cell response to nutrient overload involves phospholipid remodelling and lipid peroxidation. Diabetologia 2015, 58, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Bolognesi, A.; Chatgilialoglu, A.; Polito, L.; Ferreri, C. Membrane lipidome reorganization correlates with the fate of neuroblastoma cells supplemented with fatty acids. PLoS ONE 2013, 8, e55537. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Riahi, Y.; Shamni, O.; Guichardant, M.; Chatgilialoglu, C.; Ferreri, C.; Kaiser, N.; Sasson, S. The role of lipid peroxidation and PPARδ in amplifying glucose-stimulated insulin secretion. Diabetes 2011, 60, 2830–2842. [Google Scholar] [CrossRef] [PubMed]
- Sambuy, Y.; De Angelis, I.; Ranaldi, G.; Scarino, M.L.; Stammati, A.; Zucco, F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005, 21, 1–26. [Google Scholar] [CrossRef]
- Nano, J.L.; Nobili, C.; Girard-Pipau, F.; Rampal, P. Effect of fatty acids on the growth of Caco-2 cells. Prostaglandins Leukot. Essent. Fatty Acids 2003, 69, 207–215. [Google Scholar] [CrossRef]
- Van Greevenbroek, M.M.J.; Robertus-Teunissen, M.G.; Erkelens, D.W.; de Bruin, T.W.A. Lipoprotein secretion by intestinal Caco-2 cells is affected differently by trans and cis unsaturated fatty acids: Effect of carbon chain length and position of the double bond. Am. J. Clin. Nutr. 1998, 68, 561–567. [Google Scholar] [CrossRef]
- Balogh, G.; Maulucci, G.; Gombos, I.; Horváth, I.; Török, Z.; Péter, M.; Fodor, E.; Páli, T.; Benkő, S.; Parasassi, T.; et al. Heat Stress Causes Spatially-Distinct Membrane Re-Modelling in K562 Leukemia Cells. PLoS ONE 2011, 6, e21182. [Google Scholar] [CrossRef]
- Maulucci, G.; Troiani, D.; Eramo, S.L.M.; Paciello, F.; Podda, M.V.; Paludetti, G.; Papi, M.; Maiorana, A.; Palmieri, V.; De Spirito, M.; et al. Time evolution of noise induced oxidation in outer hair cells: Role of NAD(P)H and plasma membrane fluidity. Biochim. Biophys. Acta 2014, 1840, 2192–2202. [Google Scholar] [CrossRef]
- Zhou, H.; Das, S.; Murthy, K.S. Erk1/2- and p38 MAP kinase-dependent phosphorylation and activation of cPLA2 by m3 and m2 receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G472–G480. [Google Scholar] [CrossRef]
- Nichols, P.D.; Volkman, J.K.; Everitt, D.A. Occurrence of 6 cis-hexadecenoic fatty acid and other unusual monounsaturated fatty acids in the lipids of oceanic particulate matter. Oceanol. Acta 1989, 12, 393–403. [Google Scholar]
- Yamamoto, K.; Shibahare, A.; Nakayama, T.; Kajimoto, G. Determination of double bond positions in methylene-interrupted dienoic fatty acids by GC-MS as their dimethyl disulphide adducts. Chem. Phys. Lipids 1991, 60, 39–50. [Google Scholar] [CrossRef]
- Gaus, K.; Zech, T.; Harder, T. Visualizing membrane microdomains by Laurdan 2-photon microscopy. Mol. Membr. Biol. 2006, 23, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Landau, Z.; Forti, E.; Alcaly, M.; Birk, R. Palmitate induced lipoapoptosis of exocrine pancreas AR42J cells. Apoptosis 2006, 11, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Chajès, V.; Joulin, V.; Clavel-Chapelon, F. The fatty acid desaturation index of blood lipids, as a biomarker of hepatic stearoyl-CoA desaturase expression, is a predictive factor of breast cancer risk. Curr. Opin. Lipidol. 2010, 22, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Byberg, L.; Kilander, L.; Warensjö Lemming, E.; Michaëlsson, K.; Vessby, B. Cancer death is related to high palmitoleic acid in serum and to polymorphisms in the SCD-1 gene in healthy Swedish men. Am. J. Clin. Nutr. 2014, 99, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Picardo, M.; Ottaviani, M.; Camera, E.; Mastrofrancesco, A. Sebaceus gland lipids. Dermato-Endocrinol. 2009, 1, 68–71. [Google Scholar] [CrossRef]
- Neumann, Y.; Ohlsen, K.; Donat, S.; Engelmann, S.; Kusch, H.; Albrecht, D.; Cartron, M.; Hurd, A.; Foster, S.J. The effect of skin fatty acids on Staphylococcus aureus. Arch. Microbiol. 2015, 197, 245–267. [Google Scholar] [CrossRef]
- Moran, J.C.; Alorabi, J.A.; Horsburg, M.J. Comparative transcriptomics reveals discrete survival responses of S. aureus and S. epidermidis to sapienic acid. Front. Microbiol. 2017, 8, 1–12. [Google Scholar] [CrossRef]
