Loss of C/EBPδ Exacerbates Radiation-Induced Cognitive Decline in Aged Mice due to Impaired Oxidative Stress Response
Abstract
:1. Introduction
2. Results
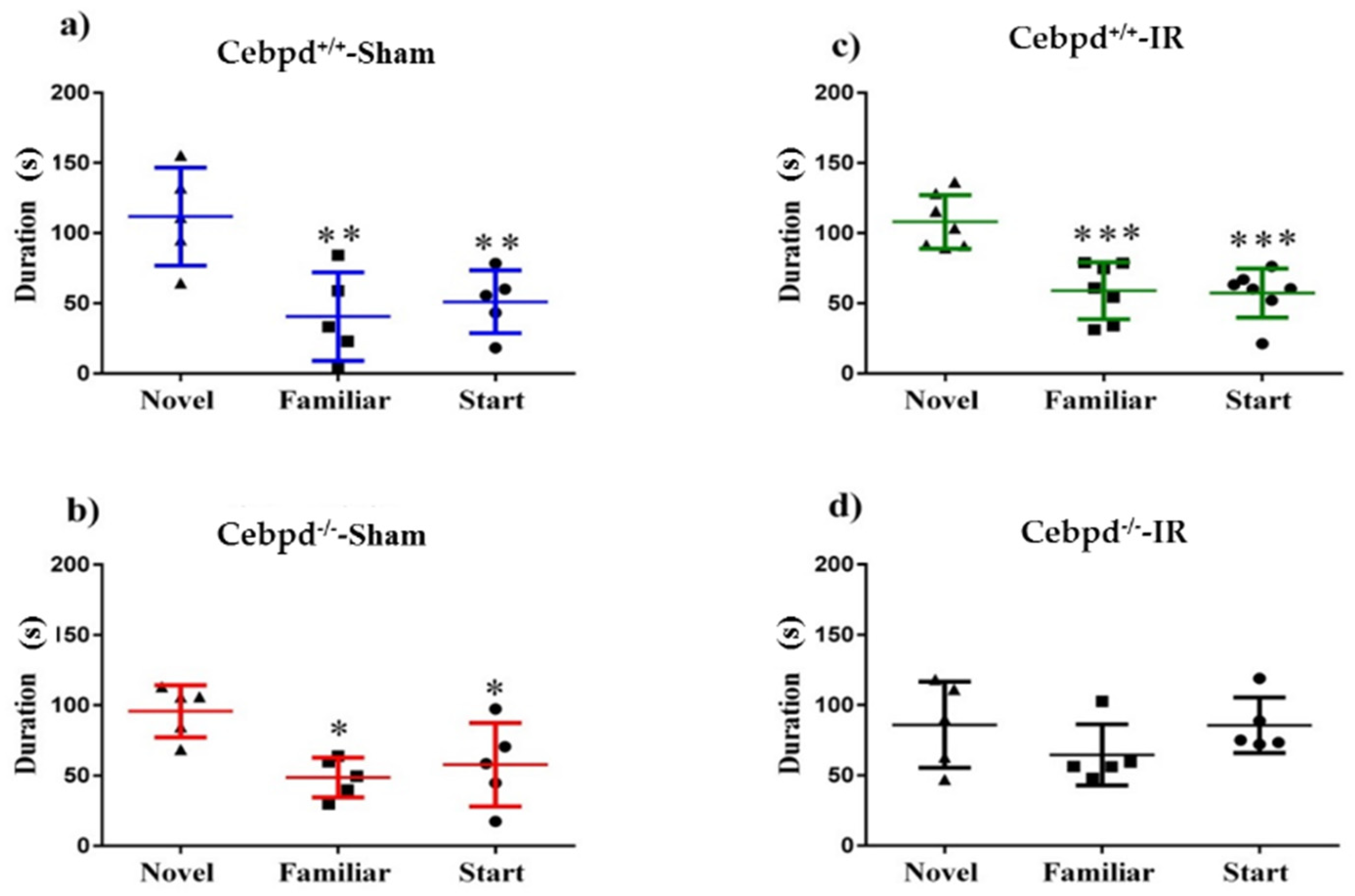
2.1. Irradiation Impairs Short-Term Memory during Y-Maze Test in Cebpd−/− Mice
2.2. Irradiation Impairs Spatial Memory in Aged Cebpd−/− Mice
2.2.1. Dendritic Morphology of Dentate Gyrus Granule Neurons is Significantly Altered in Irradiated Cebpd−/− Mice
2.2.2. Dendritic Morphology of CA1 Apical Neurons is Significantly Altered in Irradiated Cebpd−/− Mice
2.2.3. Dendritic Morphology of CA1 Basal Neurons is Significantly Altered in Irradiated Cebpd−/− Mice
2.3. Irradiated Cebpd−/− Mice Show Impaired Expression of Antioxidant Response Proteins, but no Change in the Expression of Inflammatory Markers in the Hippocampus
3. Discussion and Conclusions
4. Materials and Methods
4.1. Ethics Statement
4.2. Animals
4.3. Irradiation of Mice
4.4. Behavioral Methods

4.4.1. Y-Maze
4.4.2. Novel Object Recognition
4.4.3. Golgi Staining
4.4.4. Dendritic Morphology Quantification
4.4.5. Immunoblotting of Hippocampal Extracts
4.4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hladik, D.; Tapio, S. Effects of ionizing radiation on the mammalian brain. Mutat. Res. Rev. Mutat. Res. 2016, 770, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.; Terradas, M.; Camps, J.; Martin, M.; Tusell, L.; Genesca, A. Aging and radiation: Bad companions. Aging Cell 2015, 14, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Monje, M.L.; Toda, H.; Palmer, T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science 2003, 302, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Voloboueva, L.A.; Giffard, R.G. Inflammation, mitochondria, and the inhibition of adult neurogenesis. J. Neurosci. Res. 2011, 89, 1989–1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, T.-T.; Leu, D.; Zou, Y. Oxidative stress and redox regulation on hippocampal-dependent cognitive functions. Arch. Biochem. Biophys. 2015, 576, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betlazar, C.; Middleton, R.J.; Banati, R.B.; Liu, G.-J. The impact of high and low dose ionising radiation on the central nervous system. Redox Biol. 2016, 9, 144–156. [Google Scholar] [CrossRef] [Green Version]
- Lumniczky, K.; Szatmári, T.; Sáfrány, G. Ionizing Radiation-Induced Immune and Inflammatory Reactions in the Brain. Front. Immunol. 2017, 8, 517. [Google Scholar] [CrossRef]
- Roman, D.D.; Sperduto, P.W. Neuropsychological effects of cranial radiation: Current knowledge and future directions. Int. J. Radiat. Oncol. Biol. Phys. 1995, 31, 983–998. [Google Scholar] [CrossRef]
