Overexpression of the Stress-Inducible SsMAX2 Promotes Drought and Salt Resistance via the Regulation of Redox Homeostasis in Arabidopsis
Abstract
:1. Introduction
2. Results
2.1. Gene Cloning of SsMAX2 from Sapium sebiferum Seedlings and the Gene Expression Profile in Response to Abiotic Stresses
2.2. SsMAX2 Conferred Drought and Osmotic Stress Tolerance in Arabidopsis
2.3. SsMAX2 Conferred Salt Tolerance in Arabidopsis
2.4. SsMAX2 Promoted Seed Germination under Both Salt and Osmotic Stresses
2.5. SsMAX2 Regulated the Hydrogen Peroxide, Malondialdehyde (MDA), Proline, and Soluble Sugar Accumulation in the Seedlings in Response to the Stresses
2.6. SsMAX2 Increased the Enzyme Activity of Superoxide Dismutase (SOD), Peroxidase (POD), and Ascorbate Peroxidase (APX)
2.7. Diverse Regulation of the Abscisic Acid (ABA) Biosynthesis Genes in SsMAX2 OE Lines and max2 in Response to Drought and Salt Stress
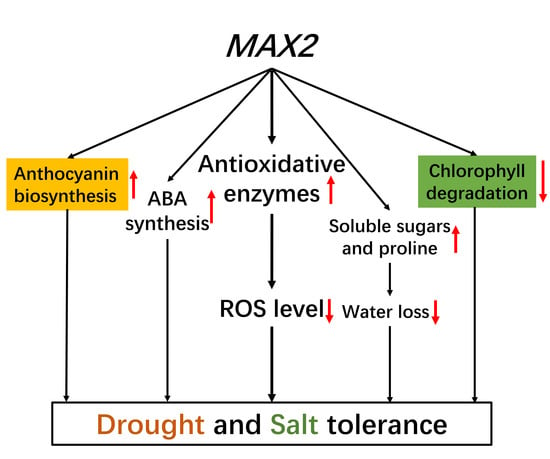
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Gene Cloning, Vector Construction, and Arabidopsis Transformation
4.3. Drought and Salt Treatment
4.4. RNA Extraction and Quantitative Real-Time PCR (qPCR)
4.5. Total Chlorophyll and Anthocyanin Determination
4.6. Determination of the Water Loss Rate
4.7. Diaminobenzidine (DAB) Staining of Hydrogen Peroxide in the Leaves
4.8. Determination of Hydrogen Peroxide, MDA, Proline and Total Soluble Sugar Level, and Antioxidant Enzyme Activity
4.9. Chlorophyll Fluorescence Measurement
4.10. Phylogenetic Analysis
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ABA | Abscisic acid |
| MDA | Malondialdehyde |
| MS | Murashige and Skoog |
| OE | Overexpression |
| PEG | polyethylene glycol |
| qPCR | Quantitative real-time PCR |
| ROS | Reactive oxygen species |
| rpm | Round per minute |
| RT-PCR | Reverse transcription PCR |
| SL | Strigolactone |
| WT | Wild-type |
References
- Zvi, P.; Eduardo, B. Hormone balance and abiotic stress tolerance in crop plants. Curr. Opin. Plant Biol. 2011, 14, 290–295. [Google Scholar]
- Raja, V.; Majeed, U.; Kang, H.; Andrabi, K.I.; John, R. Abiotic stress: Interplay between ROS, hormones and MAPKs. Environ. Exp. Bot. 2017, 137, 142–157. [Google Scholar] [CrossRef]
- Munne-Bosch, S.; Muller, M. Hormonal cross-talk in plant development and stress responses. Front. Plant Sci. 2013, 4, 529. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M. A new look at stress: Abscisic acid patterns and dynamics at high-resolution. New Phytol. 2016, 210, 38–44. [Google Scholar] [CrossRef]
- Zwack, P.J.; Rashotte, A.M. Interactions between cytokinin signalling and abiotic stress responses. J. Exp. Bot. 2015, 66, 4863–4871. [Google Scholar] [CrossRef] [Green Version]
- Kang, G.Z.; Li, G.Z.; Guo, T.C. Molecular mechanism of salicylic acid-induced abiotic stress tolerance in higher plants. Acta Physiol. Plant. 2014, 36, 2287–2297. [Google Scholar] [CrossRef]
- Korver, R.A.; Koevoets, I.T.; Testerink, C. Out of shape during stress: A key role for auxin. Trends Plant Sci. 2018, 23, 783–793. [Google Scholar] [CrossRef]
- Van Zeijl, A.; Liu, W.; Xiao, T.T.; Kohlen, W.; Yang, W.C.; Bisseling, T.; Geurts, R. The strigolactone biosynthesis gene DWARF27 is co-opted in rhizobium symbiosis. BMC Plant Biol. 2015, 15, 260. [Google Scholar] [CrossRef]
- Gomez-Roldan, V.; Fermas, S.; Brewer, P.B.; Puech-Pages, V.; Dun, E.A.; Pillot, J.P.; Letisse, F.; Matusova, R.; Danoun, S.; Portais, J.C.; et al. Strigolactone inhibition of shoot branching. Nature 2008, 455, 189–194. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Kyozuka, J. Branching hormone is busy both underground and overground. Plant Cell Physiol. 2010, 51, 1091–1094. [Google Scholar] [CrossRef]