- Cossette, C.; Patel, P.; Anumolu, J.R.; Sivedran, S.; Lee, G.J.; Gravel, S.; Graham, F.D.; Lesimple, A.; Mamer, O.A.; Rokach, J.; et al. Human neutrophils convert the sebum-derived polyunsaturated fatty acid Sebaleic acid to a potent granulocyte chemoattractant. J. Biol. Chem. 2008, 283, 11234–11243. [Google Scholar] [CrossRef]
- Simopoulos, A.P. Essential fatty acids in health and chronic disease. Am. J. Clin. Nutr. 1999, 70, 560S–569S. [Google Scholar] [CrossRef] [PubMed]
- Liebisch, G.; Ekroos, K.; Hermansson, M.; Ejsing, C.S. Reporting of lipidomics data should be standardized. Biochim. Biophys. Acta 2017, 1862, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Kallouri, R. The biology and function of exosomes in cancer. J. Clin. Investig. 2016, 126, 1208–1215. [Google Scholar] [CrossRef] [PubMed]
- Polito, L.; Bortolotti, M.; Pedrazzi, M.; Mercatelli, D.; Battelli, M.G.; Bolognesi, A. Apoptosis and necroptosis induced by stenodactylin in neuroblastoma cells can be completely prevented through caspase inhibition plus catalase or necrostatin-1. Phytomedicine 2016, 23, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Polito, L.; Mercatelli, D.; Bortolotti, M.; Maiello, S.; Djemil, A.; Battelli, M.G.; Bolognesi, A. Two Saporin-Containing Immunotoxins Specific for CD20 and CD22 Show Different Behavior in Killing Lymphoma Cells. Toxins 2017, 9, 182. [Google Scholar] [CrossRef] [PubMed]
- Polito, L.; Bortolotti, M.; Farini, V.; Pedrazzi, M.; Tazzari, P.L.; Bolognesi, A. ATG-saporin-S6 immunotoxin: A new potent and selective drug to eliminate activated lymphocytes and lymphoma cells. Br. J. Haematol. 2009, 147, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Less, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar]




| Fatty Acid | 24 h | 48 h | 72 h | 96 h |
|---|---|---|---|---|
| Palmitic acid | 218.9 | 105.5 | 99.6 | 101.1 |
| Palmitoleic acid | 240.7 | 217.0 | 214.3 | 200.5 |
| Sapienic acid | 262.1 | 245.3 | 230.2 | 232.3 |
| Fatty Acid | PO 0.5 h | SA 0.5 h | PA 0.5 h | PO 1 h | SA 1 h | PA 1 h | PO 3 h | SA 3 h | PA 3 h |
|---|---|---|---|---|---|---|---|---|---|
| 16:0 | ↑ * | ↓ * | ↓ * | ↑ * | |||||
| 6cis-16:1 | ↑ *** | ↓ * | ↑ *** | ||||||
| 9cis-16:1 | ↑ ** | ↓ ** | ↑ *** | ||||||
| 18:0 | ↓ * | ↓ * | ↓ * | ||||||
| 8cis-18:1 | ↑ * | ↓ * | ↑ ** | ↑ ** | |||||
| 5cis,8cis-18:2 | ↑ ** | ↓ ** | ↑ ** | ↓ ** | |||||
| 18:2 n-6 | ↓ * | ↓ * | |||||||
| 20:0 | ↑ ** | ↑ *** | ↑ * | ↑ * | |||||
| 20:3 n-6 | ↓ * | ↓ *** | |||||||
| 20:4 n-6 | ↓ * | ↑ * | ↓ * | ↓ * | |||||
| 20:5 n-3 | |||||||||
| 22:5 n-3 | ↓ ** | ↓ * | |||||||
| 22:6 n-3 | ↓ * | ||||||||
| SFA | ↓ * | ↓ * | |||||||
| MUFA | ↑ * | ↑ * | ↑ ** | ||||||
| Total PUFA | ↓ * | ↓ * | |||||||
| PUFA n-6 | ↓ * | ↓ *** | ↓ * | ↓ * | |||||
| PUFA n-3 | ↓ * | ↓ * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scanferlato, R.; Bortolotti, M.; Sansone, A.; Chatgilialoglu, C.; Polito, L.; De Spirito, M.; Maulucci, G.; Bolognesi, A.; Ferreri, C. Hexadecenoic Fatty Acid Positional Isomers and De Novo PUFA Synthesis in Colon Cancer Cells. Int. J. Mol. Sci. 2019, 20, 832. https://doi.org/10.3390/ijms20040832
Scanferlato R, Bortolotti M, Sansone A, Chatgilialoglu C, Polito L, De Spirito M, Maulucci G, Bolognesi A, Ferreri C. Hexadecenoic Fatty Acid Positional Isomers and De Novo PUFA Synthesis in Colon Cancer Cells. International Journal of Molecular Sciences. 2019; 20(4):832. https://doi.org/10.3390/ijms20040832
Chicago/Turabian StyleScanferlato, Roberta, Massimo Bortolotti, Anna Sansone, Chryssostomos Chatgilialoglu, Letizia Polito, Marco De Spirito, Giuseppe Maulucci, Andrea Bolognesi, and Carla Ferreri. 2019. "Hexadecenoic Fatty Acid Positional Isomers and De Novo PUFA Synthesis in Colon Cancer Cells" International Journal of Molecular Sciences 20, no. 4: 832. https://doi.org/10.3390/ijms20040832
APA StyleScanferlato, R., Bortolotti, M., Sansone, A., Chatgilialoglu, C., Polito, L., De Spirito, M., Maulucci, G., Bolognesi, A., & Ferreri, C. (2019). Hexadecenoic Fatty Acid Positional Isomers and De Novo PUFA Synthesis in Colon Cancer Cells. International Journal of Molecular Sciences, 20(4), 832. https://doi.org/10.3390/ijms20040832