- Fike, J.R.; Gobbel, G.T. Central nervous system radiation injury in large animal models. In Radiation Injury to the Nervous System; Gutin, P.H., Leibel, S.A., Sheline, G.E., Eds.; Raven Press, Ltd.: New York, NY, USA, 1991; pp. 113–135. [Google Scholar]
- Tofilon, P.J.; Fike, J.R. The radioresponse of the central nervous system: A dynamic process. Radiat. Res. 2000, 153, 357–370, Epub 2000/05/08. [Google Scholar] [CrossRef]
- Abayomi, O.K. Pathogenesis of irradiation-induced cognitive dysfunction. Acta Oncol. 1996, 35, 659–663. [Google Scholar] [CrossRef]
- Son, Y.; Yang, M.; Wang, H.; Moon, C. Hippocampal dysfunctions caused by cranial irradiation: A review of the experimental evidence. Brain Behav. Immun. 2015, 45, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Greene-Schloesser, D.; Moore, E.; Robbins, M.E. Molecular Pathways: Radiation-Induced Cognitive Impairment. Clin. Cancer Res. 2013, 19, 2294–2300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broadbent, N.J.; Squire, L.R.; Clark, R.E. Spatial memory, recognition memory, and the hippocampus. Proc. Natl. Acad. Sci. USA 2004, 101, 14515–14520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casciati, A.; Dobos, K.; Antonelli, F.; Benedek, A.; Kempf, S.J.; Bellés, M.; Balogh, A.; Tanori, M.; Heredia, L.; Atkinson, M.J.; et al. Age-related effects of X-ray irradiation on mouse hippocampus. Oncotarget 2016, 7, 28040–28058. [Google Scholar] [CrossRef] [PubMed]
- Beckervordersandforth, R.; Ebert, B.; Schäffner, I.; Moss, J.; Fiebig, C.; Shin, J.; Moore, D.L.; Ghosh, L.; Trinchero, M.F.; Stockburger, C.; et al. Role of Mitochondrial Metabolism in the Control of Early Lineage Progression and Aging Phenotypes in Adult Hippocampal Neurogenesis. Neuron 2017, 93, 560.e6–573.e6. [Google Scholar] [CrossRef]
- Knobloch, M.; Jessberger, S. Metabolism and neurogenesis. Curr. Opin. Neurobiol. 2017, 42, 45–52. [Google Scholar] [CrossRef]
- Wu, P.H.; Coultrap, S.; Pinnix, C.; Davies, K.D.; Tailor, R.; Ang, K.K.; Browning, M.D.; Grosshans, D.R. Radiation induces acute alterations in neuronal function. PLoS ONE 2012, 7, e37677. [Google Scholar] [CrossRef]
- Kumar, M.; Haridas, S.; Trivedi, R.; Khushu, S.; Manda, K. Early cognitive changes due to whole body gamma-irradiation: A behavioral and diffusion tensor imaging study in mice. Exp. Neurol. 2013, 248, 360–368. [Google Scholar] [CrossRef]
- Chakraborti, A.; Allen, A.; Allen, B.; Rosi, S.; Fike, J.R. Cranial Irradiation Alters Dendritic Spine Density and Morphology in the Hippocampus. PLoS ONE 2012, 7, e40844. [Google Scholar] [CrossRef]
- Parihar, V.K.; Limoli, C.L. Cranial irradiation compromises neuronal architecture in the hippocampus. Proc. Natl. Acad. Sci. USA 2013, 110, 12822–12827. [Google Scholar] [CrossRef] [Green Version]