- Foo, E. Auxin influences strigolactones in pea mycorrhizal symbiosis. J. Plant Physiol. 2013, 170, 523–528. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, F.; Krukowski, P.K.; Schubert, A.; Visentin, I. Strigolactones: Mediators of osmotic stress responses with a potential for agrochemical manipulation of crop resilience. J. Exp. Bot. 2018, 69, 2291–2303. [Google Scholar] [CrossRef] [PubMed]
- Ha, C.V.; Leyva-Gonzalez, M.A.; Osakabe, Y.; Tran, U.T.; Nishiyama, R.; Watanabe, Y.; Tanaka, M.; Seki, M.; Yamaguchi, S.; Dong, N.V.; et al. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc. Natl. Acad. Sci. USA 2014, 111, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Bu, Q.; Lv, T.; Shen, H.; Luong, P.; Wang, J.; Wang, Z.; Huang, Z.; Xiao, L.; Engineer, C.; Kim, T.H. Regulation of drought tolerance by the F-box protein MAX2 in Arabidopsis. Plant Physiol. 2014, 164, 424–439. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Gao, C.C.; Chen, M.S.; Pan, B.Z.; Ye, K.Q.; Xu, Z.F. Gibberellin promotes shoot branching in the perennial woody plant Jatropha curcas. Plant Cell Physiol. 2015, 56, 1655–1666. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Zhao, M.L.; Chen, M.S.; Pan, B.Z.; Tao, Y.B.; Xu, Z.F. Comparative transcriptome analysis of axillary buds in response to the shoot branching regulators gibberellin A3 and 6-benzyladenine in Jatropha curcas. Sci. Rep. 2017, 7, 11417. [Google Scholar] [CrossRef] [PubMed]
- Muhr, M.; Prufer, N.; Paulat, M.; Teichmann, T. Knockdown of strigolactone biosynthesis genes in Populus affects BRANCHED1 expression and shoot architecture. New Phytol. 2016, 212, 613–626. [Google Scholar] [CrossRef]
- Foster, T.M.; Ledger, S.E.; Janssen, B.J.; Luo, Z.W.; Drummond, R.S.M.; Tomes, S.; Karunairetnam, S.; Waite, C.N.; Funnell, K.A.; van Hooijdonk, B.; et al. Expression of MdCCD7 in the scion determines the extent of sylleptic branching and the primary shoot growth rate of apple trees. J. Exp. Bot. 2018, 69, 2379–2390. [Google Scholar] [CrossRef]
- Wang, R.; Hanna, M.A.; Zhou, W.W.; Bhadury, P.S.; Chen, Q.; Song, B.A.; Yang, S. Production and selected fuel properties of biodiesel from promising non-edible oils: Euphorbia lathyris L., Sapium sebiferum L. and Jatropha curcas L. Bioresour. Technol. 2011, 102, 1194–1199. [Google Scholar] [CrossRef]
- Xu, J.S.; Chikashige, T.; Meguro, S.; Kawachi, S. Effective utilization of stillingia or Chinese tallow-tree (Sapium sebiferum) fruits. Mok. Gakk. 1991, 37, 494–498. [Google Scholar]
- Xu, Z.; Mahmood, K.; Rothstein, S.J. ROS induces anthocyanin production via late biosynthetic genes and anthocyanin deficiency confers the hypersensitivity to ROS-generating stresses in Arabidopsis. Plant Cell Physiol. 2017, 58, 1364–1377. [Google Scholar] [CrossRef] [PubMed]
- Aarti, P.D.; Tanaka, R.; Tanaka, A. Effects of oxidative stress on chlorophyll biosynthesis in cucumber (Cucumis sativus) cotyledons. Physiol. Plant. 2010, 128, 186–197. [Google Scholar] [CrossRef]
- Liu, C.; Xu, Y.; Feng, Y.; Long, D.; Cao, B.; Xiang, Z.; Zhao, A. Ectopic expression of mulberry G-Proteins alters drought and salt stress tolerance in tobacco. Int. J. Mol. Sci. 2018, 20, 89. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Gao, F.; Bing, J.; Sun, W.; Feng, X.; Ma, X.; Zhou, Y.; Zhang, G. Overexpression of the Jojoba aquaporin gene, ScPIP1, enhances drought and salt tolerance in transgenic Arabidopsis. Int. J. Mol. Sci. 2019, 20, 153. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Chen, G.; Zhang, C. The effects of water stress on soluble protein content, the activity of SOD, POD and CAT of two ecotypes of reeds (Phragmites communis). Acta Bot. Boreal.-Occident. Sin. 2002, 22, 561–565. [Google Scholar]
