Mechanisms of PKC-Mediated Enhancement of HIF-1α Activity and its Inhibition by Vitamin K2 in Hepatocellular Carcinoma Cells
Abstract
:1. Introduction
2. Results
2.1. PKC-δ—But not PKC-α or PKC-ε—Is Involved in the Activation of HIF-1α and Inhibition by VK2 Under Both Normoxic and Hypoxic Conditions
2.2. PKC-δ is Involved in the HIF-1α Protein Expression, and VK2 Suppresses the TPA-Induced HIF-1α Protein Expression
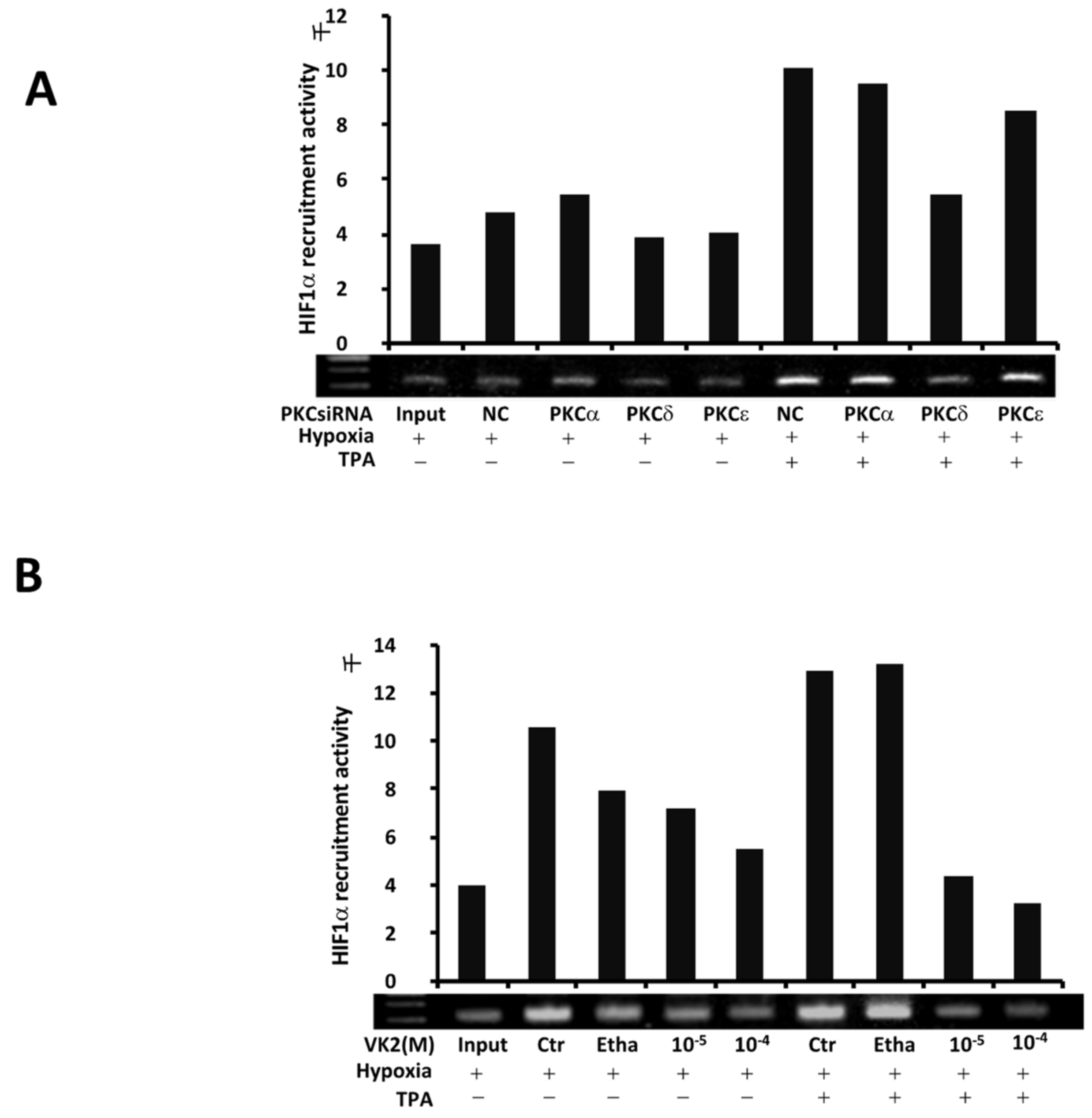
2.3. PKC-δ Regulates the TPA-Induced Recruitment of HIF-1α, and VK2 Abrogates the Induction of HIF-1α Recruitment by TPA in Huh7 Cells
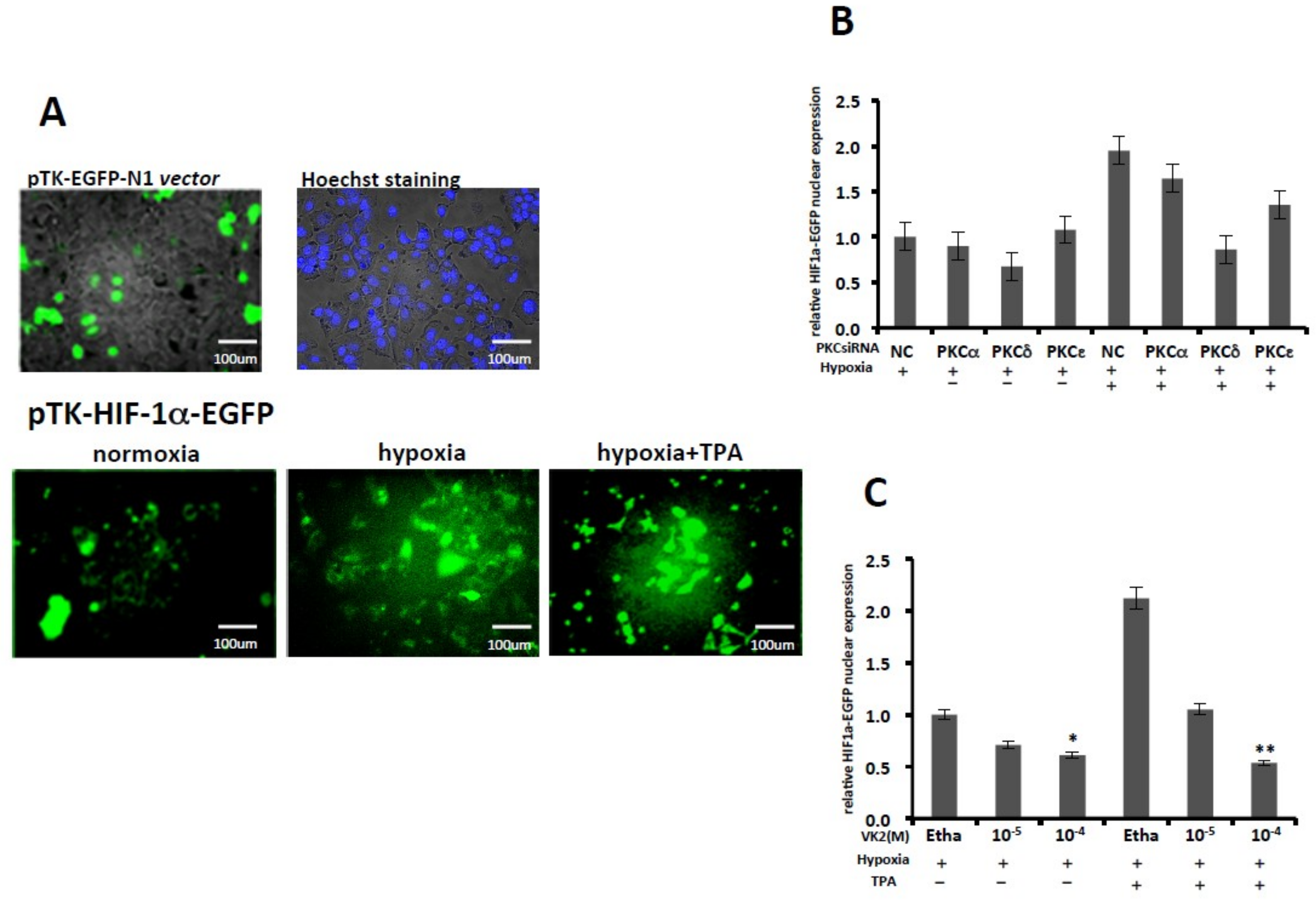
2.4. PKC-δ Knockdown and VK2 Suppresses the Nuclear Translocation of HIF-1α Stimulated by TPA
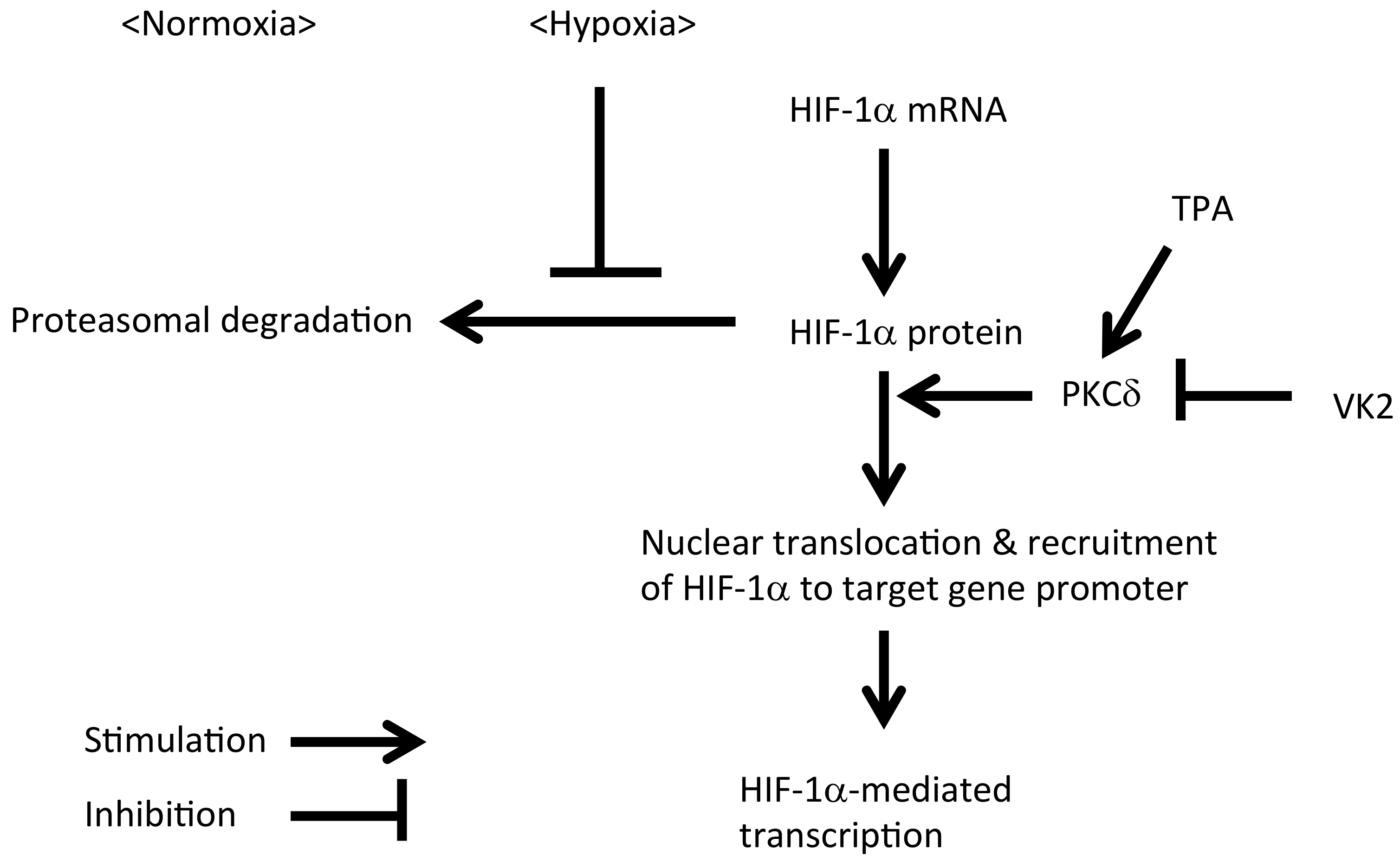
3. Discussion
4. Materials and Methods
4.1. Cell Culture, Hypoxic Conditions and Reagents
4.2. Luciferase Reporter Gene Assay
4.3. Real-Time qRT-PCR, Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction
4.4. Western Blotting
4.5. Chromatin Immunoprecipitation (ChIP) Assay
4.6. Fluorescence Microscope Assay
4.7. SiRNA-Mediated Knockdown of PKC
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HIF-1α | Hypoxia-inducible factor-1 alpha |
| PKC | Protein kinase C |
| TPA | 12-O-tetradecanoylphorbor-13-acetate |
| TF | Transcription factor |
| VK2 | Vitamin K2 |
| HRE | Hypoxia responsive element |
| VEGF | Vascular endothelial growth factor |
| EPO | Erythropoietin |
| VHL | Von Hippel-Lindau |
| NF-κB | Nuclear factor kappa B |
| FBS | Fetal bovine serum |
| DMEM | Dulbecco’s modified Eagle`s medium |
| DMSO | Dimethyl sulfoxide |
| RT-PCR | Reverse transcription polymerase chain reaction |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| ChIP assay | Chromatin immunoprecipitation assay |
| HCC | Hepatocellular carcinoma |
References
- Li, Y.; Ye, D. Cancer therapy by targeting hypoxia-inducible factor-1. Curr. Cancer Drug Targets 2010, 10, 782–796. [Google Scholar] [CrossRef] [PubMed]
- Nath, B.; Szabo, G. Hypoxia and hypoxia inducible factors: Diverse roles in liver diseases. Hepatology 2012, 55, 622–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semenza, G.L.; Wang, G.L. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at site required for transcriptional activation. Mol. Cell. Biol. 1992, 12, 5447–5454. [Google Scholar] [CrossRef]