- Parihar, V.K.; Pasha, J.; Tran, K.K.; Craver, B.M.; Acharya, M.M.; Limoli, C.L. Persistent changes in neuronal structure and synaptic plasticity caused by proton irradiation. Brain Struct. Funct. 2015, 220, 1161–1171. [Google Scholar] [CrossRef]
- Kiffer, F.; Howe, A.K.; Carr, H.; Wang, J.; Alexander, T.; Anderson, J.E.; Groves, T.; Seawright, J.W.; Sridharan, V.; Carter, G.; et al. Late effects of (1)H irradiation on hippocampal physiology. Life Sci. Space Res. 2018, 17, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Shuryak, I.; Sachs, R.K.; Brenner, D.J. Cancer risks after radiation exposure in middle age. J. Natl. Cancer Inst. 2010, 102, 1628–1636. [Google Scholar] [CrossRef] [PubMed]
- Ramji, D.P.; Foka, P. CCAAT/enhancer-binding proteins: Structure, function and regulation. Biochem. J. 2002, 365, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, T.R.; Sharan, S.; Wang, J.; Pawar, S.A.; Cantwell, C.A.; Johnson, P.F.; Morrison, D.K.; Wang, J.M.; Sterneck, E. Identification of a Src tyrosine kinase/SIAH2 E3 ubiquitin ligase pathway that regulates C/EBPdelta expression and contributes to transformation of breast tumor cells. Mol. Cell Biol. 2012, 32, 320–332. [Google Scholar] [CrossRef]
- Pawar, S.A.; Sarkar, T.R.; Balamurugan, K.; Sharan, S.; Wang, J.; Zhang, Y.; Dowdy, S.F.; Huang, A.M.; Sterneck, E. C/EBP delta targets cyclin D1 for proteasome-mediated degradation via induction of CDC27/APC3 expression. Proc. Natl. Acad. Sci. USA 2010, 107, 9210–9215. [Google Scholar] [CrossRef] [PubMed]
- Thangaraju, M.; Rudelius, M.; Bierie, B.; Raffeld, M.; Sharan, S.; Hennighausen, L.; Huang, A.M.; Sterneck, E. C/EBPdelta is a crucial regulator of pro-apoptotic gene expression during mammary gland involution. Development 2005, 132, 4675–4685. [Google Scholar] [CrossRef]
- Huang, A.M.; Montagna, C.; Sharan, S.; Ni, Y.; Ried, T.; Sterneck, E. Loss of CCAAT/enhancer binding protein delta promotes chromosomal instability. Oncogene 2004, 23, 1549–1557. [Google Scholar] [CrossRef]
- Hour, T.C.; Lai, Y.L.; Kuan, C.I.; Chou, C.K.; Wang, J.M.; Tu, H.Y.; Hu, H.T.; Lin, C.S.; Wu, W.J.; Pu, Y.S.; et al. Transcriptional up-regulation of SOD1 by CEBPD: A potential target for cisplatin resistant human urothelial carcinoma cells. Biochem. Pharmacol. 2010, 80, 325–334. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Sarkar, T.R.; Zhou, M.; Sharan, S.; Ritt, D.A.; Veenstra, T.D.; Morrison, D.K.; Huang, A.M.; Sterneck, E. CCAAT/enhancer binding protein delta (C/EBPdelta, CEBPD)-mediated nuclear import of FANCD2 by IPO4 augments cellular response to DNA damage. Proc. Natl. Acad. Sci. USA 2010, 107, 16131–16136. [Google Scholar] [CrossRef]