- Wang, C.-T.; Ru, J.-N.; Liu, Y.-W.; Li, M.; Zhao, D.; Yang, J.-F.; Fu, J.D.; Xu, Z.-S. Maize WRKY transcription factor ZmWRKY106 confers drought and heat tolerance in transgenic plants. Int. J. Mol. Sci. 2018, 19, 3046. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Zhu, L.; Bu, Q.Y.; Huq, E. MAX2 affects multiple hormones to promote photomorphogenesis. Mol. Plant 2012, 5, 750–762. [Google Scholar] [CrossRef]
- Ni, J.; Shah, F.A.; Liu, W.; Wang, Q.; Wang, D.; Zhao, W.; Lu, W.; Huang, S.; Fu, S.; Wu, L. Comparative transcriptome analysis reveals the regulatory networks of cytokinin in promoting the floral feminization in the oil plant Sapium sebiferum. BMC Plant Biol. 2018, 18, 96. [Google Scholar] [CrossRef]
- Wang, Y.Q.; Peng, D.; Zhang, L.; Tan, X.F.; Yuan, D.Y.; Liu, X.M.; Zhou, B. Overexpression of SsDGAT2 from Sapium sebiferum (L.) roxb increases seed oleic acid level in Arabidopsis. Plant Mol. Biol. Rep. 2016, 34, 638–648. [Google Scholar]
- Fu, R.; Zhang, Y.; Guo, Y.; Chen, F. Chemical composition, antioxidant and antimicrobial activity of Chinese tallow tree leaves. Ind. Crop Prod. 2015, 76, 374–377. [Google Scholar] [CrossRef]
- Divi, U.K.; Zhou, X.R.; Wang, P.H.; Butlin, J.; Zhang, D.M.; Liu, Q.; Vanhercke, T.; Petrie, J.R.; Talbot, M.; White, R.G.; et al. Deep sequencing of the fruit transcriptome and lipid accumulation in a non-seed tissue of Chinese tallow, a potential biofuel crop. Plant Cell Physiol. 2016, 57, 125–137. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luo, X.Y. Study on herbicidal activities of different organs of Sapium sebiferum. Weed Sci. 2011, 2011, 4. [Google Scholar]
- Zhu, W.; Li, X. Stress resistance of Sapium sebiferum and its forestation at wind gap. Prot. For. Sci. Technol. 2017, 2017, 10. [Google Scholar]
- El-Esawi, M.A.; Alayafi, A.A. Overexpression of rice Rab7 gene improves drought and heat tolerance and increases grain yield in rice (Oryza sativa L.). Genes 2019, 10, 56. [Google Scholar] [CrossRef] [PubMed]
- Polle, A.; Chen, S.L.; Eckert, C.; Harfouche, A. Engineering drought resistance in forest trees. Front. Plant Sci. 2019, 9, 18. [Google Scholar] [CrossRef]
- Zwanenburg, B.; Blanco-Ania, D. Strigolactones: New plant hormones in the spotlight. J. Exp. Bot. 2018, 69, 2205–2218. [Google Scholar] [CrossRef] [PubMed]
- Waters, M.T.; Gutjahr, C.; Bennett, T.; Nelson, D.C. Strigolactone signaling and evolution. Annu. Rev. Plant Biol. 2017, 68, 291–322. [Google Scholar] [CrossRef] [PubMed]
- Mostofa, M.G.; Li, W.; Nguyen, K.H.; Fujita, M.; Lam-Son Phan, T. Strigolactones in plant adaptation to abiotic stresses: An emerging avenue of plant research. Plant Cell Environ. 2018, 41, 2227–2243. [Google Scholar] [CrossRef]
- Stirnberg, P.; Furner, I.J.; Ottoline Leyser, H.M. MAX2 participates in an SCF complex which acts locally at the node to suppress shoot branching. Plant J. 2010, 50, 80–94. [Google Scholar] [CrossRef]
- Stirnberg, P.; Van, D.S.K.; Leyser, H.M. MAX1 and MAX2 control shoot lateral branching in Arabidopsis. Development 2002, 129, 1131–1141. [Google Scholar]