- Forsythe, J.A.; Jian, B.H.; Iyer, N.V.; Agani, F.; Leung, S.W.; Koos, R.D.; Semenza, G.L. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol. Cell. Biol. 1996, 16, 4603–4613. [Google Scholar] [CrossRef]
- Maxwell, P.H.; Wiesener, M.S.; Chang, G.W.; Clifford, S.C.; Vaux, E.C.; Cockman, M.E.; Wykoff, C.C.; Pugh, C.W.; Maher, E.R.; Ratcliffe, P.J. The tumor suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 1999, 399, 271–275. [Google Scholar] [CrossRef]
- Krieg, M.; Haas, R.; Brauch, H.; Acker, T.; Famme, I.; Plate, K.H. Up-regulation of hypoxia-inducible factors HIF-1alpha and HIF-2alpha under normoxic conditions in renal carcinoma cells by von Hippel-Lindu tumor suppressor gene loss of function. Oncogene 2000, 19, 5435–5443. [Google Scholar] [CrossRef]
- Yoshida, T.; Hashimura, M.; Matsumoto, T.; Tazo, Y.; Inoue, H.; Kuwata, T.; Saegusa, M. Transcriptional upregulation of HIF-1 alpha by NF-kappaB/po65 and its association with b-catenin/p300 complexes in endometrial carcinoma cells. Lab. Investig. 2013, 93, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Gorlach, A.; Bonello, S. The cross-talk between NF-kappaB and HIF-1: Further evidence for a significant liaison. Biochem. J. 2008, 412, e17–e19. [Google Scholar] [CrossRef] [PubMed]
- Datta, K.; Li, J.; Bhattacharya, R.; Gasparian, L.; Wang, E.; Mukhopadhyay, D. Protein kinase C zeta transactivates hypoxia-inducible factor alpha by promoting its association with p300 in renal cancer. Cancer Res. 2004, 64, 456–462. [Google Scholar] [CrossRef]
- Page, E.L.; Robitaille, G.A.; Pouyssegur, J.; Richard, D.E. Induction of hypoxia-inducible factor-1alpha by transcriptional and translational mechanisms. J. Biol. Chem. 2002, 277, 48403–48409. [Google Scholar] [CrossRef]