- Cardinaux, J.R.; Allaman, I.; Magistretti, P.J. Pro-inflammatory cytokines induce the transcription factors C/EBPbeta and C/EBPdelta in astrocytes. Glia 2000, 29, 91–97. [Google Scholar] [CrossRef]
- Li, R.; Strohmeyer, R.; Liang, Z.; Lue, L.F.; Rogers, J. CCAAT/enhancer binding protein delta (C/EBPdelta) expression and elevation in Alzheimer’s disease. Neurobiol. Aging 2004, 25, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Pawar, S.A.; Shao, L.; Chang, J.; Wang, W.; Pathak, R.; Zhu, X.; Wang, J.; Hendrickson, H.; Boerma, M.; Sterneck, E.; et al. C/EBP delta Deficiency Sensitizes Mice to Ionizing Radiation-Induced Hematopoietic and Intestinal Injury. PLoS ONE 2014, 9, e94967. [Google Scholar] [CrossRef]
- Banerjee, S.; Aykin-Burns, N.; Krager, K.J.; Shah, S.K.; Melnyk, S.B.; Hauer-Jensen, M.; Pawar, S.A. Loss of C/EBPδ enhances IR-induced cell death by promoting oxidative stress and mitochondrial dysfunction. Free Radic. Biol. Med. 2016, 99, 296–307. [Google Scholar] [CrossRef] [Green Version]
- Dellu, F.; Fauchey, V.; Le Moal, M.; Simon, H. Extension of a new two-trial memory task in the rat: Influence of environmental context on recognition processes. Neurobiol. Learn. Mem. 1997, 67, 112–120. [Google Scholar] [CrossRef]
- Ran, Y.; Yan, B.; Li, Z.; Ding, Y.; Shi, Y.; Le, G. Dityrosine administration induces novel object recognition deficits in young adulthood mice. Physiol. Behav. 2016, 164, 292–299. [Google Scholar] [CrossRef]
- Cohen, S.J.; Stackman, R.W., Jr. Assessing rodent hippocampal involvement in the novel object recognition task. A review. Behav. Brain Res. 2015, 285, 105–117. [Google Scholar] [CrossRef] [PubMed]
- Burke, S.N.; Wallace, J.L.; Nematollahi, S.; Uprety, A.R.; Barnes, C.A. Pattern separation deficits may contribute to age-associated recognition impairments. Behav. Neurosci. 2010, 124, 559–573. [Google Scholar] [CrossRef] [PubMed]
- Hofer, T.; Duale, N.; Muusse, M.; Eide, D.M.; Dahl, H.; Boix, F.; Andersen, J.M.; Olsen, A.K.; Myhre, O. Restoration of Cognitive Performance in Mice Carrying a Deficient Allele of 8-Oxoguanine DNA Glycosylase by X-ray Irradiation. Neurotox. Res. 2018, 33, 824–836. [Google Scholar] [CrossRef] [PubMed]
- Mizumatsu, S.; Monje, M.L.; Morhardt, D.R.; Rola, R.; Palmer, T.D.; Fike, J.R. Extreme sensitivity of adult neurogenesis to low doses of X-irradiation. Cancer Res. 2003, 63, 4021–4027. [Google Scholar] [PubMed]
- Romanko, M.J.; Rola, R.; Fike, J.R.; Szele, F.G.; Dizon, M.L.; Felling, R.J.; Brazel, C.Y.; Levison, S.W. Roles of the mammalian subventricular zone in cell replacement after brain injury. Prog. Neurobiol. 2004, 74, 77–99. [Google Scholar] [CrossRef] [PubMed]