- An, J.-P.; Li, R.; Qu, F.-J.; You, C.-X.; Wang, X.-F.; Hao, Y.-J. Apple F-Box protein MdMAX2 regulates plant photomorphogenesis and stress response. Front. Plant Sci. 2016, 7, 1685. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, H.; Maruyama, K.; Kidokoro, S.; Ito, Y.; Fujita, Y.; Shinozaki, K.; Yamaguchsi-Shinozaki, K.; Nakashima, K. The abiotic stress-responsive NAC-type transcription factor OsNAC5 regulates stress-inducible genes and stress tolerance in rice. Mol. Genet. Genom. 2010, 284, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Kim, J.E.; Park, J.A.; Eom, T.J.; Kim, W.T. Constitutive expression of abiotic stress-inducible hot pepper CaXTH3, which encodes a xyloglucan endotransglucosylase /hydrolase homolog, improves drought and salt tolerance in transgenic Arabidopsis plants. FEBS Lett. 2006, 580, 3136–3144. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Li, Y.J.; Wang, B.; Yu, H.M.; Li, Q.; Hou, B.K. The Arabidopsis UGT87A2, a stress-inducible family 1 glycosyltransferase, is involved in the plant adaptation to abiotic stresses. Physiol. Plant. 2016, 159, 416–432. [Google Scholar] [CrossRef] [PubMed]
- Keunen, E.; Remans, T.; Bohler, S.; Vangronsveld, J.; Cuypers, A. Metal-induced oxidative stress and plant mitochondria. Int. J. Mol. Sci. 2011, 12, 6894–6918. [Google Scholar] [CrossRef]
- Roy, S.J.; Tucker, E.J.; Tester, M. Genetic analysis of abiotic stress tolerance in crops. Curr. Opin. Plant Biol. 2011, 14, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.-C.; Lin, K.-H.; Ho, S.-L.; Chiang, C.-M.; Yang, C.-M. Enhancing the abiotic stress tolerance of plants: From chemical treatment to biotechnological approaches. Physiol. Plant. 2018, 164, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Eryılmaz, F. The relationships between salt stress and anthocyanin content in higher plants. Biotechnol. Biotechnol. Equip. 2006, 20, 47–52. [Google Scholar] [CrossRef]
- Naing, A.H.; Il Park, K.; Ai, T.N.; Chung, M.Y.; Han, J.S.; Kang, Y.W.; Lim, K.B.; Kim, C.K. Overexpression of snapdragon Delila (Del) gene in tobacco enhances anthocyanin accumulation and abiotic stress tolerance. BMC Plant Biol. 2017, 17, 65. [Google Scholar] [CrossRef] [PubMed]
- Lotkowska, M.E.; Tohge, T.; Fernie, A.R.; Xue, G.P.; Balazadeh, S.; Muellerroeber, B. The Arabidopsis transcription factor MYB112 promotes anthocyanin formation during salinity and under high light stress. Plant Physiol. 2015, 169, 1862–1880. [Google Scholar] [CrossRef]
- Castellarin, S.D.; Pfeiffer, A.; Sivilotti, P.; Degan, M.; Peterlunger, E.; Di Gaspero, G. Transcriptional regulation of anthocyanin biosynthesis in ripening fruits of grapevine under seasonal water deficit. Plant Cell Environ. 2007, 30, 1381–1399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, Y.W.; Jeong, H.J.; Lee, S.Y.; Choi, H.G.; Kim, S.H.; Rho, I.R. Chlorophyll fluorescence as a diagnostic tool for abiotic stress tolerance in wild and cultivated strawberry species. Hort. Environ. Biotech. 2014, 55, 280–286. [Google Scholar] [CrossRef]
- Nuccio, M.L.; Rhodest, D.; McNeil, S.D.; Hanson, A.D. Metabolic engineering of plants for osmotic stress resistance. Curr. Opin. Plant Biol. 1999, 2, 128–134. [Google Scholar] [CrossRef]
- Wani, S.H.; Gosal, S.S. Genetic engineering for osmotic stress tolerance in plants—Role of proline. J. Genet. Evol. 2011, 3, 14–25. [Google Scholar]
- Shi, H.; Wang, X.; Tan, D.X.; Reiter, R.J.; Chan, Z. Comparative physiological and proteomic analyses reveal the actions of melatonin in the reduction of oxidative stress in Bermuda grass (Cynodon dactylon (L). Pers.). J. Pineal Res. 2015, 59, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wang, Q.; Shah, F.A.; Liu, W.; Wang, D.; Huang, S.; Fu, S.; Wu, L. Exogenous melatonin confers cadmium tolerance by counterbalancing the hydrogen peroxide homeostasis in wheat seedlings. Molecules 2018, 23, 799. [Google Scholar] [CrossRef] [PubMed]