- Lee, J.W.; Park, J.A.; Kim, S.H.; Seo, J.H.; Lim, K.J.; Jeong, J.W.; Jeong, C.H.; Chun, K.H.; Lee, S.K.; Kuwon, Y.G.; et al. Protein kinase C-delta regulates the stability of hyapoxia-inducible factor-1 under hypoxia. Cancer Sci. 2007, 98, 1476–1481. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Jin, G.Y.; Li, L.C.; Yan, G.H. Inhibition of protein kinase C delta attenuates allergic airway inflammation through suppression of PI3K/Akt/mTOR/HIF-1 alpha/VEGF pathway. PLoS ONE 2013, 8, e81773. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Na, Y.R.; Kim, S.Y.; Yang, E.G. Protein Kinase C Isoforms Differentially Regulate Hypoxia-Inducible Factor-1α Accumulation in Cancer Cells. J. Cell. Biochem. 2016, 117, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Di Giulio, C.; Rapino, M.; Zingariello, M.; Antonucci, A.; Cataldi, A. PKC alpha-mediated CREB activation is oxygen and age-dependent in rat myocardial tissue. Histochem. Cell Biol. 2007, 127, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Zara, S.; Macchi, V.; De Caro, R.; Rapino, M.; Cataldi, A.; Porzionato, A. pPKCalpha mediated-HIF-1 alpha activation related to morphological modifications occurring in neonatal myocardial tissue in response to severe and mild hypoxia. Eur. J. Histochem. 2012, 56, e2. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Matsuhashi, S.; Hamajima, H.; Iwane, S.; Takahashi, H.; Eguchi, Y.; Mizuta, T.; Fujimoto, K.; Kuroda, S.; Ozaki, I. The role of PKC isoforms in the inhibition of NF-kappaB activation by vitamin K2 in human hepatocellular carcinoma cells. J. Nutr. Biochem. 2012, 23, 1668–1675. [Google Scholar] [CrossRef] [PubMed]
- Görlach, A.; Diebold, I.; Schini-Kerth, V.B.; Berchner-Pfannschmidt, U.; Roth, U.; Brandes, R.P.; Kietzmann, T.; Busse, R. Thrombin activates the hypoxia-inducible factor-1 signaling pathway in vascular smooth muscle cells: Role of the p22(phox)-containing NADPH oxidase. Circ. Res. 2001, 89, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Isaacs, J.S.; Lee, S.; Trepel, J.; Liu, Z.G.; Neckers, L. Hypoxia-inducible factor induction by tumour necrosis factor in normoxic cells requires receptor-interacting protein-dependent nuclear factor kappa B activation. Biochem. J. 2003, 370 Pt 3, 1011–1017. [Google Scholar] [CrossRef]