- Sterneck, E.; Paylor, R.; Jackson-Lewis, V.; Libbey, M.; Przedborski, S.; Tessarollo, L.; Crawley, J.N.; Johnson, P.F. Selectively enhanced contextual fear conditioning in mice lacking the transcriptional regulator CCAAT/enhancer binding protein delta. Proc. Natl. Acad. Sci. USA 1998, 95, 10908–10913. [Google Scholar] [CrossRef] [PubMed]
- Sharman, E.H.; Bondy, S.C.; Sharman, K.G.; Lahiri, D.; Cotman, C.W.; Perreau, V.M. Effects of melatonin and age on gene expression in mouse CNS using microarray analysis. Neurochem. Int. 2007, 50, 336–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarnyai, Z.; Sibille, E.L.; Pavlides, C.; Fenster, R.J.; McEwen, B.S.; Toth, M. Impaired hippocampal-dependent learning and functional abnormalities in the hippocampus in mice lacking serotonin(1A) receptors. Proc. Natl. Acad. Sci. USA 2000, 97, 14731–14736. [Google Scholar] [CrossRef] [PubMed]
- Gruber, M.J.; Gelman, B.D.; Ranganath, C. States of curiosity modulate hippocampus-dependent learning via the dopaminergic circuit. Neuron 2014, 84, 486–496. [Google Scholar] [CrossRef]
- Kiffer, F.; Alexander, T.; Anderson, J.E.; Groves, T.; Wang, J.; Sridharan, V.; Boerma, M.; Allen, A.R. Late Effects of (16)O-Particle Radiation on Female Social and Cognitive Behavior and Hippocampal Physiology. Radiat. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.C.; Lykken, C.; Tye, L.D.; Wickelgren, J.G.; Frank, L.M. Hippocampal output area CA1 broadcasts a generalized novelty signal during an object-place recognition task. Hippocampus 2014, 24, 773–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepan, J.; Dine, J.; Eder, M. Functional optical probing of the hippocampal trisynaptic circuit in vitro: Network dynamics, filter properties, and polysynaptic induction of CA1 LTP. Front. Neurosci. 2015, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, V.A.; Firestein, B.L. The dendritic tree and brain disorders. Mol. Cell. Neurosci. 2012, 50, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Nimchinsky, E.A.; Sabatini, B.L.; Svoboda, K. Structure and function of dendritic spines. Annu. Rev. Physiol. 2002, 64, 313–353. [Google Scholar] [CrossRef] [PubMed]
- Segal, M. Dendritic spines and long-term plasticity. Nat. Rev. Neurosci. 2005, 6, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Yuste, R.; Bonhoeffer, T. Morphological changes in dendritic spines associated with long-term synaptic plasticity. Annu. Rev. Neurosci. 2001, 24, 1071–1089. [Google Scholar] [CrossRef] [PubMed]