- Seiler, C.; Rajesh, K.; Reddy, P.S.; Strickert, M.; Rolletschek, H.; Scholz, U.; Wobus, U.; Sreenivasulu, N. ABA biosynthesis and degradation contributing to ABA homeostasis during barley seed development under control and terminal drought-stress conditions. J. Exp. Bot. 2011, 62, 2615–2632. [Google Scholar] [CrossRef] [Green Version]
- Vishwakarma, K.; Upadhyay, N.; Kumar, N.; Yadav, G.; Singh, J.; Mishra, R.K.; Kumar, V.; Verma, R.; Upadhyay, R.G.; Pandey, M. Abscisic acid signaling and abiotic stress tolerance in plants: A review on current knowledge and future prospects. Front. Plant Sci. 2017, 8, 161. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.G.; Kong, C.C.; Xie, Z.H. Role of abscisic acid in strigolactone-induced salt stress tolerance in arbuscular mycorrhizal Sesbania cannabina seedlings. BMC Plant Biol. 2018, 18, 74. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A.; Ni, J.; Chen, J.; Wang, Q.; Liu, W.; Chen, X.; Tang, C.; Fu, S.; Wu, L. Proanthocyanidins in seed coat tegmen and endospermic cap inhibit seed germination in Sapium sebiferum. Peer J. 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Henriques, R.; Lin, S.S.; Niu, Q.W.; Chua, N.H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat. Protoc. 2006, 1, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Adriana, P.; Gaby, T.; Sylvain, A.; Iwona, A.; Simone, M.; Thomas, M.; Karl-Hans, O.; Bernhard, K.U.; Ji-Young, Y.; Liljegren, S.J. Chlorophyll breakdown in senescent Arabidopsis leaves. Characterization of chlorophyll catabolites and of chlorophyll catabolic enzymes involved in the degreening reaction. Plant Physiol. 2005, 139, 52–63. [Google Scholar]
- Cinzia, S.; Alessandra, P.; Elena, L.; Amedeo, A.; Pierdomenico, P. Sucrose-specific induction of the anthocyanin biosynthetic pathway in Arabidopsis. Plant Physiol. 2006, 140, 637–646. [Google Scholar]
- Zhang, K.W.; Xia, X.Y.; Zhang, Y.Y.; Gan, S.S. An ABA-regulated and Golgi-localized protein phosphatase controls water loss during leaf senescence in Arabidopsis. Plant J. 2012, 69, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Yin, G.F.; Zhao, N.J.; Shi, C.Y.; Chen, S.; Qin, Z.S.; Zhang, X.L.; Yan, R.F.; Gan, T.T.; Liu, J.G.; Liu, W.Q. Phytoplankton photosynthetic rate measurement using tunable pulsed light induced fluorescence kinetics. Opt. Express 2018, 26, A293–A300. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef]










© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Ni, J.; Shah, F.; Liu, W.; Wang, D.; Yao, Y.; Hu, H.; Huang, S.; Hou, J.; Fu, S.; et al. Overexpression of the Stress-Inducible SsMAX2 Promotes Drought and Salt Resistance via the Regulation of Redox Homeostasis in Arabidopsis. Int. J. Mol. Sci. 2019, 20, 837. https://doi.org/10.3390/ijms20040837
Wang Q, Ni J, Shah F, Liu W, Wang D, Yao Y, Hu H, Huang S, Hou J, Fu S, et al. Overexpression of the Stress-Inducible SsMAX2 Promotes Drought and Salt Resistance via the Regulation of Redox Homeostasis in Arabidopsis. International Journal of Molecular Sciences. 2019; 20(4):837. https://doi.org/10.3390/ijms20040837
Chicago/Turabian StyleWang, Qiaojian, Jun Ni, Faheem Shah, Wenbo Liu, Dongdong Wang, Yuanyuan Yao, Hao Hu, Shengwei Huang, Jinyan Hou, Songling Fu, and et al. 2019. "Overexpression of the Stress-Inducible SsMAX2 Promotes Drought and Salt Resistance via the Regulation of Redox Homeostasis in Arabidopsis" International Journal of Molecular Sciences 20, no. 4: 837. https://doi.org/10.3390/ijms20040837