- Frede, S.; Stockmann, C.; Freitag, P.; Fandrey, J. Bacterial lipopolysaccharide induces HIF-1 activation in human monocytes via p44/42 MAPK and NF-kappaB. Biochem. J. 2006, 396, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Zelzer, E.; Levy, Y.; Kahana, C.; Shilo, B.Z.; Rubinstein, M.; Cohen, B. Insulin induces transcription of target genes through the hypoxia-inducible factor HIF-1alpha/ARNT. EMBO J. 1998, 17, 5085–5094. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, R.; Hirota, K.; Fan, F.; Jung, Y.D.; Ellis, L.M.; Semenza, G.L. Insulin-like growth factor 1 induces hypoxia-inducible factor 1-mediated vascular endothelial growth factor expression, which is dependent on MAP kinase and phosphatidylinositol 3-kinase signaling in colon cancer cells. J. Biol. Chem. 2002, 277, 38205–38211. [Google Scholar] [CrossRef] [PubMed]
- Tacchini, L.; De Ponti, C.; Matteucci, E.; Follis, R.; Desiderio, M.A. Hepatocyte growth factor-activated NF-kappaB regulates HIF-1 activity and ODC expression, implicated in survival, differently in different carcinoma cell lines. Carcinogenesis 2004, 25, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Bonello, S.; Zähringer, C.; BelAiba, R.S.; Djordjevic, T.; Hess, J.; Michiels, C.; Kietzmann, T.; Görlach, A. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Van Uden, P.; Kenneth, N.S.; Rocha, S. Regulation of hypoxia-inducible factor-1alpha by NF-kappaB. Biochem. J. 2008, 412, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Ohsaki, Y.; Shirakawa, H.; Miura, A.; Giriwono, P.E.; Sato, S.; Ohashi, A.; Iribe, M.; Goto, T.; Komai, M. Vitamin K suppresses the lipopolysaccharide-induced expression of inflammatory cytokines in cultured macrophage-like cells via the inhibition of the activation of nuclear factor κB through the repression of IKKα/β phosphorylation. J. Nutr. Biochem. 2010, 21, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Dihingia, A.; Ozah, D.; Baruah, P.K.; Kalita, J.; Manna, P. Prophylactic role of vitamin K supplementation on vascular inflammation in type 2 diabetes by regulating the NF-κB/Nrf2 pathway via activating Gla proteins. Food Funct. 2018, 9, 450–462. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M. Systemic Therapy for Hepatocellular Carcinoma: Latest Advances. Cancers (Basel) 2018, 10, 412. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.P.; Ho, R.L.; Chen, G.G.; Lai, P.B. Sorafenib inhibits hypoxia-inducible factor-1α synthesis: Implications for antiangiogenic activity in hepatocellular carcinoma. Clin. Cancer Res. 2012, 18, 5662–5671. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Zheng, T.; Song, R.; Wang, J.; Yin, D.; Wang, L.; Liu, H.; Tian, L.; Fang, X.; Meng, X.; et al. Hypoxia-mediated sorafenib resistance can be overcome by EF24 through Von Hippel-Lindau tumor suppressor-dependent HIF-1α inhibition in hepatocellular carcinoma. Hepatology 2013, 57, 1847–1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, D.; Wu, J. Hypoxia inducible factor in hepatocellular carcinoma: A therapeutic target. World J. Gastroenterol. 2015, 21, 12171–12178. [Google Scholar] [CrossRef] [PubMed]
- Semenza, G.L. HIF-1 and tumor progression: Pathophysiology and threrapeutics. Trends Mol. Med. 2002, 8 (Suppl. 4), S62–S67. [Google Scholar] [CrossRef]
- Wu, J.M.; Sheng, H.; Saxena, R.; Skill, N.J.; Bhat-Nakshatri, P.; Yu, M.; Nakshatri, H.; Maluccio, M.A. NF-kappaB inhibition in human hepatocellular carcinoma and its potential as adjunct to sorafenib based therapy. Cancer Lett. 2009, 278, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Lo, J.; Lau, E.Y.; Ching, R.H.; Cheng, B.Y.; Ma, M.K.; Ng, I.O.; Lee, T.K. Nuclear factor kappa B-mediated CD47 up-regulation promotes sorafenib resistance and its blockade synergizes the effect of sorafenib in hepatocellular carcinoma in mice. Hepatology 2015, 62, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Liebman, H.A.; Furie, B.C.; Tong, M.J.; Blanchard, R.A.; Lo, K.J.; Lee, S.D.; Coleman, M.S.; Furie, B. Des-gamma-carboxy (abnormal) prothrombin as a serum marker of primary hepatocellular carcinoma. N. Engl. J. Med. 1984, 310, 1427–1431. [Google Scholar] [CrossRef] [PubMed]
- Koike, Y.; Shiratori, Y.; Sato, S.; Obi, S.; Teratani, T.; Imamura, M.; Yoshida, H.; Shiina, S.; Omata, M. Des-r-carboxy prothrombin as a useful predisposing factor for the development of portal venous invasion in patients with hepatocellular carcinoma. A prospective analysis of 227 patients. Cancer 2001, 91, 561–569. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Dietrich, M.; Akman, S.; Block, J.B. Vitamin K3 inhibition of malignant murine cell growth and human tumor colony formation. Cancer Treat. Rep. 1985, 69, 527–532. [Google Scholar] [PubMed]