- Raber, J.; Allen, A.R.; Sharma, S.; Allen, B.; Rosi, S.; Olsen, R.H.; Davis, M.J.; Eiwaz, M.; Fike, J.R.; Nelson, G.A. Effects of Proton and Combined Proton and (56)Fe Radiation on the Hippocampus. Radiat. Res. 2016, 185, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Sydow, A.; Hochgrafe, K.; Konen, S.; Cadinu, D.; Matenia, D.; Petrova, O.; Joseph, M.; Dennissen, F.J.; Mandelkow, E.M. Age-dependent neuroinflammation and cognitive decline in a novel Ala152Thr-Tau transgenic mouse model of PSP and AD. Acta Neuropathol. Commun. 2016, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.B. Ionizing radiation and aging: Rejuvenating an old idea. Aging 2009, 1, 887–902. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Wilhelm, I.; Nyul-Toth, A.; Kozma, M.; Farkas, A.E.; Krizbai, I.A. Role of pattern recognition receptors of the neurovascular unit in inflamm-aging. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H1000–H1012. [Google Scholar] [CrossRef]
- Banerjee, S.F.Q.; Shah, S.K.; Ponnappan, U.; Melnyk, S.B.; Hauer-Jensen, N.; Pawar, S.A. Role of TLR4 in the pathogenesis of radiation-induced intestinal injury in C/EBPδ-knockout mice. In SHOCK; Lippincott Williams & Wilkins: Fort Lauderdale, FL, USA, 2017. [Google Scholar]
- Robello, E.; Bonetto, J.G.; Puntarulo, S. Cellular Oxidative/Antioxidant Balance in gamma-Irradiated Brain: An Update. Mini-Rev. Med. Chem. 2016, 16, 937–946. [Google Scholar] [CrossRef]
- Fishman, K.; Baure, J.; Zou, Y.; Huang, T.-T.; Andres-Mach, M.; Rola, R.; Suarez, T.; Acharya, M.; Limoli, C.L.; Lamborn, K.R.; et al. Radiation-induced reductions in neurogenesis are ameliorated in mice deficient in CuZnSOD or MnSOD. Free Radic. Biol. Med. 2009, 47, 1459–1467. [Google Scholar] [CrossRef] [Green Version]
- Dai, D.F.; Chiao, Y.A.; Martin, G.M.; Marcinek, D.J.; Basisty, N.; Quarles, E.K.; Rabinovitch, P.S. Mitochondrial-Targeted Catalase: Extended Longevity and the Roles in Various Disease Models. Prog. Mol. Biol. Transl. Sci. 2017, 146, 203–241. [Google Scholar]
- Parihar, V.K.; Allen, B.D.; Tran, K.K.; Chmielewski, N.N.; Craver, B.M.; Martirosian, V.; Morganti, J.M.; Rosi, S.; Vlkolinsky, R.; Acharya, M.M.; et al. Targeted overexpression of mitochondrial catalase prevents radiation-induced cognitive dysfunction. Antioxid. Redox Signal. 2015, 22, 78–91. [Google Scholar] [CrossRef]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.H.; Zheng, G.; Wang, T.; Du, K.J.; Han, X.; Luo, W.J.; Shen, X.F.; Chen, J.Y. Low-level Gestational Lead Exposure Alters Dendritic Spine Plasticity in the Hippocampus and Reduces Learning and Memory in Rats. Sci. Rep. 2018, 8, 3533. [Google Scholar] [CrossRef] [PubMed]
- Mikolaenko, I.; Rao, L.M.; Roberts, R.C.; Kolb, B.; Jinnah, H.A. A Golgi study of neuronal architecture in a genetic mouse model for Lesch-Nyhan disease. Neurobiol. Dis. 2005, 20, 479–490. [Google Scholar] [CrossRef] [PubMed]
- Groves, T.R.; Wang, J.; Boerma, M.; Allen, A.R. Assessment of Hippocampal Dendritic Complexity in Aged Mice Using the Golgi-Cox Method. J. Vis. Exp. JoVE 2017. [Google Scholar] [CrossRef] [PubMed]