- Wu, F.Y.; Liao, W.C.; Chang, H.M. Comparison of antitumor activity of vitamins K1, K2 and K3 on human tumor cells by two (MTT and SRB) cell viability assays. Life Sci. 1993, 52, 1797–1804. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, M.; Finn, F.; Carrj, B.I. The growth inhibitory effects of vitamins K and their actions on gene expression. Hepatology 1995, 22, 876–882. [Google Scholar] [PubMed]
- Habu, D.; Shiomi, S.; Tamori, A.; Takeda, T.; Tanaka, T.; Kubo, S.; Nishiguchi, S. Role of vitamin K2 in the development of hepatocellular carcinoma in women with viral cirrhosis of the liver. JAMA 2004, 292, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, T.; Ozaki, I.; Eguchi, Y.; Yasutake, T.; Kawazoe, S.; Fujimoto, K.; Yamamoto, K. The effect of menatetrenone, a vitamin K2 analog, on disease recurrence and survival in patients with hepatocellular carcinoma after curative treatment: A pilot study. Cancer 2006, 106, 867–872. [Google Scholar] [CrossRef] [PubMed]
- Kakizaki, S.; Soharaj, N.; Sato, K.; Suzuki, H.; Yanagisawa, M.; Nakajima, H.; Takagi, H.; Naganuma, A.; Otsuka, T.; Takahashi, H.; et al. Preventive effects of vitamin K on recurrent disease in patients with hepatocellular carcinoma arising from hepatitis C viral infection. J. Gastroenterol. Hepatol. 2007, 22, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.H.; Mo, X.S.; Xiang, B.D.; Yuan, W.P.; Jiang, J.F.; Xie, G.S.; Li, L.Q. Postoperative use of the chemopreventive vitamin K2 analog in patients with hepatocellular carcinoma. PLoS ONE 2013, 8, e58082. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Shiratori, Y.; Kudo, M.; Shiina, S.; Mizuta, T.; Kojiro, M.; Yamamoto, K.; Koike, Y.; Saito, K.; Koyanagi, N.; et al. Effect of vitamin K2 on the recurrence of hepatocellular carcinoma. Hepatology 2011, 54, 532–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalmeijer, G.W.; van der Schouw, Y.T.; Magdeleyns, E.; Ahmed, N.; Vermeer, C.; Beulens, J.W. The effect of menaquinone-7 supplementation on circulating species of matrix Gla protein. Atherosclerosis 2012, 225, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Theuwissen, E.; Cranenburg, E.C.; Knapen, M.H.; Magdeleyns, E.J.; Teunissen, K.J.; Schurgers, L.J.; Smit, E.; Vermeer, C. Low-dose menaquinone-7 supplementation improved extra-hepatic vitamin K status, but had no effect on thrombin generation in healthy subjects. Br. J. Nutr. 2012, 108, 1652–1657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theuwissen, E.; Magdeleyns, E.J.; Braam, L.A.; Teunissen, K.J.; Knapen, M.H.; Binnekamp, I.A.; van Summeren, M.J.; Vermeer, C. Vitamin K status in healthy volunteers. Food Funct. 2014, 5, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Knapen, M.H.J.; Jardon, K.M.; Vermeer, C. Vitamin K-induced effects on body fat and weight: Results from a 3-year vitamin K2 intervention study. Eur. J. Clin. Nutr. 2018, 72, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Hartley, L.; Clar, C.; Ghannam, O.; Flowers, N.; Stranges, S.; Rees, K. Vitamin K for the primary prevention of cardiovascular disease. Cochrane Database Syst. Rev. 2015, 9, CD011148. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, J.; Ozaki, I.; Matsuhashi, S.; Kuwashiro, T.; Takahashi, H.; Anzai, K.; Mizuta, T. Mechanisms of PKC-Mediated Enhancement of HIF-1α Activity and its Inhibition by Vitamin K2 in Hepatocellular Carcinoma Cells. Int. J. Mol. Sci. 2019, 20, 1022. https://doi.org/10.3390/ijms20051022
Xia J, Ozaki I, Matsuhashi S, Kuwashiro T, Takahashi H, Anzai K, Mizuta T. Mechanisms of PKC-Mediated Enhancement of HIF-1α Activity and its Inhibition by Vitamin K2 in Hepatocellular Carcinoma Cells. International Journal of Molecular Sciences. 2019; 20(5):1022. https://doi.org/10.3390/ijms20051022
Chicago/Turabian StyleXia, Jinghe, Iwata Ozaki, Sachiko Matsuhashi, Takuya Kuwashiro, Hirokazu Takahashi, Keizo Anzai, and Toshihiko Mizuta. 2019. "Mechanisms of PKC-Mediated Enhancement of HIF-1α Activity and its Inhibition by Vitamin K2 in Hepatocellular Carcinoma Cells" International Journal of Molecular Sciences 20, no. 5: 1022. https://doi.org/10.3390/ijms20051022