- Sholl, D.A. Dendritic organization in the neurons of the visual and motor cortices of the cat. J. Anat. 1953, 87, 387–406. [Google Scholar] [PubMed]
- Morley, B.J.; Mervis, R.F. Dendritic spine alterations in the hippocampus and parietal cortex of alpha7 nicotinic acetylcholine receptor knockout mice. Neuroscience 2013, 233, 54–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]






| Cell Type and Measurements | Cebpd+/+-Sham (mean ± SEM) | Cebpd−/−-Sham (mean ± SEM) | Cebpd+/+-IR (mean ± SEM) | Cebpd−/−-IR (mean ± SEM) |
|---|---|---|---|---|
| DG | ||||
| Total Dendritic Length | 1224 ± 77.77 | 1313 ± 86.06 | 987.4 ± 144.5 | 587.7 ± 36.45 |
| Total Number of Branch Points | 8.92 ± 0.57 | 8.08 ± 0.57 | 5.8 ± 0.72 | 3.8 ± 0.21 |
| Complexity | 30796 ± 6401 | 38947 ± 3424 | 15985 ± 5436 | 7050 ± 1350 |
| Cell Type and Measurements | Cebpd+/+-Sham (mean ± SEM) | Cebpd−/−-Sham (mean ± SEM) | Cebpd+/+-IR (mean ± SEM) | Cebpd−/−-IR (mean ± SEM) |
|---|---|---|---|---|
| CA1 Apical | ||||
| Total Dendritic Length | 839.8 ± 82.52 | 677.6 ± 74.02 | 519.1 ± 43.86 | 414.6 ± 32.46 |
| Total Number of Branch Points | 8.00 ± 0.96 | 7.13 ± 0.58 | 5.3 ± 0.39 | 4.05 ± 0.45 |
| Complexity | 43356 ± 9937 | 29426 ± 6042 | 17154 ± 1491 | 8699 ± 1649 |
| CA1 Basal Measurements | ||||
| Total Dendritic Length | 1301 ± 173.34 | 823.01 + 52.91 | 788.8 ± 22.93 | 472.6 ± 28.61 |
| Total Number of Branch Points | 9.90 ± 1.35 | 7.00 + 0.64 | 6.95 ± 0.49 | 3.85 ± 0.52 |
| Complexity | 20722 ± 5219 | 8366 + 1556 | 9275 + 1671 | 3749 ± 540.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banerjee, S.; Alexander, T.; Majumdar, D.; Groves, T.; Kiffer, F.; Wang, J.; Gorantla, A.; Allen, A.R.; Pawar, S.A. Loss of C/EBPδ Exacerbates Radiation-Induced Cognitive Decline in Aged Mice due to Impaired Oxidative Stress Response. Int. J. Mol. Sci. 2019, 20, 885. https://doi.org/10.3390/ijms20040885
Banerjee S, Alexander T, Majumdar D, Groves T, Kiffer F, Wang J, Gorantla A, Allen AR, Pawar SA. Loss of C/EBPδ Exacerbates Radiation-Induced Cognitive Decline in Aged Mice due to Impaired Oxidative Stress Response. International Journal of Molecular Sciences. 2019; 20(4):885. https://doi.org/10.3390/ijms20040885
Chicago/Turabian StyleBanerjee, Sudip, Tyler Alexander, Debajyoti Majumdar, Thomas Groves, Frederico Kiffer, Jing Wang, Akshita Gorantla, Antiño R. Allen, and Snehalata A. Pawar. 2019. "Loss of C/EBPδ Exacerbates Radiation-Induced Cognitive Decline in Aged Mice due to Impaired Oxidative Stress Response" International Journal of Molecular Sciences 20, no. 4: 885. https://doi.org/10.3390/ijms20040885





